Abstract
Blood vessels normally maintain stereotyped lumen diameters and their stable structures are crucial for vascular function. However, very little is known about the molecular mechanisms controlling the maintenance of vessel diameters and the integrity of endothelial cells. We investigated this issue in zebrafish embryos by a chemical genetics approach. Small molecule libraries were screened using live Tg(kdrl:GRCFP)zn1 transgenic embryos in which endothelial cells are specifically labeled with GFP. By analyzing the effects of compounds on the morphology and function of embryonic blood vessels after lumen formation, PP1, a putative Src kinase inhibitor, was identified as capable of specifically reducing vascular lumen size by interrupting endothelial-cell integrity. The inhibitory effect is not due to Src or general VEGF signaling inhibition because another Src inhibitor and Src morpholino as well as several VEGFR inhibitors failed to produce a similar phenotype. After profiling a panel of 22 representative mammalian kinases and surveying published data, we selected a few possible new candidates. Combinational analysis of these candidate kinase inhibitors established that PP1 induced endothelial collapse by inhibiting both the VEGFR2 and MAP kinase pathways. More importantly, combinatory use of two clinically approved drugs Dasatinib and Sunitinib produced the same phenotype. This is the first study to elucidate the pathways controlling maintenance of endothelial integrity using a chemical genetics approach, indicating that endothelial integrity is controlled by the combined action of the VEGFR2 and MAP kinase pathways. Our results also suggest the possible side effect of the combination of two anticancer drugs on the circulatory system.
Keywords: PP1, endothelial-cell integrity, vascular lumen, zebrafish
Introduction
The formation and maintenance of vascular lumens by endothelial cells are very important for the establishment of a functional vertebrate circulatory system. Vascular lumens are mainly formed by two means, endothelial-cell hollowing or endothelial-cell-cord hollowing 1, 2, 3, 4. In vitro studies indicate that this process is regulated by integrin- and cdc42/Rac1-dependent pinocytic events downstream of the integrin-extracellular matrix signaling pathway 5, 6, 7. However, the mechanism of vascular lumen maintenance is still largely unknown 8.
The major challenge facing investigations into this question is that classic loss-of-function and gain-of-function genetic approaches are not applicable because the initial defects in vasculogenesis or angiogenesis usually impair lumen formation as well. Although conditional gene knockouts in mice may overcome this shortcoming, analysis of vessel diameter in live embryos is made difficult by in utero development. In this study, we used a transgenic zebrafish model coupled with a temporally controlled chemical genetics approach to elucidate the mechanism of vascular lumen maintenance. Vascular development of zebrafish embryos has been well studied 9, 10. The large axial vessels (dorsal aorta and posterior cardinal vein) and caudal plexus are formed by vasculogenesis while other vessels are formed by angiogenesis. By 24 hpf (hours post fertilization), the heart begins to beat and blood cells circulate in the axial vessels. Furthermore, unlike mammal models, zebrafish embryos can survive and develop for up to about 1 week without any blood circulation owing to its small body that is readily accessible to the diffusion of oxygen, CO2, and nutrients 11. This ability offers a unique opportunity to examine lumen maintenance defects that usually cause lethality in mammals. In addition, several endothelial-cell-specific GFP transgenic zebrafish lines have been generated with VEGFR2 (also known as flk or kdrl) or fli1a promoters, which render easy, fast, and continuous observation of blood vessel development under a fluorescence microscope 12.
Small-molecule chemicals offer easier and more precise temporal control of gene function by allowing the addition and removal of a given compound at preselected time points. A chemical capable of specifically modifying a biological process is not only a useful molecular and biological tool but also a potential drug candidate. Zebrafish has been used for chemical library screens, including antiangiogenesis screens, but most of them have been conducted by adding compounds as early as 2 hpf or at prevasculogenesis or angiogenesis stages. To identify small molecules that can specifically regulate the maintenance of the vascular system, we performed a screen using two libraries consisting of ∼1700 chemical compounds by adding each compound to embryos placed in 96-well plates at 30 hpf, when blood circulation is well established, and examined changes in blood circulation at 48 hpf. One of the compounds, PP1, a previously known inhibitor of Src kinase, stopped circulation but did not have any morphological effect on the whole embryo. Further examination of Tg(kdrl:GRCFP)zn1 transgenic embryos treated by PP1 revealed that blood vessels, in particular the dorsal aorta and intersegmental vessels (ISV), had reduced or closed vessel lumens through endothelial-cell collapse. We show here that pathways triggered by PP1 in the context of blood vessel lumen regulation are not Src-dependent but rather involve a combinatory action of VEGFR and MAP kinase signaling pathways.
Results
Identification and characterization of PP1 as a molecule that regulates vascular lumen maintenance
Through screening ∼1 700 small-molecule compounds of the BioMol and Prestwick libraries, PP1 was identified as one of the compounds that stopped blood circulation but had no effect on heart beat and overall body morphology (Figure 1 and supplementary information, Figure S1). Angiography with a 2 000-kDa green fluorescent dye showed that the dye was completely blocked in the heart and did not enter the blood vessels (Figure 1D). To further reveal the activity of PP1 in regulating blood vessel formation as well as function, it was added to embryos at the shield, 30 hpf, 2 dpf, 3 dpf, and 4 dpf stages. When added at the shield stage, PP1 inhibited the growth of intersegmental vessel in a dose-dependent manner, completely blocking ISV sprouting at 5 μM concentration (Supplementary information, Figure S2). When added at later stages of 30 hpf, 2 dpf, 3 dpf, and 4 dpf, 10 μM PP1 could completely block the already-established circulation after about 16 h of treatment (Supplementary information, Movies S1 and S2).
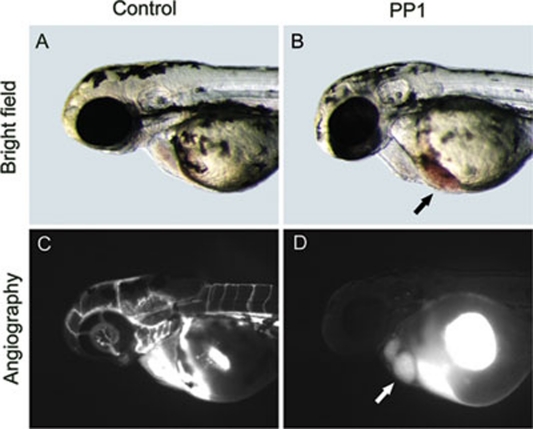
Figure 1.
PP1 blocked established blood circulation. PP1 was added to embryos at 30 hpf when blood circulation was already established. At 48 hpf the blood circulation of PP1-treated embryos stopped. (A and C) Control embryos. (B and D) PP1-treated embryos. Except blood cells stuck at sinus venous (black arrow), PP1-treated embryos looked normal. (C) Fluorescein isothiocyanate dextran (MW=2 000 000 Da) was observed in circulation, labeling the whole vasculature. (D) Fluorescein isothiocyanate dextran was stuck in heart (white arrow), indicating the lumen of dorsal aorta reduced and did not allow the dye to pass.
To study the unique activity of PP1 in regulating the late maintenance of blood vessels, we chose to add it at 3 dpf and observe the phenotype at 4 dpf. Lateral-view images taken by confocal microscopy showed that the dorsal aorta of PP1-treated embryos was thinner than that of the control, implying lumen size reduction (Figure 2B). Cross sections confirmed that PP1 indeed caused vascular lumens to reduce. In PP1-treated embryos, both the dorsal aorta and cardinal vein were narrower, with the dorsal aorta shrinking more severely. The lumen of the dorsal aorta was almost absent (Figure 3B and 3F). The blockage of blood vessels was further demonstrated by microangiography with tetramethylrhodamine dextran (2 000 kDa) in Tg(kdrl:GRCFP)zn1 embryos. In PP1-treated embryos, tetramethylrhodamine dextran failed to enter circulation (Supplementary information, Figure S3). To rule out the possibility that the absence of blood flow and blood pressure could lead to collapse of vascular lumens, a myosin-ATPase inhibitor BDM (2, 3-butanedione 2-monoxime) was used to stop the heart beat of 3 dpf zebrafish embryos 13. After incubation of embryos in BDM for 24 h, vascular lumen appeared intact in the absence of heart beat (Supplementary information, Figure S4). Moreover, PP1 treatment did not stop heart beat. Together, these data indicate that lack of blood circulation caused by PP1 was the consequence of the reduction of vascular lumens.
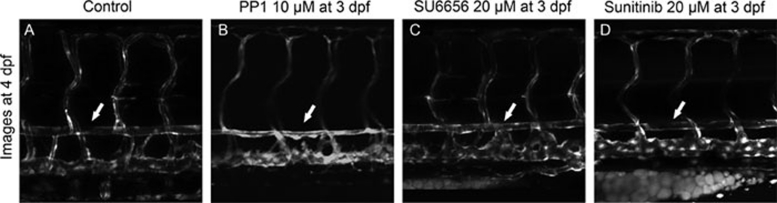
Figure 2.
PP1 caused dorsal aorta to reduce while SU6656 and Sunitinib did not. All these three small molecules were added to Tg(kdrl:GRCFP)zn1 embryos at 3 dpf and images were taken at 4 dpf. The trunk region above yolk extension was shown. White arrows point to the dorsal aorta. In PP1-treated embryos the dorsal aorta looked thinner than in control. In SU6656- or Sunitinib-treated embryos, the dorsal aortas remained the same.
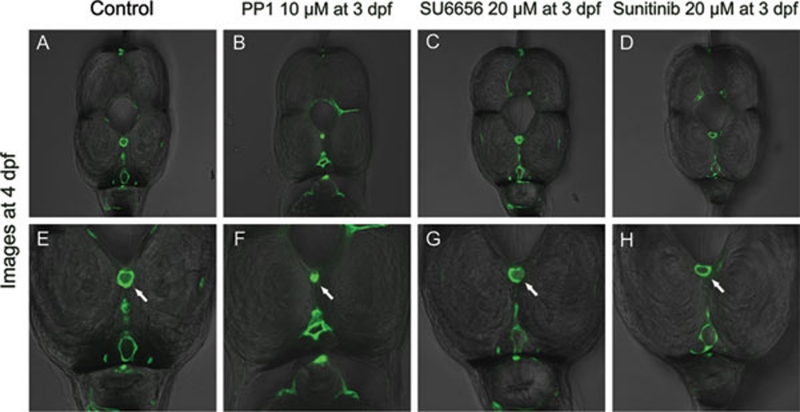
Figure 3.
Cross section showed PP1 did cause vascular lumens to collapse. The lower row is the magnification of upper row. White arrows point to the lumen of dorsal aorta. In PP1-treated embryos, both the dorsal aorta and cardinal vein collapsed, while the dorsal aorta collapsed more severely than the cardinal vein. The lumen of dorsal aorta disappeared. In other groups, the dorsal aorta and cardinal vein remained intact.
PP1 did not appear to kill endothelial cells as the green fluorescence of either Tg(kdrl:GRCFP)zn1 or Tg(fli1a:nEGFP)y7 embryos remained intact, suggesting the presence of live endothelial cells. To further confirm this conclusion, whole mount TUNEL assay was performed with DMSO (vehicle control) or PP1-treated Tg(fli1a:nEGFP)y7embryos and no difference in apoptosis of endothelial cells was observed (Supplementary information, Figure S5). Under the same PP1 treatment condition, the pronephric duct lumen appeared intact (Supplementary information, Figure S6), indicating that the activity of PP1 on endothelial cells was specific.
To examine structural changes in endothelial-cell morphology induced by PP1 administration, we analyzed blood vessels by electron microscopy. Compared to the control embryos at 4 dpf (Figure 4A), the PP1-treated embryos exhibited non-functional aortic lumens and no blood cells in the dorsal aorta (Figure 4B). The diameter of the aortic lumen was smaller than a red blood cell (Figure 4B), consequently prohibiting circulation. The endothelial cells in dorsal aorta, posterior cardinal vein, and ISVs in PP1-treated embryos were collapsed (Figure 4 and Supplementary information, Figures S6 and S7). In the control embryos, they were squamous shaped (Figure 4C), whereas in PP1-treated embryos, they were shorter and thicker, and lacked tight adhesions with surrounding cells (Figure 4D). The cell junctions between the endothelial cells were also abnormal in PP1-treated embryos (Figure 4D, black stars) compared with control embryos (Figure 4C, white star). The inhibitory activities of PP1 were not reversible as circulation failed to recover in PP1-treated embryos after washing with fresh fish water.
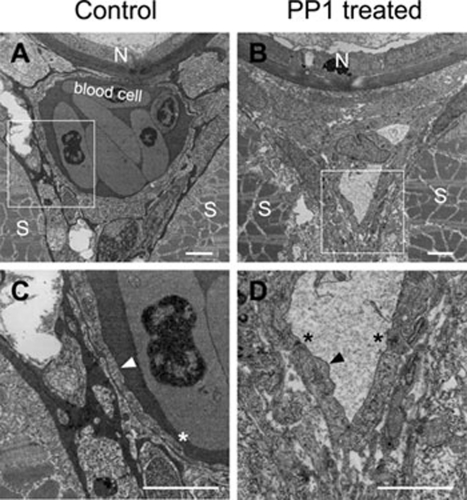
Figure 4.
The ultra-structural changes of endothelial cells in aorta revealed by electron microscopy. The images were transverse sections with dorsal to the top. The lumen changes between the control embryos (A) and the PP1-treated embryos (B) at 4 dpf. (C and D) The structural changes of endothelial cells boxed in A and B, respectively. Note that the endothelial cell in D (black arrow head) was short and thick compared with that in C (white arrow head). The stars indicated the cell junctions between endothelial cells. N, notochord; S, somite. Scale bars are 2 μm.
To investigate if PP1 could also cause vascular lumen regression in mammalian systems, we added it to established network of HUVEC (human umbilical vascular endothelial cell) in Matrigel. As shown in Supplementary information, Figure S8, the HUVEC network was disrupted in a dose-dependent manner.
Vessel lumen reduction by PP1 is neither through Src kinase nor VEGF pathway alone
PP1 was initially reported as an ATP-competitive Src kinase inhibitor 14. To study the mode of action of PP1 in zebrafish, we first compared it with SU6656 (Supplementary information, Figure S1), the most selective Src kinase inhibitor reported so far 15, 16. Unexpectedly, we did not observe any lumen diameter reduction induced by SU6656, suggesting a non-Src mechanism (Figures 2C, 3C and 3G). To confirm this idea, we knocked down the zebrafish Src gene by injecting morpholino that specifically blocks the translation of the Src protein 17. Again, no lumen diameter reduction was observed. Since PP1 inhibited angiogenic ISV formation when added earlier at the shield stage, which can also be caused by inhibiting VEGF signaling (Supplementary information, Figure S2) 12, we tested if Sunitinib 18, 19, a kinase inhibitor that has strong activity on multiple VEGFRs could also reduce lumen size like PP1 when added at late stages. As shown in Figures 2 and 3, Sunitinib did not produce the same defective phenotype as PP1 did. These studies suggest that PP1 has activity in regulating other targets required for maintaining blood vessel lumens.
Combined action of VEGFR and MAP kinase pathways maintains vessel lumen integrity
The ATP-analogous structure of PP1 suggests that its primary targets are most likely kinases. To explore other kinases inhibited by PP1 as potential targets involved in blood vessel lumen maintenance, we profiled its activity against a panel of 22 representative kinases. As shown in Table 1, PP1 inhibited ABL1 (89% at 5 μM), LCK (93% at 5 μM), and VEGFR2 (92% at 5 μM). As mentioned above, inhibition of VEGFR2 alone failed to reproduce the phenotype of late PP1 treatment. It has been previously reported that PP1 has multiple targets 20, as confirmed here in our kinase profiling study. We therefore tested the combinatory action of these potential candidates using different selective kinase inhibitors. However, combined testing of ABL1, LCK and VEGFR2 using specific chemical inhibitors (Bcr-abl Inhibitor for ABL1, SU6656 for LCK, and Sunitinib for VEGFR2) did not reproduce the same phenotype of PP1 treatment (n = 60, embryos treated at 3 dpf, none showed block of circulation).
Table 1. PP1 kinase profiling.
| Kinase | Inhibition (%) | Kinase | Inhibition (%) |
|---|---|---|---|
| ABL1 | 89 | MAP2K1 (MEK1) | 20 |
| CAMK1D (CaMKI delta) | 6 | MAP3K9 (MLK1) | 29 |
| CAMK2B (CaMKII beta) | -1 | MAPK1 (ERK2) | 3 |
| CDK2/cyclin A | 5 | MAPK8 (JNK1) | 7 |
| CHEK2 (CHK2) | 8 | MAPKAPK2 | 2 |
| CLK1 | 10 | MYLK2 (skMLCK) | 5 |
| CSNK1D (CK1 delta) | 68 | NEK2 | 2 |
| FLT3 | 56 | PLK1 | -1 |
| GSK3B (GSK3 beta) | 0 | RPS6KA1 (RSK1) | 9 |
| KDR (VEGFR2) | 92 | SGK (SGK1) | 4 |
| LCK | 93 | STK3 (MST2) | 13 |
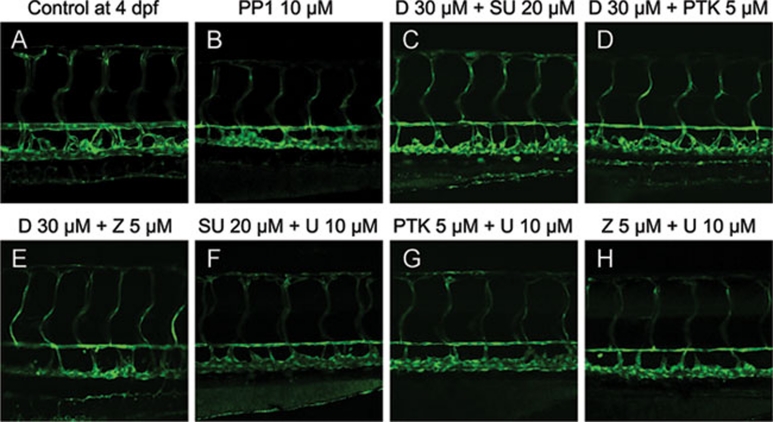
It has been noticed that PP1 can inhibit the MAP kinase pathway in HUVEC 21. In another study, anthrax toxin, whose targets include MEK1/2 22, appeared to reduce vessel lumen size in zebrafish 23. During earlier developmental stages, MEK1/2 is also involved in artery specification in both zebrafish and mice 24, 25. Together these findings imply that MEK1/2 may cooperate with VEGFR to regulate blood vessel lumen diameter. We examined this hypothesis by combining VEGFR inhibitor Sunitinib and MEK1/2 inhibitor U0126. Indeed, U0126 (10 μM) combined with Sunitinib (20 μM) resulted in a reduction of vessel lumen size (Figure 5F), whereas individually they did not. Combined treatment of another MAP kinase signaling pathway inhibitor Dasatinib (20 μM) with Sunitinib (20 μM) produced a similar phenotype as PP1, whereas each individual inhibitor did not result in any vessel shrinkage even at much higher concentrations (Figure 5C).
Figure 5.
Combinational treatment of kinase inhibitors induces the similar phenotype produced by PP1. All images are lateral view with dorsal to the top and anterior to the left. The combinational treatment of Dasatinib (D) or U0126 (U) with Sunitinib (SU), PTK787 (PTK), or ZM323881 (Z) resulted in the shrinkage of dorsal aorta.
Since there are three major VEGF receptors, it is desirable to determine which receptor is involved in combinatory action with MEK1/2. To address this issue, additional highly selective VEGFR inhibitors PTK787 and ZM323881 were tested. Both of these inhibitors (PTK787 at 5 μM or ZM323881 at 5 μM), when individually combined with Dasatinib or U0126, induced a reduction of vessel lumen size (Figure 5D, 5E, 5G, and 5H). However, they did not lead to this phenotype when added alone. Given that PTK787 does not efficiently inhibit VEGFR3 26 and ZM323881 is inactive against VEGFR1 27, 28, VEGFR2 is therefore the most likely factor involved in maintaining lumen size. Collectively, our results indicate that PP1 may induce reduction of the blood vessel lumen size by inhibiting both the VEGFR2 and MAP kinase pathways.
Discussion
To the best of our knowledge, this study represents the first analysis of mechanistic pathways involved in maintaining blood vessel lumen diameter after the establishment of functional circulation. The temporal chemical genetics approach coupled with transgenic zebrafish technology offers a novel entry point toward analyzing this late function of blood vessels. Through testing PP1 at a series of developmental stages, we establish that adding compounds to embryos at 3 dpf and examining their effect at 4 dpf is an effective protocol to test candidates involved in regulating the maintenance of endothelial cells. With circulation fully established and the dorsal aorta and posterior cardinal vein separated completely after 3 dpf, observation of circulation is very achievable. When added to embryos at 3 dpf, PP1 specifically caused endothelial cells to lose their integrity. It will be interesting to further analyze endothelial cell junction with antibody staining but nonetheless the present electron microscopic data suggest that the junctions between endothelial cells were dramatically changed by PP1 administration (Figure 4D).
As reported previously and confirmed by kinase profiling performed in this study, PP1 is an ATP-competitive kinase inhibitor targeting multiple kinases including Src family members, Abl, and VEGFR. Numerous studies have shown that the Src family and VEGFR are involved in vascular development, especially the VEGF pathway, which is the master regulator of angiogenesis. However, analysis with highly specific small-molecule inhibitors targeting the Src family or VEGFR showed that the application of each alone does not cause endothelial cells to collapse, implying additional unknown targets in the mode of PP1 action. We show here that activity of MEK1/2 (MAP2K) in collaboration with VEGFR2 is involved in the maintenance of endothelial cells. Since PP1 does not directly inhibit MAP2K in vitro (Table 1), it may exert its effect on endothelial cells through regulating other factors that consequently result in the reduction of MAP2K in vivo.
Since the mechanisms of endothelial-cell maintenance are likely conserved in humans, our findings on Dasatinib and Sunitinib may have clinical implications. Dasatinib (marketed as Sprycel by Bristol-Myers Squibb) is a dual ABL and Src family kinase inhibitor approved by the FDA for patients with imatinib-resistant chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Sunitinib (marketed as Sutent by Pfizer) is a multi-target receptor tyrosine kinase inhibitor approved by the FDA for the treatment of renal-cell carcinoma and imatinib-resistant gastrointestinal stromal tumor. Since the combined treatment of Dasatinib and Sunitinib caused vascular lumen reduction and circulation blockage, potential side effects should be considered if these two drugs are used together.
Materials and methods
Zebrafish stocks and chemical library screening
The wild-type AB strain zebrafish and transgenic lines Tg(kdrl:GRCFP)zn1, Tg(fli1a:nEGFP)y7, and Tg(gata1:dsRed)sd2 were used in this study. Embryos were raised under standard condition and staged according to description by Kimmel et al. 29. Live embryos were placed into 96-well plates, 6 embryos per well with 200 μl fresh fish water containing 1× Antibiotic-Antimycotic Solution (Mediatech, Manassas, VA). Chemical libraries were added to the embryos at the concentration of approximately 10 μM at 30 hpf, and circulation and morphology of the embryos were observed at 48 hpf.
Chemical libraries and compounds
BIOMOL chemical library consisting of ∼500 compounds of bioactive lipids, endocannabinoid, ion-channel ligands, enzyme inhibitors, phosphatase and kinase inhibitors, and orphan ligands (BIOMOL); and Prestwick library of 1120 compounds consisting of 85% FDA-approved drugs (Prestwick Chemical, Inc) were screened for compounds that regulate blood vessel function after circulation was established. PP1 was purchased from BIOMOL; Dasatinib, PTK787 and Sunitinib were purchased from Selleck; U0126 and BDM were purchased from Sigma-Aldrich; ZM323881 was purchased from Tocris; SU6656 and Bcr-abl Inhibitor were purchased from Calbiochem. High-concentration stocks of these organic compounds were made in DMSO. Working solutions were diluted from DMSO stocks into fish water.
TUNEL assay
Apoptosis in the endothelial cells of Tg(fli1a:nEGFP)y7 transgenic embryos was examined using terminal transferase-mediated dUTP nick end-labeling (TUNEL) assay as per manufacturer's protocol with some modifications (In situ Cell Death Detection Kit, TMR Red, Roche Applied Science). After PP1 treatment, 4 dpf embryos were fixed overnight in 2% paraformaldehyde in PBS at 4 °C. Embryos were then washed with PBST buffer twice and stored in methanol at −20 °C overnight. Embryos were rehydrated, permeabilized by proteinase K (Sigma) (50 μg/ml) for 40 min, and refixed in 2% paraformaldehyde in PBS for 10 min at room temperature. Embryos were then washed 3 × 5 min in PBST and incubated in the TUNEL reaction mix for 3 h at 37 °C in darkness. After reaction, embryos were washed 3 × 30 min in PBST at room temperature and stored in PBST at 4 °C.
Tubular network degeneration assay with HUVEC
Matrigel (growth factor reduced) was thawed at 4 °C overnight, and each well of prechilled 96-well plates was coated with 50 μl PBS-diluted Matrigel (1:1) and incubated at 37 °C for 30 min. HUVECs ( 15 000 cells per well) were added into each Matrigel-treated well and cultured in 0.1 ml ECM (supplemented with 0.5% FBS and 40 ng/ml VEGF). After incubation at 37 °C and 5% CO2 for 4 h, tube-like structures were formed, and then treated by PP1 at different concentrations for 18 h. The density of tubular structure was quantified by manual counting of the length of endothelial network in high-power fields (200×).
Kinase profiling
A panel of 22 representative mammalian kinases was tested for inhibition by PP1 using SelectScreen Kinase Profiling Service (Invitrogen, Carlsband, CA). The concentration of PP1 tested was 5 μM in 1% DMSO.
Microangiography
Fluorescein isothiocyanate dextran (MW=2 000 000 Da, Sigma) or tetramethylrhodamine dextran (MW=2 000 000 Da, Invitrogen) dissolved in double-distilled water was microinjected into the sinus venous of zebrafish embryos at 48 hpf or 4 dpf, respectively.
Light microscopy
Pictures of zebrafish embryos were taken with either a confocal microscope (Zeiss LSM510 Meta and Axiovert 200M), or AxioImager A1 microscope and AxioCam digital camera (Zeiss, Oberkochen, Germany), and edited with Photoshop 7.0 (Adobe Systems, San Jose, CA).
Electron microscopy
Embryos for the transmission electron microscopy were fixed in 2% (v/v) glutaraldehyde (Sigma-Aldrich) and 2% (w/v) paraformaldehyde (Sigma-Aldrich) dissolved in 0.1 M sodium cacodylate buffer (pH 7.4) at 4 °C overnight, and post-fixed in 2% (w/v) osmium tetroxide at room temperature for 4 h. After dehydrating through serial ethanol (15%, 30%, 50%, 75%, 85%, 95%, and 100%), the embryos were embedded in Spurr's resin (SPI-Chem). Sections of 75 nm were obtained with a microtome (Leica), and stained with 1% (w/v) uranyl acetate and 0.5% (w/v) lead citrate. Images were obtained with an electron microscope (JEOL).
Acknowledgments
We thank Zahra Tehrani for editing the manuscript. This work was supported by research grants from the Peking University Shenzhen Graduate School (to Hanbing Zhong), the Project 31071281 supported by National Natural Science Foundation of China (to Hanbing Zhong), the 973 Program from MOST of China (2009CB941203 to Hanbing Zhong) and (2009CB940904 to Song Li), and the National Institutes of Health (R01 DK54508 to Shuo Lin).
Glossary
- hpf
hours post fertilization
- dpf
days post fertilization
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
Molecular structures of compounds used.
PP1 potently inhibited the growth of ISV when added at the shield stage in a dose-dependent manner.
Angiography with tetramethylrhodamine dextran (2 000 kD) in Tg(kdrl:GRCFP)zn1 embryos showed that the blood vessels were malfunctioned in PP1 treated embryos.
Vascular lumen reduction caused by PP1 was not due to lack of heart beating and circulation. The 3 dpf Tg(kdrl:GRCFP)zn1 embryos were treated with BDM (20 mM).
PP1 did not induce excess apoptosis of endothelial cells.
Ultra-structural changes of posterior cardinal vein induced by PP1.
Ultra-structural changes of ISV induced by PP1.
PP1 caused the developed tubes of HUVEC to degenerate in a dose-dependent manner.
References
- Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- Blum Y, Belting HG, Ellertsdottir E, Herwig L, Lüders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316:312–322. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Ellertsdottir E, Lenard A, Blum Y, et al. Vascular morphogenesis in the zebrafish embryo. Dev Biol. 2010;341:56–65. doi: 10.1016/j.ydbio.2009.10.035. [DOI] [PubMed] [Google Scholar]
- Strilic B, Kucera T, Eglinger J, et al. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell. 2009;17:505–515. doi: 10.1016/j.devcel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Bayless KJ, Salazar R, Davis GE. RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5)beta(1) integrins. Am J Pathol. 2000;156:1673–1683. doi: 10.1016/s0002-9440(10)65038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci. 2002;115(Pt 6):1123–1136. doi: 10.1242/jcs.115.6.1123. [DOI] [PubMed] [Google Scholar]
- Davis GE, Bayless KJ. An integrin and Rho GTPase-dependent pinocytic vacuole mechanism controls capillary lumen formation in collagen and fibrin matrices. Microcirculation. 2003;10:27–44. doi: 10.1038/sj.mn.7800175. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16:222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- Childs S, Chen JN, Garrity DM, Fishman MC. Patterning of angiogenesis in the zebrafish embryo. Development. 2002;129:973–982. doi: 10.1242/dev.129.4.973. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Weinstein BM, Detrich HW 3rd, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–3150. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- Cross LM, Cook MA, Lin S, Chen JN, Rubinstein AL. Rapid analysis of angiogenesis drugs in a live fluorescent zebrafish assay. Arterioscler Thromb Vasc Biol. 2003;23:911–912. doi: 10.1161/01.ATV.0000068685.72914.7E. [DOI] [PubMed] [Google Scholar]
- Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Blake RA, Broome MA, Liu X, et al. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge SA. Role of Src in signal transduction pathways. The Jubilee Lecture. Biochem Soc Trans. 2002;30:11–17. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Jopling C, den Hertog J. Fyn/Yes and non-canonical Wnt signalling converge on RhoA in vertebrate gastrulation cell movements. EMBO Rep. 2005;6:426–431. doi: 10.1038/sj.embor.7400386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Roskoski R Jr. Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun. 2007;356:323–328. doi: 10.1016/j.bbrc.2007.02.156. [DOI] [PubMed] [Google Scholar]
- Apsel B, Blair JA, Gonzalez B, et al. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol. 2008;4:691–699. doi: 10.1038/nchembio.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merhi-Soussi F, Dominguez Z, Macovschi O, et al. Mechanisms involved in the stimulation of prostacyclin synthesis by human lymphocytes in human umbilical vein endothelial cells. Br J Pharmacol. 2003;139:321–328. doi: 10.1038/sj.bjp.0705253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesbery NS, Webb CP, Leppla SH, et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- Bolcome RE 3rd, Sullivan SE, Zeller R, Barker AP, Collier RJ, Chan J. Anthrax lethal toxin induces cell death-independent permeability in zebrafish vasculature. Proc Natl Acad Sci U S A. 2008;105:2439–2444. doi: 10.1073/pnas.0712195105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CC, Peterson QP, Hong JY, Peterson RT. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol. 2006;16:1366–1372. doi: 10.1016/j.cub.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Deng Y, Mukhopadhyay A, et al. ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. J Clin Invest. 2010;120:1217–1228. doi: 10.1172/JCI39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JM, Bold G, Buchdunger E, et al. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–2189. [PubMed] [Google Scholar]
- Endo A, Fukuhara S, Masuda M, Ohmori T, Mochizuki N. Selective inhibition of vascular endothelial growth factor receptor-2 (VEGFR-2) identifies a central role for VEGFR-2 in human aortic endothelial cell responses to VEGF. J Recept Signal Transduct Res. 2003;23:239–254. doi: 10.1081/rrs-120025567. [DOI] [PubMed] [Google Scholar]
- Whittles CE, Pocock TM, Wedge SR, et al. ZM323881, a novel inhibitor of vascular endothelial growth factor-receptor-2 tyrosine kinase activity. Microcirculation. 2002;9:513–522. doi: 10.1038/sj.mn.7800164. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Molecular structures of compounds used.
PP1 potently inhibited the growth of ISV when added at the shield stage in a dose-dependent manner.
Angiography with tetramethylrhodamine dextran (2 000 kD) in Tg(kdrl:GRCFP)zn1 embryos showed that the blood vessels were malfunctioned in PP1 treated embryos.
Vascular lumen reduction caused by PP1 was not due to lack of heart beating and circulation. The 3 dpf Tg(kdrl:GRCFP)zn1 embryos were treated with BDM (20 mM).
PP1 did not induce excess apoptosis of endothelial cells.
Ultra-structural changes of posterior cardinal vein induced by PP1.
Ultra-structural changes of ISV induced by PP1.
PP1 caused the developed tubes of HUVEC to degenerate in a dose-dependent manner.