MHC molecules have been shown to play key roles in the immune system including regulating T-cell repertoire development through the process of positive and negative selection. MHC molecules also function to bridge the innate and adaptive immune system through the presentation of processed antigenic peptides to T-cells. Strategies employed by tumour cells and viruses that downregulate expression of MHC molecules to avoid immune detection highlight the crucial role that these molecules play in maintaining immune homeostasis. Along with the well defined classical functions of MHC molecules several studies have demonstrated non-classical roles for MHC molecules in immunity, such as regulating B-cell survival in addition to driving cytokine production in macrophages. The critical importance of these non-classical roles of MHC molecules has been highlighted in a recent article in Nature Immunology, in which Liu et al. 1 elegantly demonstrate a novel role for intracellular MHC Class II in promoting the full activation of TLR responses via direct interaction with the tyrosine kinase Btk 1.
Toll like receptors (TLRs) are specialized innate immune receptors that have evolved to recognize distinct highly conserved molecular motifs on pathogens and in doing so, drive the production of inflammatory cytokines or type I interferons and the activation of transcription factors NFκB and IRF3/7, respectively 2. Differential recruitment of key adapter molecules to the intracellular domain of the various TLRs ensures that an appropriate antiviral or antibacterial response is initiated. For example, TLR3, 7 and 9 traffic to endosomal compartments following viral or bacterial nucleic acid recognition where they interact with MyD88 in order to regulate both NFκB- and IRF7-dependent cytokine induction. In contrast, TLR4, the receptor for gram negative LPS, requires MyD88 in complex with Mal and signals from the cell membrane in order to activate NFκB, whilst relocation of TLR4 in complex with adaptors TRIF and TRAM to the endosome is required for activation of the IFN pathway. Thus, TLR signaling is both spatially and temporally regulated, ensuring a pathogen-specific immune response. Investigations into non-canonical pathways that are activated downstream of the TLRs have brought Bruton's tyrosine kinase (Btk), a non-receptor tyrosine kinase, to the fore as key signaling molecule in this respect. In these studies Btk has been demonstrated to interact with the adaptor molecules MyD88 and Mal downstream of TLR4, 6, 8, and 9 resulting in NFκB activation and the production of TNF-α and IL-6 3, 4, 5, 6.
In the context of macrophages, TLRs not only regulate cytokine production but also enhance the expression of antigen presentation and co-stimulatory molecules that facilitate the clearance of pathogen and the induction of an adaptive immune response. The idea that crosstalk between MHC molecules and TLRs is important in the immune response is not new, although prior to this study the exact mechanism was not clearly defined. One such study shows that MHC class II expression enhanced TLR-mediated cytokine production via co-localization of the receptors in lipid raft domains at the membrane 7. Another study demonstrated that, in human monocytes, ligation of MHC class II with specific antibodies or staphylococcal enterotoxin induced MyD88 expression followed by NFκB activation and pro-inflammatory cytokine production. However, no direct interaction between MHC class II and MyD88 was established 8. Therefore, although it has been previously appreciated that there is crosstalk between these two pathways, the exact underlying mechanism has remained elusive.
In the current study, Liu et al. demonstrate that loss of MHC Class II (H2−/− mice) results in reduced production of pro-inflammatory cytokines and type I IFNs in response to intraperitoneal administration of LPS (TLR4), CpG (TLR9) and poly(I:C) (TLR3), extending previous findings by Frei et al. and Kissner et al., suggesting that MHC class II is playing a fundamental role in driving TLR-mediated inflammatory responses 7, 8. Importantly, reconstitution of bone marrow cells from H2+/+ or H2−/− mice into lethally irradiated wild-type mice clearly demonstrated that protection from endotoxic shock in H2−/− mice was independent of T-cells and was in fact due to lack of MHC class II expression on antigen presenting cells (APCs) which resulted in impaired MyD88-dependent and TRIF-dependent TLR signaling. In search of a mechanism to explain this phenomenon, the authors performed a screen of kinases known to be involved in TLR signaling. This revealed impaired phosphorylation of the tyrosine kinase Btk following TLR3, 4 and 9 stimulation of peritoneal macrophages from H2−/− mice compared with controls. Importantly, overexpression of MHC class II α and β-chains restored Btk activation in H2−/− peritoneal macrophages, underlining the critical importance of MHC class II in TLR-induced Btk activation. In addition TNF-α, IL-6 and IFN-β were all reduced in Btk−/− macrophages, suggesting that MHC class II molecules facilitate TLR-triggered inflammatory responses via regulating the activity of Btk. Whereas previous studies have demonstrated that Btk is activated downstream of TLR4 and 9, this is the first indication that Btk is activated downstream of TLR3 and that MHC class II is critically required for these responses. In addition, this data strongly suggests that Btk regulates the TRIF-driven pathway, leading to IRF3 activation and IFN-β production, a previously unreported observation.
MHC class II can be found both at the plasma membrane and in intracellular compartments. Interestingly, as mentioned previously, TLR3, 7 and 9 traffic from the ER and signal from endosomal compartments once ligated, whereas TLR4 is internalized from the cell membrane to drive TRIF-driven NFκB and IRF3 activity from the endosome. Importantly trafficking of the TLRs examined in this study to endosomes following stimulation was not affected in H2−/− mice, however, a difference in the signaling complexes formed by MHC class II at the plasma membrane and in intracellular compartments was revealed. Specifically, the association between Btk and MHC class II was also found to occur in intracellular compartments (later identified as endosomes) and not at the plasma membrane, indicating that the location of MHC class II molecules related to their function. Another piece of the puzzle was solved by the finding that activation of Btk in these complexes was shown to require CD40, a finding consistent with previous studies in innate B1-cells that demonstrated that ligation of CD40 resulted in activation of Btk 9. With respect to the mechanism by which this complex contributes to the TLR response, association of Btk with the key pathway mediators MyD88 and TRIF was examined. These studies found diminished association of Btk with MyD88 or TRIF in H2−/− macrophages compared to wild type following LPS stimulation. Given previous findings that Btk can interact with and phosphorylate MyD88 (as can MHC class II 8), this result may not be too surprising. However, the finding that the association between Btk and MyD88 (and TRIF) is reduced in the absence of MHC class II suggests that this complex requires MHC class II and occurs at the endosome, implying a novel role for Btk in regulating signals emanating from the endosome such as those regulating IFN-β production, a previously underappreciated aspect of Btk function.
Thus, Liu et al. have demonstrated that there is crosstalk between the two pathways with MHC class II functioning as a novel adaptor molecule to form a complex with Btk and CD40 at the endosome and further enhance the TLR response. This study clearly shows that in the absence of this complex, responses to TLR3, 4 and 9 are blunted and that for full robust response, formation of this endosomal MHC class II-Btk containing signalsome is required. Interestingly, crosstalk between the pathways works both ways – for example, in dendritic cells TLR stimulation enhances MHC class II expression and stability as a result of reduced turnover of the receptor by MARCH-1, a membrane associated E3 ligase 10. Therefore, we now potentially have a situation where TLR signaling not only provides the stimulus to initiate the production of type I IFNs and pro-inflammatory cytokines, but also prevents the degradation of MHC class II molecules, facilitating the formation of the Btk-CD40-MHC class II signalling complex at the endosome that further amplifies TLR driven responses (Figure 1). The role of Btk in TLR-signaling is somewhat controversial, with a number of studies demonstrating no defects either in mice lacking Btk or in patients with X-linked agammaglobulemia (XLA). These discrepancies may now be explained in light of these new findings that demonstrate the association between MHC class II and Btk – for example, MHC class II expression is limited largely to APCs and tightly regulated, with activation of many cells required to observe expression. Neutrophils, one cell type for example where a role for Btk has been called into question, only express MHC class II following stimulation 11. Therefore, the context in which these experiments were performed and their interpretation must now be re-evaluated given this new data.
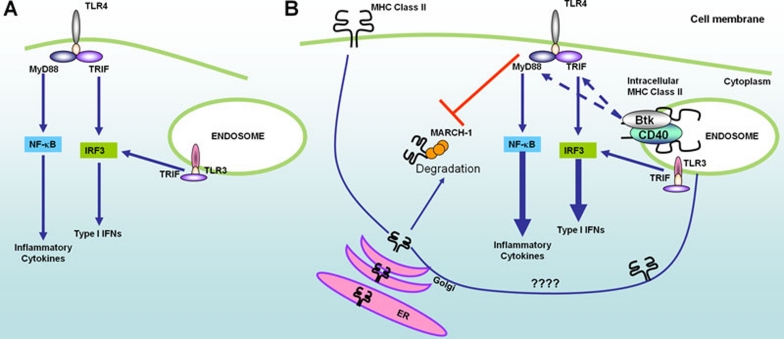
Figure 1.
Schematic of crosstalk between TLR pathway and MHC molecules. (A) The traditional view of TLR signaling which holds that TLR ligation initiates several signaling cascades within the cell that drive the production of type I IFNs and pro-inflammatory cytokines that are essential for both innate and adaptive immune responses resulting in pathogen clearance. (B) Recent evidence has shown that a second pathway involving crosstalk between the TLR pathway and MHC pathway is initiated. This crosstalk results in the formation the Btk-CD40-MHC class II signaling complex at the endosome that enhances TLR-driven cytokine production. Ligation of TLRs also results in the inhibition of MARCH-1-mediated ubiquitination and degradation of MHC class II molecules, potentially increasing the pool of intracellular MHC class II molecules available to form the MHC-Btk signalosome at the endosome.
Given the pathogenic role of TLR-driven pro-inflammatory cytokines and type I IFNs in autoimmune conditions such as rheumatoid arthritis and systemic lupus erythrematosus, understanding the relative contribution of these pathways to the overall response and the control points on this positive feedback loop is of critical importance. TLR9 has been implicated in the pathogenesis of SLE through over-production of pro-inflammatory cytokines and type I IFNs following recognition of self-nucleic acids complexed with autoantibodies. Whilst Btk is known to regulate TLR9 signaling, a recent study by Kubo et al. demonstrated that inhibition of Btk activity following TLR9 stimulation by the paired immunoglobulin-like receptor B (PIR-B) in B1-cells was critical to prevent autoimmunity. Interestingly, the ligand for PIR-B is MHC class I, demonstrating an additional link between MHC molecules, Btk and immune regulation 12. The findings of Liu et al. therefore support the case for Btk being an excellent target for therapeutic intervention for the treatment of SLE and underline the need for further investigation into the mechanisms that control Btk activation with a view to understanding its contribution to autoimmune conditions in greater detail.
References
- Liu X, Zhan Z, Li D, et al. Intracellular MHC class II molecules promote TLR-triggered innate immune respnses by maintaining activation of the kinase Btk. Nature Immunol. 2011;12:416–424. doi: 10.1038/ni.2015. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Horwood NJ, Page TH, McDaid JP, et al. Bruton's tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J Immunol. 2006;176:3635–3641. doi: 10.4049/jimmunol.176.6.3635. [DOI] [PubMed] [Google Scholar]
- Doyle SL, Jefferies CA, Feighery C, O'Neill LA. Signaling by Toll-like receptors 8 and 9 requires Bruton's tyrosine kinase. J Biol Chem. 2007;282:36953–36960. doi: 10.1074/jbc.M707682200. [DOI] [PubMed] [Google Scholar]
- Doyle SL, Jefferies CA, O'Neill LA. Bruton's tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during NFkappaB activation by lipopolysaccharide. J Biol Chem. 2005;280:23496–23501. doi: 10.1074/jbc.C500053200. [DOI] [PubMed] [Google Scholar]
- Jefferies CA, Doyle S, Brunner C, et al. Bruton's tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. J Biol Chem. 2003;278:26258–26264. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- Frei R, Steinle J, Birchler T, et al. MHC class II molecules enhance Toll-like receptor mediated innate immune responses. PLoS One. 2010;5:e8808. doi: 10.1371/journal.pone.0008808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissner TL, Ruthel G, Alam S, Ulrich RG, Fernandez S, Saikh KU. Activation of MyD88 signaling upon staphylococcal enterotoxin binding to MHC class II molecules. PLoS One. 2011;6:e15985. doi: 10.1371/journal.pone.0015985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner C, Avots A, Kreth HW, Serfling E, Schuster V. Bruton's tyrosine kinase is activated upon CD40 stimulation in human B lymphocytes. Immunobiology. 2002;206:432–440. doi: 10.1078/0171-2985-00192. [DOI] [PubMed] [Google Scholar]
- Walseng E, Furuta K, Goldszmid RS, Weih KA, Sher A, Roche PA. Dendritic cell activation prevents MHC class II ubiquitination and promotes MHC class II survival regardless of the activation stimulus. J Biol Chem. 2010;285:41749–41754. doi: 10.1074/jbc.M110.157586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marron TU, Rohr K, Martinez-Gallo M, Yu J, Cunningham-Rundles C. TLR signaling and effector functions are intact in XLA neutrophils. Clin Immunol. 2010;137:74–80. doi: 10.1016/j.clim.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Uchida Y, Watanabe Y. Augmented TLR9-induced Btk activation in PIR-B-deficient B-1 cells provokes excessive autoantibody production and autoimmunity. J Exp Med. 2009;206:1971–1982. doi: 10.1084/jem.20082392. [DOI] [PMC free article] [PubMed] [Google Scholar]