Abstract
Background
Few population-based epidemiological data are available on Merkel cell carcinoma (MCC), a rare lethal non-melanoma skin cancer. We analysed multiple-cause-of-death records to describe MCC mortality and trends and the association with other primary cancers.
Methods
We reviewed all 6,713,059 death certificates in Italy (1995–2006) to identify those mentioning MCC. We evaluated the association with other primary cancers by calculating the ratio of observed to expected deaths, using a standardized mortality ratio (SMR)-like analysis. We also evaluated the geographic distribution of deaths.
Results
We identified 351 death certificates with the mention of MCC. The age-adjusted mortality was 0.031/100,000, with a significant trend of increase and a slight north–south gradient. There was a significant deficit for solid cancers (SMR = 0.15) and a non-significant excess for lymphohematopoietic malignancies (SMR = 1.62). There were significant excesses for chronic lymphocytic leukemia (SMR = 4.07) and Waldenström’s macroglobulinemia (SMR = 27.2) and a non-significant excess for chronic myeloid leukemia (SMR = 5.12).
Conclusions
The increase in MCC mortality reflects the incidence trend in the literature. The association with chronic lymphocytic leukemia confirms the importance of immunologic factors in MCC. Regarding Waldenström’s macroglobulinemia, an association with MCC has never been reported.
Keywords: Multiple-cause-of-death, Non-melanoma skin cancer, Waldenström’s macroglobulinemia, Chronic lymphocytic leukemia, Neuroendocrine carcinoma
Introduction
Merkel cell carcinoma (MCC) is a rare neuroendocrine cancer of the skin that usually affects elderly persons [1–3]. MCC has been recently shown to be associated with a virus, “Merkel-cell-polyomavirus” [4]. An increased risk of MCC has been reported among solid-organ transplant recipients [5, 6] and persons with HIV/AIDS [6, 7], and some studies have reported an association between MCC and other cancers, such as chronic lymphocytic leukemia [8–11]. MCC is considered to be highly aggressive and lethal [1, 2]; in fact, more than half of the persons with non-localized MCC die within 1 year of diagnosis [11].
Given the rarity of MCC, few population-based epidemiological data are available, and these data, which derive from cancer registries, have become available only recently. In Europe, the only published data for individual countries are incidence data for Denmark and Sweden [11, 12]. For other rare tumours or diseases for which few data are available, multiple-cause-of-death records have been used as a source of data [13, 14]. The advantage to using these records is that they are a readily available source of population-based information on the health status of a given population and that they contain, in addition to the underlying cause of death, those other diseases or conditions that contributed to death [15].
With specific regard to Italy, the only data on the incidence of MCC are those derived by applying the morphology code for MCC (M8247/3) (International Classification of Diseases for Oncology; Edition 3; ICD-O-3) to data from the Italian Association of Cancer Registries (AIRTUM) (0.28 per 100,000 population in 2001–2005; 95% CI: 0.25–0.32; AIRTUM, personal communication). However, these data are unpublished in this form (i.e., MCC is generally pooled with other types of non-melanoma skin cancer), and the Italian network of registries only covers about one-third of the country’s population (AIRTUM; http://www.registri-tumori.it/cms/). In light of the limited data available, we conducted a study based on the analysis of the multiple-cause-of-death database. The objective of the study was to use mortality data as an epidemiological indicator for MCC in Italy. In particular, the data were used to estimate the occurrence of MCC, including its geographic distribution and temporal trends, and to identify other concomitant conditions and potential associations with other primary cancers.
Materials and methods
Identification of deceased persons with MCC
To identify deceased persons with MCC, we used the multiple-cause-of-death database, which is managed by Italy’s National Institute of Statistics (ISTAT; www.istat.it). This database consists of individual anonymous electronic records that contain the underlying cause of death and all causes as typewritten out in full by the medical examiner on the death certificate; the causes of death are not coded. This database currently contains records for the period 1995–2006 and covers Italy’s entire population (about 57,000,000). We considered both persons with MCC recorded as the underlying cause on the death certificate (i.e., death due to MCC) and those with MCC recorded as another cause or condition but who had another underlying cause (i.e., death with MCC). We reviewed all 6,713,059 records in the period 1995–2006 and identified those with the term “Merkel” written anywhere on the death certificate. In this way, 345 records were identified, yet 8 were eliminated because we deemed that the term “Merkel” was a misspelling of the name of another pathology [“Merkel diverticulum” (n = 6), “Merkel cave” (n = 1), and “Merkeloma of the lung” (n = 1)]. We included another 14 death certificates on which “Merkel” was written, yet incorrectly [“Meckel cell carcinoma” (n = 9), “Mekel cell carcinoma” (n = 2), and “Mechel cell carcinoma” (n = 3)]. Thus, a total of 351 records were identified.
For the 351 records, we conducted a descriptive analysis, which included demographic information, the anatomical site of MCC, and the diffusion of MCC at death (i.e., whether it was localized or non-localized and whether there were regional lymph node metastases or distant metastases). We also considered the most relevant diseases or conditions other than MCC mentioned on the death certificates; when more than one of these diseases or conditions was recorded on the death certificate, we identified the most important one based on its position on the certificate (i.e., underlying cause, other causes resulting in the underlying cause, other significant conditions, or immediate/final cause of death) and on the hierarchy of conditions used in ICD-10. To determine whether the age distribution significantly differed by gender, we conducted a non-parametric Mann–Whitney test [16]. We also performed a geographic analysis of the place of death by calculating the standardized mortality ratio (SMR) for three macroareas (northern Italy, central Italy, and southern Italy/the islands); the reference population was Italy’s general population in the same period (1995–2006).
Mortality rate and temporal trends
To evaluate temporal trends in MCC mortality, based on the multiple-cause-of-death database, we calculated the crude mortality rate for each year and the mean mortality for the entire period, assuming that the population remained stable during the study period. This calculation was performed using the Cuzick test for trend, which is an extension of the Wilcoxon rank-sum test [17, 18], and considering deaths due to MCC (n = 298). We also calculated the age-adjusted mortality rate (and the 95% confidence intervals; 95% CI) for the entire period using a direct method of standardization to account for differences in age distribution, considering the European population as reference.
Association between MCC and other cancers
To evaluate the potential association between MCC and other primary cancers, we determined whether there was an excess or deficit of deaths due to these cancers among deceased persons with MCC by making comparisons with the general population of deceased persons. However, only the underlying cause of death (and not other causes) is available for the general population. Thus for this analysis, we considered only the underlying cause of death. In particular, we compared the observed number of deaths due to other primary cancers to the expected number. The observed number was the number of deceased persons with MCC who had a given primary cancer as the underlying cause; the expected number was the number of deceased persons with the given cancer as the underlying cause in the same age general population of persons who died in the same period. For the expected number of deaths, the data source was Italy’s National Mortality Database; until 2003, ICD-9 was used to code the underlying cause of death in this database; since then, ICD-10 has been used (www.who/int/classifications/icd/en/). The ratio of observed-to-expected cases can be considered as standardised mortality ratio (SMR)-like (herein referred to as “SMR”) [13]. The SMR was calculated for major groups of cancers and for individual tumours. The 90% confidence intervals were calculated using either the Byar formula (for cancers for which there were at least three deaths) [19] or assuming a Poisson distribution of observed cases (for cancers with fewer than three deaths).
Results
Information on the 351 deaths with MCC is reported in Table 1. MCC mainly affected the elderly population (median age at death: 75 years for men and 80 years for women); 6.3% of the deaths occurred under 55 years of age. The age distribution differed by gender (p < 0.05): for persons under 55 years of age, there were twice as many men; for persons older than 85 years, there were three times more women. For the 238 (67.8%) deaths with known MCC stage, in most cases (n = 214) it was non-localized (i.e., regional lymph nodes or distant site). The sites of the distant metastases were skin, subcutaneous tissue, distant lymph nodes, liver, lung, bone, central nervous system (brain, leptomeninges, cerebellum, and spinal cord), stomach, oesophagus, bone marrow, pleura, peritoneum, and unspecified site. Of the 24 deaths with localized MCC, for 18 of them MCC was the underlying cause of death; in one-third of these cases, MCC was localized on the face.
Table 1.
Distribution of 351 deaths with Merkel cell carcinoma in Italy (1995–2006)
| Characteristics | n (%) |
|---|---|
| Gender | |
| Men | 179 (51.0) |
| Women | 172 (49.0) |
| Age at death | |
| <60 | 33 (9.4) |
| 60–74 | 110 (31.3) |
| 75+ | 208 (59.3) |
| Anatomical site | |
| Head and neck | 21 (6.0) |
| Upper limb | 11 (3.1) |
| Lower limb | 39 (11.1) |
| Buttock | 10 (2.8) |
| Unspecified | 270 (76.9) |
| Stage at death | |
| Local | 24 (6.8) |
| Regional | 27 (7.7) |
| Distant | 187 (53.3) |
| Unstaged | 113 (32.2) |
| Underlying cause of death | |
| Merkel cell carcinoma | 298 (84.9) |
| Primary cancers | 26 (7.4) |
| Cardiovascular diseases | 16 (4.6) |
| Digestive diseases | 3 (0.9) |
| Respiratory diseases | 3 (0.9) |
| Other causes | 5 (1.4) |
Of the 351 deaths with MCC, the underlying cause was MCC for 298 deaths (Table 1). When considering other relevant diseases or conditions reported anywhere on the death certificate (data not shown in Table 1), for 190 deaths (54.1%) none were recorded, whereas for the remaining 161 deaths, the other diseases or conditions were other primary cancers (n = 38 certificates), cardiovascular disease (n = 45), genitourinary diseases (n = 27), diabetes (n = 20), respiratory disease (n = 6), digestive disease (n = 5) and other diseases (n = 14); 7 death certificates mentioned a condition associated with chronic immune suppression [HIV/AIDS (n = 3), kidney transplant (n = 2), and autoimmune disease (Bechet’s disease (n = 1), and autoimmune haemolytic anemia (n = 1))].
Thirty-eight certificates mentioned 41 primary cancers in addition to MCC (Table 2), as underlying cause (n = 26) or other causes (n = 15). Triple primary cancers (i.e., two cancers in addition to MCC) were recorded on 3 certificates: chronic lymphocytic leukemia and Kaposi’s sarcoma; thyroid and adrenal gland cancers; and larynx cancer and non-Hodgkin’s lymphoma.
Table 2.
Additional primary cancers in deaths with Merkel cell carcinoma
| Cancer type or site | ICD_9 | ICD_10 | Underlying cause of death | Other’s cause of death | ||
|---|---|---|---|---|---|---|
| n | SMR | 90% CI | n | |||
| Solid cancers | 140–199, 210–239 | C00–C79 | 13 | 0.15 | 0.09–0.23 | 9 |
| Digestive organs | 150–159 | C15–C26 | 7 | 0.20 | 0.09–0.37 | – |
| Stomach | 151 | C16 | 1 | 0.14 | 0.01–0.67 | 1 |
| Colon | 153 | C18 | 4 | 0.52 | 0.19–1.31 | 1 |
| Liver | 155 | C22 | 1 | 0.16 | 0.01–0.77 | – |
| Pancreasa | 157 | C25 | 1 | 0.19 | 0.01–0.93 | – |
| Lung, larynx | 161–162 | C32–C34 | 2 | 0.09 | 0.02–0.30 | – |
| Female breast | 174 | C50 | 1 | 2.97 | 0.17–15.80 | 2 |
| Skin, non-melanomab | 173 | C44 | 1 | 0.14 | 0.01–0.67 | – |
| Kaposi’s sarcoma | – | C46 | – | – | – | 1 |
| Uterine cervix | 180.1 | C53.1 | 1 | 16.80 | 0.50–47.44 | – |
| Prostate | 185 | C61 | 1 | 0.22 | 0.01–0.67 | – |
| Kidney | 189 | C64 | – | – | – | 1 |
| Thyroid | 193 | C73 | – | – | – | 1 |
| Adrenal gland | 194.0 | C74 | – | – | – | 1 |
| Soft tissue sarcomac | 171 | C49 | – | – | – | 1 |
| Lymphohaematopoietic cancers | 200–208 | C81–C96 | 13 | 1.62 | 0.96–2.58 | 6 |
| Non-Hodgkin’s lymphoma | 200, 202 | C83, C85 | 3 | 1.05 | 0.28–2.67 | 1 |
| Multiple myeloma | 203 | C90 | 1 | 0.62 | 0.03–2.97 | – |
| Acute leukemia | 204.0, 205.0 | C91.0, C92.5 | 1d | 4.10 | 0.25–23.72 | 1e |
| Chronic lymphocytic leukemia | 204.1 | C91.1 | 3 | 4.07 | 1.17–11.08 | 4 |
| Chronic myeloid leukemia | 205.1 | C92.1 | 2 | 5.12 | 0.89–15.74 | – |
| Waldenström’s macroglobulinemia | 273.3 | C88 | 2 | 27.20 | 3.55–62.9 | – |
| Primary myelofibrosis | – | – | 1 | –f | – | – |
| All cancers | 140–239 | C00–D48 | 26 | 0.26 | 0.18–0.36 | 15 |
aNeuroendocrine carcinoma
bSquamous cell carcinoma
cSarcoma of large systemic blood vessels
dMyelomonocytic leukemia
eLymphoid leukemia
fSMR could not be calculated for this entity
Regarding the association between MCC and other primary cancers, there was a significant deficit for all solid tumours combined and individually, except for female breast cancer and uterine cervix cancer, for which a non-significant excess was found. A non-significant (though borderline) excess was found for malignant neoplasms of lymphocytic and haematopoietic tissue; however, significant excesses were found for the single entities chronic lymphocytic leukemia and Waldenström’s macroglobulinemia and non-significant excesses for chronic myeloid leukemia, acute leukemia and non-Hodgkin’s lymphoma.
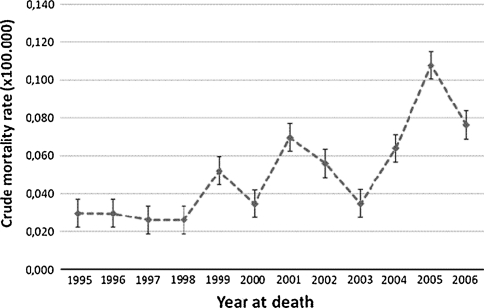
The crude mortality rate for the study period was 0.04 per 100,000 population; the age-adjusted rate was 0.027 per 100,000 population (95% CI: 0.027–0.034). There was a significant trend of increase in the crude mortality rate (p < 0.005) (Fig. 1).
Fig. 1.
Mortality trend for Merkel cell carcinoma in Italy (1995–2006)
The geographic analysis showed a significant excess in MCC deaths in northern Italy (n = 185, SMR = 1.21, 90% CI: 1.06–1.36), a non-significant excess in central Italy (n = 76, SMR = 1.11, 90% CI: 0.91–1.34) and a significant deficit in southern Italy and the islands (n = 90, SMR = 0.70, 90% CI: 0.58–0.83).
Discussion
Because MCC is so rare, there are few population-based epidemiological data, most of which derive from cancer registries and have only recently become available. In Europe, the crude incidence was estimated to be 0.13 per 100,000 population in 1995–2002, based on pooled data on “endocrine carcinoma of the skin” from a network of cancer registries participating in the RARECARE project on rare cancers (http://www.rarecare.eu/rare_indicators/rare_indicators.asp; Technical Report); the only published data on MCC incidence for individual European countries are from Denmark (crude incidence = 0.22) and Sweden (0.42 for men and 0.33 for women) [11, 12]. In Italy, the crude incidence in 2001–2005 was 0.28 (95% CI: 0.25–0.32), and the age-adjusted incidence (using the standard European population) was 0.16 (95% CI: 0.14–0.19) (AIRTUM, personal communication). In both Europe and the United States, there seems to be a trend of increase in incidence [3, 11, 12, 20].
To contribute to the knowledge of the occurrence of this tumour and its relationship with other diseases, we used mortality data, which are exhaustive in terms of population coverage and are readily available. In particular, we used the multiple-cause-of-death database, given that in the official National Mortality Database, it is not possible to distinguish MCC because it is coded among “other malignant neoplasms of skin” (also known as “non-melanoma skin cancer”).
We report one of the highest numbers of deaths of MCC in Europe. In fact, we found, in a single country, 351 MCC deaths in 12 years (1995–2006), compared to the estimated 457 deaths in 8 years (1995–2002) for all of the European countries included in RARECARE (www.rarecare.eu). We also found a significant increase over time in the crude mortality rates, which is consistent with the increases in incidence in a similar time period in both Europe and the United States [3, 11, 12, 20].
Our crude mortality rate for MCC in 1995–2006 (0.04) is consistent with the 0.05 crude mortality rate for “endocrine carcinoma of the skin” for 1995–2002 reported by RARECARE, which, however, was not based on actual mortality data and was estimated based on incidence rates multiplied by the fatality proportion, under the assumption of constant incidence and survival rates (www.rarecare.eu). However, both our standardized mortality (0.027) and crude mortality (0.04), as well as the rate reported by RARECARE, are much lower than the 0.16 standardized incidence estimated for 2001–2006 in Italy (AIRTUM) and the crude incidence estimated for Europe (0.13, based on 1,082 cases). These findings are unexpected, given that MCC is generally considered to be highly aggressive and lethal, and mortality would be expected to reflect incidence. There are a number of possible explanations for this, at least with regard to Italy. In particular, the mortality rate for MCC based on death certificates may constitute an underestimate. Moreover, the period of analysis in our study was longer than the periods considered in the incidence estimates, and both incidence and mortality have increased in recent years. Another explanation could be that the incidence estimate for Italy is based on data from the cancer registries, which cover only one-third of the national population. Finally, these findings could suggest that MCC is not as lethal as currently believed.
To the best of our knowledge, this is the only nationwide population-based mortality rate based on actual data, although a rate has been reported for Western Australia [21].
Our results confirm that MCC mainly affects the elderly population [1–3]. Moreover, MCC was associated with a significant decrease in the median age at death (men: 75 years for MCC vs. 80 years for the general population; women: 80 and 85.7 years, respectively). Although this decrease could be considered as an indication of the lethality of the disease, this cannot be thoroughly evaluated because we have no information on the time of diagnosis.
Regarding the anatomical site of MCC, it must be considered that this information was not provided on more than three-fourths of the death certificates, and it is likely that in some of these cases the MCC was occult (nodal or visceral disease with no primary tumour identified), which has been reported in a number of MCC series [22]. Regarding the diffusion of the tumour, for which data were more complete, in most cases the metastases were multiple metastases beyond regional lymph nodes, both in typical sites (e.g., liver) and less typical sites (e.g., gastrointestinal tract), as well as some unusual sites (e.g., spinal cord). The finding that MCC was the underlying cause of death for most of the 24 deaths with localized tumour can be explained by the fact that MCC is known to invade deep extradermal structures. On two death certificates, MCC involving the parotid gland was reported as the underlying cause of death, yet we did not calculate the excess because they were probably not salivary gland primaries. However, in a recent study on MCC, salivary gland cancer was considered as a second primary and a significant excess was found, though the authors discuss the difficulty in making this distinction [8]. In fact, the presence of salivary gland cancer and MCC could be indicative of: (1) MCC that developed in the skin and infiltrated the parotid gland; (2); MCC-like primary small cell carcinoma of the parotid gland, which is closely related to cutaneous MCC [23]; or (3) parotid metastases of MCC. In fact, we found a third death certificate with non-localized MCC mentioning parotid metastases of MCC.
The non-neoplastic diseases reported on the death certificates are typical causes of death in the elderly. With regard to diabetes, though there is no way of differentiating type I from type II on death certificates, 3 of the 20 certificates mentioning diabetes reported type I, which is considered to be an autoimmune disease. MCC has been shown to be associated with autoimmune diseases [24], solid-organ transplantation [5, 6] and, though with conflicting results, AIDS/HIV-infection [6, 7]; in our study, we only identified a few cases of these conditions.
In comparing our results on associations between MCC and other cancers to the results of other studies, it must be considered that all of the other studies are based on incidence data [8, 9, 11]. In our analysis, we were only able to consider the tumour indicated as the underlying cause of death. Moreover, death certificates do not provide information on temporal order (i.e., whether MCC occurs as first or second primary). The excess found for lymphatic/haematologic malignancies, though not significant, is consistent with the findings of previous studies [8, 9, 11]. It is also consistent with our finding that 19 of the 41 tumours found anywhere on the death certificate were lymphatic/haematologic malignancies. We also found a significant deficit for solid tumours overall; in the only previous study that calculated the risk for all solid tumours, a slight (though not significant) risk was found [8]. We also found a four fold excess for chronic lymphocytic leukemia, which is the only cancer for which all of the available incidence studies consistently report a significant association with MCC [8, 25]; our finding is the first to confirm this association with mortality data. The association between MCC and chronic lymphocytic leukemia is consistent with studies that have demonstrated the importance of immunologic factors in MCC [8–11]. Of interest is the finding that chronic lymphocytic leukemia was also indicated on another 4 death certificates as a non-underlying cause. We also found a slight, yet not significant, excess for non-Hodgkin’s lymphoma, as also reported in Finland [9]; significant excesses were found in Denmark and the SEER study [8, 20]. Regarding the excess for Waldenström’s macroglobulinemia (a rare lymphoplasmacytic lymphoma), an association with MCC has never been reported previously. This is of interest because certain immune-related and/or infectious conditions are strongly associated with an increased risk of Waldenström’s macroglobulinemia [26]. Although it was not possible to determine the temporal sequence of these tumours, our results could indicate that these diseases share some etiologic or risk factors. We also found non-significant excesses for chronic myeloid leukemia and acute leukemia.
Differently from incidence studies [9], we did not observe an excess for squamous or basal cell carcinoma of the skin. However, this is not surprising, in that non-lethal tumours are generally not reported on death certificates. The indication of pancreatic neuroendocrine neoplasm as the underlying cause on a single certificate is of interest because of the rarity of this neoplasm as a primary cancer; however, a metastatic MCC mimicking primary pancreatic endocrine tumour cannot be ruled out. Of the three certificates that mentioned three primary cancers each, that which mentioned MCC, chronic lymphocytic leukemia and Kaposi’s sarcoma is of interest in light of the detection of Merkel-cell-polyomavirus in a number of cases of Kaposi’s sarcoma [4, 27] and similar case reports [28].
The pattern of additional cancers revealed by our results is similar to that found in a previous study on classic Kaposi’s sarcoma [13], another virus-related skin cancer. In particular, for both MCC and classic Kaposi’s sarcoma, there was a significant deficit for solid cancers, an excess for chronic lymphocytic leukemia, and advanced age at death. However, there were also some differences: different percentage of deaths due to the disease (85% for MCC vs. 12% for classic Kaposi’s sarcoma); a predominance of men for Kaposi’s sarcoma; and a greater association with immune suppression for Kaposi’s sarcoma.
The north–south gradient in the risk of MCC mortality seems to be contradictory, in that sun exposure (i.e., one of the few known risk factors for MCC [2, 3]) is higher in southern Italy. Although we cannot explain this finding, we can speculate that it is related to differences in the geographic distribution of oncology treatment centres and the rate of MCC underdiagnosis.
In drawing conclusions, some potential limits need to be mentioned. Although this study includes data from a large population over a reasonably long period of time, since MCC is very rare the numbers were extremely small when evaluating the association with other primary cancers, which may have affected the SMR estimates. Moreover, we were not able to evaluate associations with non-lethal tumours or cancer that was cured in the past, in that these conditions are not included on death certificates. Regarding ascertainment bias, we are reasonably sure that there were no false-positives, simply because of the peculiarity of the term “Merkel”. We also paid particular attention to all of the possible misspellings and pathologies whose name could have created confusion. However, we cannot exclude the possibility of false-negatives, a limitation that is inherent to death certificates. We may also have failed to identify cases indicated as “trabecular carcinoma of the skin” and “cutaneous neuroendocrine carcinoma” (without the term “Merkel”). However, the most prevalent name currently used dates back to after 1992, when the importance of staining for cytokeratin-20 was published for the differential diagnosis of MCC [29], and our data refer to 1995 and beyond.
Despite these limitations, the in-depth review of multiple-cause-of-death records can be an important source of clinical information. Although using mortality data does not allow us to identify all persons with MCC, they do provide us with a conservative population-based estimate of its occurrence and of its association with other primary cancers.
Acknowledgments
We wish to thank AIRTUM for providing us with the MCC incidence data for Italy.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Goessling W, McKee PH, Mayer RJ. Merkel cell carcinoma. J Clin Oncol. 2002;20:588–598. doi: 10.1200/JCO.20.2.588. [DOI] [PubMed] [Google Scholar]
- 2.Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: etiological similarities and differences. Cancer Epidemiol Biomark Prev. 1999;8:153–158. [PubMed] [Google Scholar]
- 3.Agelli M, Clegg LX, Becker JC, Rollison DE. The etiology and epidemiology of merkel cell carcinoma. Curr Probl Cancer. 2010;34:14–37. doi: 10.1016/j.currproblcancer.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koljonen V, Kukko H, Tukiainen E, Bohling T, Sankila R, Pukkala E, et al. Incidence of Merkel cell carcinoma in renal transplant recipients. Nephrol Dial Transpl. 2009;24:3231–3235. doi: 10.1093/ndt/gfp334. [DOI] [PubMed] [Google Scholar]
- 6.Lanoy E, Costagliola D, Engels EA. Skin cancers associated with HIV infection and solid-organ transplantation among elderly adults. Int J Cancer. 2010;126:1724–1731. doi: 10.1002/ijc.24931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanoy E, Dores GM, Madeleine MM, Toro JR, Fraumeni JF, Jr, Engels EA. Epidemiology of nonkeratinocytic skin cancers among persons with AIDS in the United States. Aids. 2009;23:385–393. doi: 10.1097/QAD.0b013e3283213046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard RA, Dores GM, Curtis RE, Anderson WF, Travis LB. Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol Biomark Prev. 2006;15:1545–1549. doi: 10.1158/1055-9965.EPI-05-0895. [DOI] [PubMed] [Google Scholar]
- 9.Koljonen V, Kukko H, Tukiainen E, Bohling T, Sankila R, Joensuu H, et al. Second cancers following the diagnosis of Merkel cell carcinoma: a nationwide cohort study. Cancer Epidemiol. 2010;34:62–65. doi: 10.1016/j.canep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Brenner B, Sulkes A, Rakowsky E, Feinmesser M, Yukelson A, Bar-Haim E, et al. Second neoplasms in patients with Merkel cell carcinoma. Cancer. 2001;91:1358–1362. doi: 10.1002/1097-0142(20010401)91:7<1358::AID-CNCR1139>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Kaae J, Hansen AV, Biggar RJ, Boyd HA, Moore PS, Wohlfahrt J, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102:793–801. doi: 10.1093/jnci/djq120. [DOI] [PubMed] [Google Scholar]
- 12.Hussain SK, Sundquist J, Hemminki K. Incidence trends of squamous cell and rare skin cancers in the Swedish national cancer registry point to calendar year and age-dependent increases. J Invest Dermatol. 2010;130:1323–1328. doi: 10.1038/jid.2009.426. [DOI] [PubMed] [Google Scholar]
- 13.Ascoli V, Minelli G, Kanieff M, Crialesi R, Frova L, Conti S. Cause-specific mortality in classic Kaposi’s sarcoma: a population-based study in Italy (1995–2002) Br J Cancer. 2009;101:1085–1090. doi: 10.1038/sj.bjc.6605265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziade N, Jougla E, Coste J. Population-level influence of rheumatoid arthritis on mortality and recent trends: a multiple cause-of-death analysis in France, 1970–2002. J Rheumatol. 2008;35:1950–1957. [PubMed] [Google Scholar]
- 15.Redelings MD, Wise M, Sorvillo F. Using multiple cause-of-death data to investigate associations and causality between conditions listed on the death certificate. Am J Epidemiol. 2007;166:104–108. doi: 10.1093/aje/kwm037. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG. Practical statistics for medical research. London: Chapman and Hall; 1991. [Google Scholar]
- 17.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 18.Conover WJ. Practical nonparametric statistics. New York: Wiley; 1999. [Google Scholar]
- 19.Breslow NE, Day NE (1987) Statistical methods in cancer research. The design and analysis of cohort studies. IARC Sci Publ 2:1–406 [PubMed]
- 20.Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2009;37:20–27. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 21.Girschik J, Fritschi L, Threlfall T, Slevin T. Deaths from non-melanoma skin cancer in Western Australia. Cancer Causes Control. 2008;19:879–885. doi: 10.1007/s10552-008-9150-9. [DOI] [PubMed] [Google Scholar]
- 22.Gupta SG, Wang LC, Penas PF, Gellenthin M, Lee SJ, Nghiem P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685–690. doi: 10.1001/archderm.142.6.685. [DOI] [PubMed] [Google Scholar]
- 23.Nagao T, Gaffey TA, Olsen KD, Serizawa H, Lewis JE. Small cell carcinoma of the major salivary glands: clinicopathologic study with emphasis on cytokeratin 20 immunoreactivity and clinical outcome. Am J Surg Pathol. 2004;28:762–770. doi: 10.1097/01.pas.0000126776.65815.48. [DOI] [PubMed] [Google Scholar]
- 24.Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112–114. doi: 10.1038/sj.bjc.6605733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koljonen V, Kukko H, Pukkala E, Sankila R, Bohling T, Tukiainen E, et al. Chronic lymphocytic leukaemia patients have a high risk of Merkel-cell polyomavirus DNA-positive Merkel-cell carcinoma. Br J Cancer. 2009;101:1444–1447. doi: 10.1038/sj.bjc.6605306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kristinsson SY, Koshiol J, Bjorkholm M, Goldin LR, McMaster ML, Turesson I, et al. Immune-related and inflammatory conditions and risk of lymphoplasmacytic lymphoma or Waldenstrom macroglobulinemia. J Natl Cancer Inst. 2010;102:557–567. doi: 10.1093/jnci/djq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katano H, Ito H, Suzuki Y, Nakamura T, Sato Y, Tsuji T, et al. Detection of Merkel cell polyomavirus in Merkel cell carcinoma and Kaposi’s sarcoma. J Med Virol. 2009;81:1951–1958. doi: 10.1002/jmv.21608. [DOI] [PubMed] [Google Scholar]
- 28.Cottoni F, Montesu MA, Lissia A, Dore F, Posadino AM, Farris A, et al. Merkel cell carcinoma, Kaposi’s sarcoma, basal cell carcinoma and keratoacanthoma: multiple association in a patient with chronic lymphatic leukaemia. Br J Dermatol. 2002;147:1029–1031. doi: 10.1046/j.1365-2133.2002.49859.x. [DOI] [PubMed] [Google Scholar]
- 29.Moll R, Lowe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992;140:427–447. [PMC free article] [PubMed] [Google Scholar]