Dear Editor,
Enterovirus 71 (EV71), which belongs to the genus Enterovirus of the family Picornaviridae, is one of the major pathogens that cause hand, foot and mouth disease, primarily in infants and young children. In recent years, epidemic and sporadic outbreaks of neurovirulent EV71 infections have been reported in many countries and regions, including Japan, China and Taiwan 1. However, there is still no effective antiviral treatment against severe EV71 infections, and no vaccine is available.
During their life cycle, viruses can induce many changes in their host cells including increased plasma membrane permeability 2. Although its detailed mechanism remains largely unknown, this effect on the plasma membrane could be due to the expression of many virus-encoded proteins 3, 4, 5. The expression of protein 2B from polioviruses and coxsackieviruses can increase the permeability of the cell membrane to the translational inhibitor hygromycin B 6, 7. A recent study that focused on protein 2B from coxsackievirus B3 (CVB3) showed that 2B can facilitate virus release by increasing the concentration of free cytosolic Ca2+ 8. However, little is known about 2B proteins from other enteroviruses, and the detailed mechanisms of how 2B changes cellular ion homeostasis and, thus, promotes virion release remain unclear.
Because the 2B protein of CVB3 can form homodimers and homotetramers that are located primarily in the cell membrane system, including the Golgi complex 9, 10, we asked whether the 2B proteins of EV71 might share similar characteristics. To address this question, the sequences of the 2B genes from these two viruses were compared. These sequences have relatively high similarity, especially within their two transmembrane domains (TM1 and TM2), structures that are essential for the function of viroporins (Supplementary information, Figure S1A). Next, we examined the subcellular localization of the EV71 2B protein in human rhabdomyosarcoma (RD) cells and found that it colocalized with the Golgi complex (Supplementary information, Figure S1B). Finally, when the 2B protein was fused to an HA tag and transiently overexpressed in 293T cells, a ∼13-kDa monomer, a putative 26-kDa dimer and a putative 52-kDa tetramer were detected after pull down in an immunoprecipitation assay using an anti-HA antibody. After treatment with β-mercaptoethanol (β-ME), which reduces disulfide bonds within proteins, the putative dimer and tetramer bands were no longer detected (Supplementary information, Figure S1C). These data indicate that the 2B protein in EV71 has characteristics in common with ion channels.
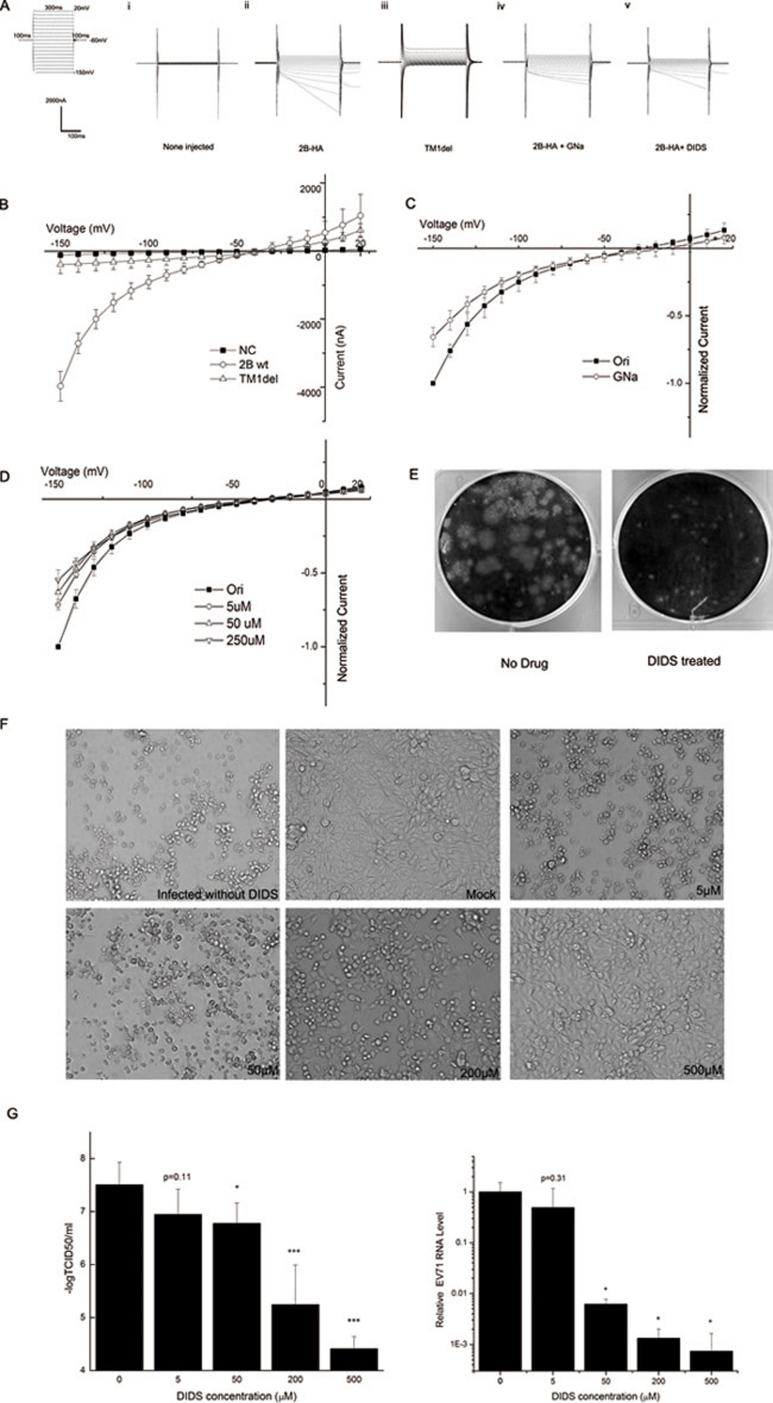
To test whether the EV71 2B protein indeed forms an ion channel, we used two-electrode voltage-clamp recordings to measure membrane currents in Xenopus oocytes that were injected with complementary RNA (cRNA) encoding 2B protein with a C-terminal HA tag. Expression of 2B-HA in oocytes was confirmed by immunostaining with an anti-HA antibody. Using confocal microscopy, the 2B-HA protein was shown to mainly localize at the cell membrane, suggesting that 2B is an integral membrane protein (Supplementary information, Figure S2). Thirty-six to forty-eight hours after injection, a significant increase in membrane conductance was detected in the oocytes (Figure 1Ai and Aii). We also generated a dysfunctional mutant in which the entire TM1 domain was deleted. When injected into oocytes, this mutant showed a dramatic decrease in current, indicating that the current is not induced simply by RNA injection (Figure 1Aiii). An I-V curve generated from four oocytes that were injected with 25 ng of cRNA showed that the 2B protein induced a current that was ∼4 000 nA at −150 mV and the reversal potential is around −30 mV (Figure 1B). These results suggest that the 2B protein might function as an ion channel in the oocyte membrane, thereby generating the observed current.
Figure 1.
(A) The general protocol used in the voltage-clamp experiments is shown in the left panel. (i, ii) At 24 to 36 h after cRNA injection, currents were recorded using a two-electrode voltage clamp, and injected and non-injected oocytes were compared. (iii) Raw current in an oocyte expressing the dysfunctional mutant TM1del. (iv) Raw current in an oocyte expressing 2B-HA after the extracellular NaCl in the oocyte Ringer solution (ORi) was replaced with equimolar sodium gluconate (GNa). (v) Raw current in an oocyte expressing 2B-HA and treated with 250 μM 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS). (B) Current-voltage (I-V) curves of the 2B-HA-mediated currents. The normalized currents from the chloride replacement experiment are plotted in C as the current at each voltage normalized to the peak current at −150 mV. The corresponding normalized currents following the application of a given dose of DIDS are shown in D. The data in B-D are presented as mean ± SD and were generated from at least three separate experiments. (E) A plaque assay was performed in cells treated with or without 10 μM DIDS. (F) RD cells were mock infected or infected with EV71 at an MOI of 0.002 and then cultured in medium containing the indicated concentration of DIDS for an additional 48 h. The cytopathic effect (CPE) was observed using microscopy (at 100× magnification). (G) RD cells were infected with the EV71 virus at an MOI of 0.002 for 30 min, and cultured in fresh medium containing indicated concentration of inhibitor. Two days after infection, the supernatant was collected for tissue culture infective dose (TCID50) analysis (left panel) or real-time RT-PCR analysis (right panel). The data are presented as mean ± SD and were generated from at least three separate experiments. (*P < 0.05, ***P < 0.001, compared with the no DIDS group)
To explore the ion selectivity of the 2B-mediated current, ion replacement experiments were performed. Both the inward and outward currents decreased when NaCl was replaced with sodium gluconate (Figure 1Aiv and 1C), and the reversal potential shifted from approximately −30 mV to −10 mV. In contrast, replacing the external potassium or sodium with N-methyl-D-glucamine only had a slight effect on the current (data not shown). Together, these data led to the hypothesis that chloride is the principal ion carried in the 2B-mediated current.
To further test whether 2B-mediated conductance is carried by chloride, we examined the effect of chloride channel inhibitors. The 2B-mediated current was partially inhibited by 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS; Figure 1Av). This inhibitory effect was concentration dependent (Figure 1D) but was difficult to recover. (We washed the oocyte with standard bath solution for about 3 min to attempt recovery.) Taken together, these data indicate that the 2B protein induces a chloride-dependent current that can be partially blocked by DIDS.
Finally, we tested whether DIDS can affect virus life cycle in a cell culture system. A plaque assay revealed that even a relatively low concentration (10 μM) of DIDS significantly inhibits virus production, as the plaque size was dramatically reduced after treatment (Figure 1E). Next, RD cells were infected with the EV71 virus, followed by the replacement of fresh medium containing DIDS 30 min later. Forty-eight hours later, an EV71-induced cytopathic effect (CPE) was observed microscopically, and this effect was significantly smaller in the DIDS-treated cells compared with the control cells. The inhibition of CPE by DIDS was concentration dependent, with a range of 5-500 μM (Figure 1F). A volume of 500 μM DIDS conferred the maximum protective effect against CPE. Higher concentrations (800 and 1 000 μM, data not shown) were tested; however, as was seen for the 50% tissue culture infective dose (TCID50), these higher concentrations did not cause significantly more inhibition than 500 μM. Moreover, at these higher concentrations, DIDS exerted morphological effects on the cells. In addition, we collected the cell supernatant for TCID50 (Figure 1G, left panel) and real-time RT-PCR (Figure 1G, right panel) analyses. The results show that both the viral titer and the RNA levels in the supernatant markedly decreased after DIDS treatment, and this effect was concentration dependent. In conclusion, DIDS appears to protect RD cells from EV71 viral infection by inhibiting virus production.
Many viruses encode small hydrophobic proteins that form a pore-like structure and increase the membrane permeability of cells. These proteins were initially described as viroporins and are generally considered to be promising targets for antiviral drugs. In this study, we observed a similarity between the sequences of the 2B proteins from EV71 and CVB3, and then investigated their potential function as ion channels in Xenopus oocytes. Because they express low levels of endogenous ion channels, oocytes are a good system for testing the putative ion channels properties of proteins. However, we observed that the subcellular localization of 2B is different in oocytes compared with RD cells. This may be due to different expression patterns in these two systems. Moreover, it has been reported that the localization of 2B proteins is different when they are coexpressed with other viral products rather than expressed alone 11. Therefore, the putative ion channel function and the native localization of the 2B protein should be detected simultaneously in an infection-permeability coupled assay.
A recent study has suggested that the CVB3 2B protein can mediate cytosolic calcium elevation 8. However, our data demonstrate that the current induced by the EV71 2B protein is mainly carried by chloride ions. In this regard, the calcium elevation in the cytoplasm is likely to be an indirect phenomenon induced by the 2B protein. Some investigators have reported that certain anion channels in the Golgi complex may serve as the source of counter anions for H+-ATP transporters 12. Moreover, the calcium uptake mechanism requires ATP to maintain homeostasis in the Golgi complex 13. Thus, the 2B-mediated, chloride-dependent current may perturb anion homeostasis in the Golgi complex and ultimately lead to calcium leakage into the cytoplasm. Nevertheless, whether or not calcium ions can directly pass through a 2B channel needs more investigations. It remains unclear how 2B is involved in the viral life cycle. As a cytolytic virus, EV71 may primarily release its progeny via cell lysis. The 2B expression would perturb ion homeostasis and may disrupt cellular function 8. Some studies have suggested that 2B induces cell apoptosis via a caspase-dependent pathway 14, which may explain the role of 2B in cell lysis. However, little direct evidence has been provided to establish a link between changes in permeability and 2B-induced apoptosis.
In this study, DIDS potently inhibited virus production in RD cells. We hypothesize that DIDS suppresses virus release by inhibiting the 2B protein-mediated chloride-dependent current. However, as a classic anion exchanger blocker, DIDS also has effects on the cells themselves 15. Nevertheless, in our study, DIDS had no significant effect on either cell growth or morphology at relatively low concentrations (≤ 500 μM), suggesting that the inhibition of DIDS on EV71 production can be primarily attributed to its effects on the 2B-mediated current. Clearly, additional experiments are needed to further test this hypothesis.
In summary, we report that the 2B protein may mediate a chloride-dependent current in oocytes, and DIDS, an inhibitor of this current, blocks virus production and virus-induced CPE in RD cells. This study provides a new approach for identifying potential anti-EV71 drugs. Moreover, understanding the properties of the 2B protein as an ion channel may help shed light on the life cycle of this virus.
Acknowledgments
We thank Prof FJ van Kuppeveld (Radboud University Nijmegen Medical Centre) for useful discussions and Dr Jun-ichi Miyazaki (Osaka University) for providing the pCAGGS plasmid. We also thank Dr Sheri Skinner for English editing. This work was supported by a CAS project (KSCX2-YW-R-161), a grant from the National Ministry of Science and Technology (20072714), grants from the National Natural Science Foundation of China (30950002, 30623003, 30721065, 30801011, 30870126 and 90713044), grants from the Science and Technology Commission of Shanghai Municipality (08DZ2291703, 088014199 and 08431903004), a National Science and Technology Major Project (2008ZX10002-014, 2008ZX10004-002 and 2009ZX10004-105), a grant from the National 973 Key Project (2007CB512404), grants from SPHRF (SPHRF2008001 and SPHRF2009001), a grant from the National 863 Project (2006AA02A247) and a grant from the Li Kha Shing Foundation.
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
The EV71 2B protein has many similarities with the CBV3 2B protein.
Immunofluorescent detection of 2B proteins in Xenopus oocytes.
Materials and Methods
References
- Weng KF, Chen LL, Huang PN, Shih SR. Neural pathogenesis of enterovirus 71 infection. Microbes Infect. 2010;12:505–510. doi: 10.1016/j.micinf.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Carrasco L. Modification of membrane permeability by animal viruses. Adv Virus Res. 1995;45:61–112. doi: 10.1016/S0065-3527(08)60058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez ME, Carrasco L. Viroporins. FEBS Lett. 2003;552:28–34. doi: 10.1016/s0014-5793(03)00780-4. [DOI] [PubMed] [Google Scholar]
- Fischer WB, Kruger J. Viral channel-forming proteins. Int Rev Cell Mol Biol. 2009;275:35–63. doi: 10.1016/S1937-6448(09)75002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Xie S, Sun B. Viral proteins function as ion channels. Biochim Biophys Acta. 2011;1808:510–515. doi: 10.1016/j.bbamem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens JR, Kirkegaard K. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 1995;14:894–907. doi: 10.1002/j.1460-2075.1995.tb07071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kuppeveld FJ, Melchers WJ, Kirkgaard K, Doedens JR. Structure-function analysis of coxsackie B3 virus protein 2B. Virology. 1997;227:111–118. doi: 10.1006/viro.1996.8320. [DOI] [PubMed] [Google Scholar]
- van Kuppeveld FJ, Hoenderop JG, Smeets RL, et al. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997;16:3519–3532. doi: 10.1093/emboj/16.12.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong AS, Melchers WJ, Glaudemans DH, Willems PH, van Kuppeveld FJ. Mutational analysis of different regions in the coxsackievirus 2B protein: requirements for homo-multimerization, membrane permeabilization, subcellular localization, and virus replication. J Biol Chem. 2004;279:19924–19935. doi: 10.1074/jbc.M314094200. [DOI] [PubMed] [Google Scholar]
- de Jong AS, Wessels E, Dijkman HB, et al. Determinants for membrane association and permeabilization of the coxsackievirus 2B protein and the identification of the Golgi complex as the target organelle. J Biol Chem. 2003;278:1012–1021. doi: 10.1074/jbc.M207745200. [DOI] [PubMed] [Google Scholar]
- Suhy DA, Giddings TH, Kirkegaard K. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J Virol. 2000;74:8953–8965. doi: 10.1128/jvi.74.19.8953-8965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen MH, Jones SM, Howell KE, Caldwell JH. GOLAC: an endogenous anion channel of the Golgi complex. Biophys J. 2000;78:2918–2928. doi: 10.1016/S0006-3495(00)76832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baelen K, Dode L, Vanoevelen J, et al. The Ca2+/Mn2+ pumps in the Golgi apparatus. Biochim Biophys Acta. 2004;1742:103–112. doi: 10.1016/j.bbamcr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Madan V, Castello A, Carrasco L. Viroporins from RNA viruses induce caspase-dependent apoptosis. Cell Microbiol. 2008;10:437–451. doi: 10.1111/j.1462-5822.2007.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Sjoholm C, Hoffmann EK. Identification of the anion exchange protein of Ehrlich cells: a kinetic analysis of the inhibitory effects of 4,4′-diisothiocyano-2,2′-stilbene-disulfonic acid (DIDS) and labeling of membrane proteins with 3H-DIDS. J Membr Biol. 1986;92:195–205. doi: 10.1007/BF01869388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The EV71 2B protein has many similarities with the CBV3 2B protein.
Immunofluorescent detection of 2B proteins in Xenopus oocytes.
Materials and Methods