Abstract
The effects of long-term cranberry consumption on age-related changes in endocrine pancreas are not fully understood. Here we treated male Fischer 344 rats with either 2% whole cranberry powder supplemented or normal rodent chow from 6 to 22 month old. Both groups displayed an age-related decline in basal plasma insulin concentrations, but this age-related decline was delayed by cranberry. Cranberry supplementation led to increased β-cell glucose responsiveness during the oral glucose tolerance test. Portal insulin concentration was 7.6-fold higher in rats fed cranberry, coupled with improved β-cell function. However, insulin resistance values were similar in both groups. Total β-cell mass and expression of pancreatic and duodenal homeobox 1 and insulin within islets were significantly enhanced in rats fed cranberry relative to controls. Furthermore, cranberry increased insulin release of an insulin-producing β-cell line, revealing its insulinotropic effect. These findings suggest that cranberry is of particular benefit to β-cell function in normal aging rats.
Keywords: Cranberry, Insulin release, Pancreatic β-cell function, Pancreatic β-cell mass, Aging
DURING normal aging, the pancreatic islet undergoes morphological and functional decline. Morphological evidence indicates that pancreatic islet size increases with aging, and part of this change is due to a dramatic increase in the amount of connective tissue within the islets, that is, a multinodular islet, in which clusters of endocrine cells are widely separated from each other by traversing bands of connective tissue (1). Corresponding changes in islet structure occur in pancreatic β-cell function, which are expressed in a variety of responses. For instance, glucose-stimulated insulin secretion per given unit of β-cell mass decreases in a linear fashion with age at a rate of ∼0.7% per year (2). This decline is coupled with a progressive decrease in glucose tolerance (3) and a relative defect of insulin secretion that together results in a high incidence of type 2 diabetes during aging (4). A multicenter retrospective study of 957 nondiabetic participants indicates that age per se is positively related to progressive declines in both basal insulin release and insulin clearance after controlling for body mass, fasting plasma glucose, and waist-to-hip ratio (5). Furthermore, inhibition of basal insulin release leads to an increase of hepatic glucose production and a decrease of peripheral glucose uptake, as well as intracellular glucose oxidation in healthy individuals (6) and type 2 diabetic patients (7). Conversely, a constant basal insulin supplement improves β-cell function in diabetes (8). Although there is debate about whether insulin secretory dysfunction is a cause or consequence of normal aging or a disease state, it has become increasingly apparent that changes in β-cell mass can lead to insulin secretory dysfunction.
The β-cell mass refers to the total volume of β-cells within the pancreas without regard to cell number or size. In the adult, β-cell mass is plastic, and its regulation through the expansion of preexisting β cells and the formation of new islets contributes to a balance between insulin supply and metabolic demand. However, the contribution made by each regulatory pathway is variable and may change at different stages of life. It is known that the regenerative capacity of β-cells decreases with age (9). A study of pancreatic tissue from 124 autopsies, for example, convincingly shows a decrease in the formation of new islets with aging (10). Indeed, β-cell mass decreases during normal aging when apoptosis ultimately outweighs β-cell replication and neogenesis (11), and most individuals with type 2 diabetes, whether obese or lean, demonstrate a net decrease in β-cell mass due to the increased vulnerability of replicating β-cells to apoptosis (10). This may explain, at least in part, why susceptibility to type 2 diabetes increases with aging. These findings also suggest that interventions promoting the preservation of functional β-cell mass might yield protection against type 2 diabetes.
Diet, together with genetic and environmental factors, profoundly influences longevity and susceptibility to age-related disease. Growing evidence suggests that polyphenolic phytochemicals, when consumed at appropriate levels as part of a varied diet, can provide certain health benefits (12). Cranberry (Vaccinium macrocarpon) contains a class of flavonoid polyphenols, including quercetin, catechin, and resveratrol (13) that are generally considered to be non-nutritive agents. Interest in the phytochemicals of cranberry stems partly from their reported benefits in the prevention and treatment of urinary tract infections (14), cardiovascular disease and carcinogenesis (15,16), and type 2 diabetes (17). These effects are thought to be mediated by biochemical and molecular mechanisms, linked to the antioxidant-responsive metabolic pathways (18). Moreover, dietary intake of cranberry may protect against the development of diabetic complications by inhibiting two enzymes, alpha-amylase and alpha-glucosidase (19). These enzymes are required for digestion of starch to maltodextrins and, thereby conversion into absorbable monosaccharides. Thus, inhibition of these enzymes improves metabolic homeostasis and comprises a tractable target for the delay or prevention of diabetes and diabetic complications. On the basis of these findings, the present study was undertaken to evaluate the influence of a cranberry rich diet on the aging of pancreatic β-cells structure and function in Fischer 344 rats.
MATERIALS AND METHODS
Animal Protocols
Twenty-four male Fischer 344 rats at 6 months of age were purchased from the National Institute on Aging/Harlan colony (Harlan Teklad, Indianapolis, IN) and housed individually on a 12-h light/dark schedule with ad libitum access to food and water. After 1-week acclimation, rats were randomly assigned to either cranberry (CB) diet chow (n = 12) or NIH-31 standard (N) rodent chow (n = 12). Considering that mean life span of Fischer 344 rats is approximately 24 months (20), rats in this study were maintained on their respective diets for 16 months in order to assess the effects of cranberry supplementation on age-related changes. The CB diet was prepared by incorporating whole cranberry powder (PACran, Decas Botanical Synergies, LLC., Carver, MA) into standard NIH-31 standard rodent chow (Harlan Teklad) at 2% by weight, followed by pelleting. The whole cranberry powder was prepared by the Decas Botanical Synergies LLC through a manufacturing process that complies with all the provisions of the Food, Drug, and Cosmetic Act. The cranberry contained 100% whole cranberry solids without preservatives, flavorings, or colorings. A number of studies have shown that supplementation of 2% various botanical extracts, such as blueberry, strawberry, cranberry, açai, nectarine, and an oregano-cranberry mixture, provides health benefits in rodents and flies (21–26). Therefore, we chose to supplement the rodent diet with 2% cranberry powder in the present study. Nutrient composition of the cranberry powder is shown in Table 1.
Table 1.
Typical Nutrient Composition of Whole Cranberry Powder and Rodent Diets
| Nutritional Analysis | Whole Cranberry Powder (per 100 g) | NIH-31 Rodent Control Diet (per 100 g)* | CB Supplemented Rodent Diet (per 100 g)† | Nutritional Changes in CB Diet‡ | % of Nutritional Changes in CB Diet§ |
| Total calorie | 432 kcal | 300 kcal | 308.64 kcal | 8.64 kcal | 2.88 |
| Calorie from fat | 99 kcal | 42 kcal | 43.98 kcal | NA | 4.71 |
| Total fat | 10.99 g | 4.7 g | 4.92 g | 0.22 g | 4.68 |
| Saturated fat | 0.92 g | 0.9 g | 0.92 g | 0.02 g | 2.04 |
| Cholesterol | 0.0 mg | 5 mg | 5 mg | 0.0 mg | 0.00 |
| Phytosterols | 2.8 mg | NA | NA | NA | NA |
| Total carbohydrates | 77.87 g | 46.5 g | 48.06 g | 1.56 g | 3.35 |
| Sugars | 13.21 g | NA | NA | NA | NA |
| Dietary fiber | 45.9 g | 13.6 g | 14.51 g | 0.91 g | 6.75 |
| Protein | 5.38 g | 18 g | 18.11 g | 0.11 g | 0.60 |
| Moisture | 4.60 g | NA | NA | NA | NA |
| Ash | 1.16 g | 6.2 g | 6.223 g | 0.023 g | 0.37 |
| Vitamin A | 892 IU | 24,200 IU | 24,217.84 IU | 17.84 IU | 0.07 |
| Vitamin C | 19.4 mg | NA | NA | 0.39 mg | NA |
| Vitamin D | 0 IU | 420 IU | 420 IU | 0 IU | 0.00 |
| Vitamin E | 0 IU | 4,100 IU | 4,100 IU | 0 IU | 0.00 |
| Calcium | 60.4 mg | 1,100 mg | 1,101.2 mg | 1.20 mg | 0.11 |
| Iron | 6.61 mg | 27 mg | 27.13 mg | 0.13 mg | 0.49 |
| Copper | 0.4 mg | 1.3 mg | 1.31 | 0.008 mg | 0.62 |
| Magnesium | 49.5 mg | 200 mg | 200.00 mg | 0.99 mg | 0.50 |
| Niacin | 0.75 mg | 8.7 mg | 8.715 mg | 0.015 mg | 0.17 |
| Phosphorus | 139.4 mg | 1,000 mg | 1,002.79 mg | 2.79 mg | 0.28 |
| Iodine | 0.0 mg | 0.2 mg | 0.2 mg | 0 mg | 0.00 |
| Riboflavin | 0.70 mg | 0.7 mg | 0.714 mg | 0.014 mg | 2.00 |
| Sodium | 3.7 mg | 300 mg | 300.074 mg | 0.074 mg | 0.02 |
| Potassium | 444.2 mg | 600 mg | 608.88 mg | 8.88 mg | 1.48 |
| Thiamine | 0.24 mg | 7.6 mg | 7.6048 mg | 0.0048 mg | 0.06 |
| Zinc | 1.8 mg | 4.7 mg | 4.736 mg | 0.036 mg | 0.77 |
| Total phenolics | 2.00–5.00 g | NA | NA | 40–100 mg | NA |
| Total anthocyanins | 0.15–1.00 g | NA | NA | 3–20 mg | NA |
| Antioxidant (ORAC) | 17.5–40.0 mmole Trolox | NA | NA | 0.35–0.8 mmole Trolox | NA |
| Ellagic acid | 20.0–41.5 mg | NA | NA | 0.4–0.83 mg | NA |
Notes: NA = not available.
Nutritional information is based on the NIH-31 rodent diet from Harlan Laboratories (www.harlan.com).
CB diet contains 2% cranberry powder in weight.
The values indicate the amount of extra calorie or nutrients in CB diet compared with normal NIH-31 rodent diet.
The values indicate extra percentages of calorie or nutrients in CB diet compared with normal NIH-31 rodent diet.
Body weights and food intake were recorded monthly for the first 11-month treatment considering the length of the intervention in the present study. At the end of the experiment (16 months after initiation of CB diet), animals were sacrificed following an overnight fasting. Abdominal fat content was estimated by weighing dissected epididymal white adipose tissue and intrascapular brown adipose tissue deposits and expressing those as percent fat mass weight to terminal body weight. Efficiency of conversion of ingested food to unit of body weight (feed efficiency) was calculated by full body weight that included contents of the gastrointestinal track (grams) divided by weekly gross energy intake (kilocalories) calculated by the average nutrient composition of diet. Animal procedures for this study were reviewed and approved by the Animal Care and Use Committee at the Biomedical Research Center, National Institute on Aging, National Institutes of Health at Baltimore, MD.
Blood Glucose and Plasma Insulin Assays
At each experimental time point, blood glucose obtained by tail snip after overnight fasting was measured with a glucometer (Bayer Corporation, Elkhart, IN). Additional blood samples were collected in ethylenediaminetetraacetic acid (K3)–coated microcapillary tubes for determination of peripheral plasma insulin levels. At the end of the experiment, fasting portal vein blood samples were collected in the tubes containing 7.5% ethylenediaminetetraacetic acid (K3) for determination of portal plasma insulin levels. Plasma insulin concentrations were determined using an enzyme-linked immunosorbent assay kit according to the protocol suggested by the manufacturer with rat insulin as the standard (ALPCO Diagnostics, Windham, NH).
Oral Glucose Tolerance Test
The Oral Glucose Tolerance Test (OGTT) reflects the body’s efficiency in disposing of glucose after an oral glucose load, a response mediated by a complex dynamic process including glucose absorption, insulin secretion, and its metabolic activity. At 12 and 18 months of age (6 and 12 months after CB diet), OGTTs were performed after overnight fasting. Two grams of D-glucose per kilogram body weight were administered orally in a 50% solution, and blood glucose levels were measured at 0, 15, 30, 60, and 120 minutes with a glucometer (Bayer Corporation, Elkhart, IN). Blood samples from snipped tails were collected in ethylenediaminetetraacetic acid (K3)–coated microcapillary tubes at 0, 15, 30, 60, and 120 minutes during OGTT. Plasma insulin concentrations were measured as described earlier.
Homeostasis Model Assessment
Insulin resistance and deficient β-cell function can be estimated by Homeostasis Model Assessment (HOMA) indices that are highly correlated with whole-body insulin resistance in humans (27) and in rats (28). At the end of the present experiment, plasma insulin and blood glucose concentrations were determined from single samples under a fasted condition (steady state). Based on the values of fasting glucose and fasting insulin, the indices for insulin resistance (HOMA-IR) and β-cell function (HOMA-β) were calculated according to the following formulas: HOMA-IR = fasting insulin (μU ml−1) × fasting glucose (mM)/22.5; HOMA-β = 20 × fasting insulin (μU ml−1)/(fasting glucose [mM] − 3.5). If the value of fasting glucose in a sample was ≤3.5 mM, a maximum value of HOMA-β derived from the same group was given to this sample.
Cell Culture and Insulin Secretion Assay
The insulin-producing β-cell line, INS-1, was seeded at equal density into 12-well plates and grown in standard RPMI medium supplemented with 10% heat-inactivated fetal calf serum, 5.5 mM glucose, and other additions as previously described (29). After 6–7 days (∼70%–80% confluence), cells were incubated in the same medium containing a soluble cranberry powder at a concentration of 0, 0.25, and 0.5 mg/mL, respectively, for 72 hours. Cells were then starved for 6 hours with serum- and glucose-free growth medium and subsequently washed twice with Krebs–Ringer bicarbonate HEPES buffer containing 143.5 mM Na+, 5.8 mM K+, 2.5 mM Ca+, 25 mM CO32−, 0.3% bovine serum albumin (Fraction V; ICN, Lisle, IL), 25 mM HEPES (pH 7.4), and 3.3 mM glucose, followed by a 30-minute preincubation in 1 mL of oxygenated Krebs–Ringer bicarbonate HEPES buffer. After preincubation, cells were shifted to 1 mL of the same buffer, and insulin release was measured using static incubation for a 60-minute period. At the end of the incubation, supernatant was collected, cleared by centrifugation, and stored at −80°C until insulin assay. Cells from these wells were trypsinized, washed, and DNA content was measured using a DNA quantitative kit (Bio-Rad Laboratories, Hercules, CA) with calf thymus DNA as a standard. Insulin secretion into the medium was measured with a commercially available enzyme-linked immunosorbent assay kit (ALPCO Diagnostics), normalized to total DNA content, and represented as nanograms per microgram DNA per 60 minutes.
Quantitative Morphometric and Confocal Image Analysis
At the end of the experiment, overnight fasted animals were sacrificed under deep isoflurane anesthesia. Each pancreas was excised and cleared of extraneous lymph nodes and fat, weighed and cassetted with the same anatomic orientation and then placed in 10% buffer-neutralized formalin and processed for paraffin embedding according to standard protocols. Two sets of five serial sections (5–7 μm thick) were obtained at intervals of ∼250 μm. The first set of sections was deparaffinized and immunostained using a commercially available ABC kit (Vector Laboratories, Burlingame, CA) and 3, 3-diaminobenzidine for visualization. The primary antibody was polyclonal guinea pig antiporcine insulin, 1:200 (ZYMED Laboratories, San Francisco, CA). Background of pancreatic tissue was stained with Methyl Green. Using an established point-counting morphometric method (30), the relative volumes of β-cells per pancreas were determined at an original magnification of 200×. Briefly, starting at a random point, in one corner of each section, the relative volumes of β-cells were scored in every other field using a 100-point grid with a minimum of 10,000 points in 100 fields counted per tissue block and then calculated as the number of intercepts over the target tissue as a proportion of the total counts positioned over pancreatic tissue. Intercepts falling on blood vessels, fat ducts, or interlobular spaces were subtracted to give the total pancreatic counts. To obtain absolute mass (milligrams), the relative volume of β-cells was multiplied by the weight of pancreas. For islet size distribution, the size of islets per tissue block was measured at an original magnification of 100× using AxioVision software (Carl Zeiss, Inc.). The images were calibrated with a stage micrometer taken at appropriate magnifications. For each tissue block, 60–140 randomly selected islets were measured. To minimize interrating variations, a single observer (M.Z.) carried out the morphometric analysis (coefficient of variation <7%).
For confocal image analysis, a second set of tissue sections was incubated overnight at 4°C with the primary antibodies at the following dilutions: guinea pig antiinsulin, 1:500 (Millipore, Billerica, MA); mouse antiglucagon, 1:1,000 (Sigma, St Louis, MO); and rabbit antipancreatics and duodenal homeobox 1 (PDX-1), 1:5,000 (Abcam, Cambridge, MA). Sections were then incubated with secondary antibodies (Alexa Fluor 488 goat antiguinea pig, 1:1,000; Cy5 goat antimouse, 1:1,000; Alexa Fluor 568 goat antirabbit, 1:1,000, Invitrogen, Carlsbad, CA) for 1 hour at room temperature. The triple-stained sections were examined with a Zeiss confocal microscope equipped with a krypton–argon laser and analyzed using Matlab software. Intensity readings of each image ranged from 0 to 255, with 255 being the greatest pixel density and hence the highest staining intensity. The region of interest comprised areas of specific immunoreactivity within each islet following background subtraction. The pixels within the bounds of the region of interest and above an intensity threshold of 25 were selected from which actual islet area was calculated. For each tissue block, a minimum of 60 randomly selected islets were randomly measured. The normalized variance of the region of interest was used to calculate an artificial ellipse from which the major and minor axes were determined. All confocal images were acquired by the same operator (W.K.)
Data Presentation and Statistics
Unless mentioned otherwise, data are presented as means ± standard error of the mean. Group data were statistically compared by two-tailed unpaired t or Mann–Whitney U tests as appropriate. Because the islet size values were not normally distributed, these values were logarithmically transformed for statistical comparison of difference between means. However, for practical reasons, raw data for islet size were given in figures. A Kolmogorov–Smirnov test was used for statistical comparison of variable distributions between two groups. A p value of <.05 was deemed statistically significant. All analyses were performed with STATVIEW software (SAS Institute, Inc., Cary, NC).
RESULTS
Effects of Cranberry-Rich Diet on Body Weight, Body Composition, and Energy Balance
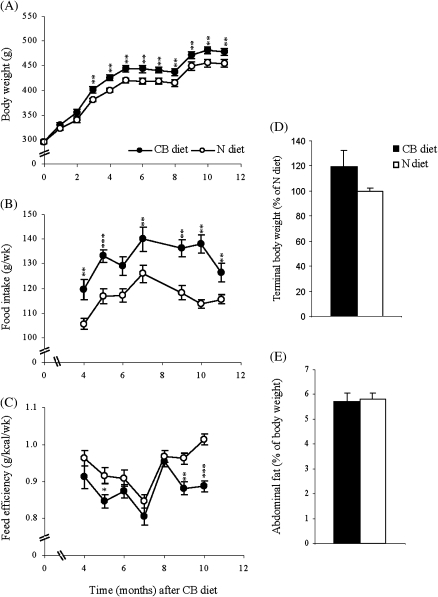
To investigate the effect of long-term cranberry supplementation on age-related changes, we fed Fischer 344 rats of 6 month old the rodent diet supplemented with or without 2% whole cranberry powder. As shown in the nutrition information of the cranberry powder in Table 1, supplementation of 2% cranberry resulted in very small changes in calorie content (<3%) and nutrient composition of the rodent diet, indicating that cranberry rich diet and normal rodent diet were isocaloric. Dietary fiber, total carbohydrate, and total fat were increased by 6.75%, 3.35%, and 4.68% due to supplemented cranberry, respectively (Table 1). We monitored body weight monthly over the course of 11 months in rats fed either the CB or the N diet. In the fed state, rats fed CB diet were significantly heavier than controls beginning 3 months after intervention (Figure 1A). In contrast, the terminal body weight measured after overnight fasting and the accumulation of abdominal fat after normalized to the terminal body weight were not significantly different between the two groups (Figure 1D and E). These findings ruled out the possibility that increased body weight gain in rats fed CB diet under the fed state was derived from abdominal fat mass that is a risk factor associated with insulin resistance. In addition, rats fed CB diet exhibited a significant increase in food intake throughout the observation period (Figure 1B). However, when body weight and food intake were considered together as the efficiency of conversion of ingested food to unit of body weight (feed efficiency), CB rats displayed lower feed efficiency (Figure 1C). This difference was prominently reflected in the analysis of total area under the curve: 185.464 ± 0.961 for CB diet versus 197.901 ± 1.979 for N diet (p < .0001).
Figure 1.
Body weight, body composition, and energy balance. Male Fisher-344 rats were randomly assigned to either a 2% cranberry diet (CB) or a normal diet (N) and maintained from 6 to 22 months of age (16 months of cranberry diet). Body weight (A) and food intake (B) were recorded monthly over the course of first 11 months of treatment. Efficiency of conversion of ingested food to unit of body weight (feed efficiency) was calculated by full body weight that included contents in the gastrointestinal track (grams) divided by weekly gross energy intake (kilocalories) calculated by the average nutrient composition of diet (C). At the time of sacrifice after overnight fasting, final body weight (D) and abdominal fat deposits (E) including epididymal white adipose tissue and intrascapular brown adipose tissue were determined. Values are means ± standard error of 10–12 rats per each group. *p < .05, **p < .01, and ***p < .001 versus age-matched N rats according to unpaired t test.
Effects of Cranberry-Rich Diet on Basal Blood Glucose and Plasma Insulin As Well As Portal Value of Insulin
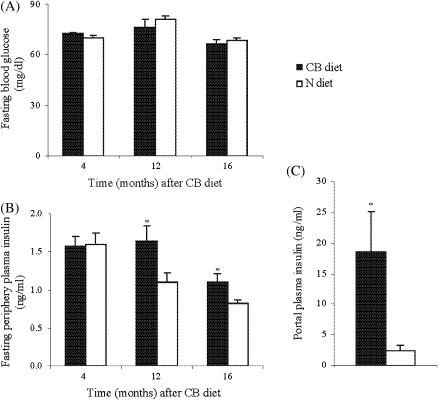
Glucose regulation in both liver and peripheral tissues (eg, muscle and adipose tissue) critically depends on adequate basal insulin secretion. We next studied whether cranberry could affect blood glucose and insulin levels in a fasted state. As shown in Figure 2, blood glucose levels were comparable between the two groups measured at 4, 12, and 16 months after cranberry supplementation (Figure 2A). In contrast, rats of both groups displayed an age-related decline in basal insulin concentrations, but this age-related effect was delayed by CB diet such that insulin levels in rats fed CB diet were significantly greater than in controls after 12 months of treatment (Figure 2B). At the end of the experiment (16 months after CB diet), portal insulin concentration in rats fed CB diet was 7.6-fold higher than in those fed N diet (Figure 2C). These results indicate that the decline in basal insulin levels typically observed during aging is potently reversed by a cranberry-rich diet.
Figure 2.
Basal blood glucose, plasma insulin levels, and portal plasma insulin concentration. At each experimental point (4, 12, and 16 months of treatment), animals were fasted overnight. Blood glucose from snipped tail was measured using a portable glucose meter (A). Peripheral blood samples were taken from snipped tail for measuring plasma insulin concentration (B). At the time of sacrifice (16 months of treatment), after overnight fasting, blood sample from portal vein was collected for measuring portal insulin concentration (C). Values are means ± standard error of 10–12 rats per group. *p < .05 versus age-matched N rats according to unpaired t test.
Effects of Cranberry-Rich Diet on Postprandial Insulin Release After Glucose Challenge
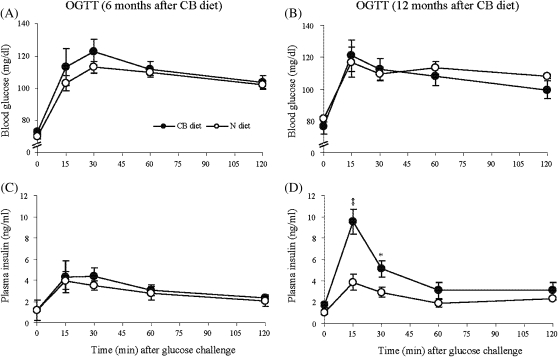
Postprandial insulin secretion is critical for regulation of glucose metabolism when carbohydrates are abundant and must be metabolized in order to maintain homeostasis. In order to evaluate the effect of cranberry-rich diet on the efficiency of the body to dispose of glucose and insulin release after glucose challenge, the OGTT was performed as described in “Materials and Methods.” The initial OGTT, administered after 6 months of treatment, showed no significant effects of cranberry diet on both blood glucose level and insulin release at all time points (Figure 3A and C). However, the second OGTT, performed after 12 months of treatment, revealed that the early-phase insulin release (15 minutes) in rats fed CB diet exceeded those in control rats by an average of 147% (Figure 3D), whereas blood glucose values did not differ significantly (Figure 3B). The differential effect of CB diet on corresponding insulin release was particularly evident from an analysis of total area under the curve, 514.997 ± 73.732 in rats fed CB diet versus 283.639 ± 10.512 for rats fed N diet (p = .006; Figure 3D), whereas total area under the blood glucose response curve (Figure 3B) was similar between the two groups (3,327.69 ± 173.09 in rats fed CB diet versus 3,362 ± 119.39 in rats fed N diet, p = .872). Thus, these findings suggest that long-term cranberry supplementation improves glucose responsiveness of β-cells.
Figure 3.
Blood glucose levels and insulin release after glucose challenge. Oral glucose tolerance tests were performed at 6 months (A and C) and 12 months (B and D) of cranberry treatment, respectively. After overnight fasting, D-glucose (2 g/kg body weight) was administrated orally. Blood samples were taken from snipped tail at different time points during Oral Glucose Tolerance Test for measurement of blood glucose (A and B) and plasma insulin (C and D). Values are means ± standard error of seven rats per group. *p < .05 and **p < .01 versus age-matched N rats according to unpaired t test.
Effects of Cranberry-Rich Diet on Insulin Resistance (HOMA-IR) and β-cell Function (HOMA-β)
Any combination of insulin resistance and β-cell deficit provides a unique set of plasma insulin and glucose concentrations. We further analyzed the relationship between fasting insulin and blood glucose concentration with degree of HOMA-IR and different degrees of HOMA-β by using HOMA from the single samples under a fasting condition. Notably, the index of HOMA-IR was not significantly affected by cranberry-rich diet, showing that HOMA-IR values were similar in both groups (3.7 ± 0.2 in rats fed CB diet vs 3.4 ± 0.21 in controls, p = .385). In contrast, the HOMA estimated value of β-cell function (HOMA-β) in rats fed CB diets had a trend of improvement, which was 1.6-fold higher in rats fed CB diet than those fed N diet. However, this improvement of β-cell function did not reach statistically significance due to variability (3,295 ± 1,038 for rats fed CB diet vs 2,110 ± 707 for controls, p = .380). Moreover, there was little additional glucose drop in response to increased insulin secretion in rats fed CB diet, with the exception of two rats whose fasting blood glucose levels were ≤3.5 mM. These findings suggest that cranberry supplementation does not lead to insulin resistance.
Effects of Cranberry-Rich Diet on Total Pancreatic β-Cell Mass, Hormone Levels, and Islet Morphology
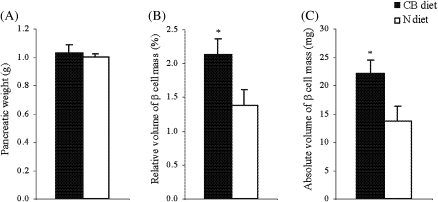
Because β-cells are the sole source of insulin production in the body, we performed morpholometric histological analyses to determine absolute β-cell mass as a function of insulin synthesis and storage, as well as β-cell growth (ie, hypertrophy and replication). As shown in Figure 4A, pancreatic weight was comparable between the two groups, suggesting that exocrine pancreas was not affected by cranberry because exocrine pancreas contributes to almost 99% of total pancreatic weight. The relative volume of β-cells mass (percent relative to pancreas), however, was significantly greater in rats fed CB diet than in controls (2.134 ± 0.235 for rats fed CB diet vs 1.382 ± 0.238 for controls, p < .05; Figure 4B). Furthermore, absolute β-cell mass (milligrams), calculated as a product of the relative β-cell volume and pancreatic weight in rats fed CB diet, was 1.6-fold higher than in controls (22.181 ± 2.267 for rats fed CB diet vs 13.818 ± 2.499 for controls; p < .05; Figure 4C).
Figure 4.
Pancreatic weight and total pancreatic β-cell mass. At the end of experiment (16 months of treatment), each pancreas was excised, cleared of extraneous lymph nodes and fat, and then weighed (A) and cassetted with the same anatomic orientation. After fixative, the pancreas was processed for paraffin embedding using a standard protocol. Two sets of five serial sections (5–7 μm) were obtained at intervals of 250 μm and immunostained by using an ABC kit for insulin, followed by background staining with Methyl Green. Using point-counting morphometric technique, the relative volumes of β-cells (percent relative to pancreas) were quantified (B). Absolute β-cell mass (C) was then calculated by multiplying the relative β-cell volume times the tissue weight. Values are means ± standard error of 10 rats per group. *p < .05 versus aged-matched controls according to unpaired t test.
Quantitative measurement of islet profile area revealed a negative kurtosis for logarithmical distributions of islet size in both groups of rats, but the shape of the log distribution in the rats fed CB diet appeared to be different and had lighter tails, a flatter center, and heavier shoulders (Figure 5B) relative to those fed N diet (Figure 5A). However, these differences in the distribution of islet size between the two groups did not reach a significantly different level as determined by the Kolmogorov–Smirnov test (p = .1133). This indicates that long-term cranberry supplementation did not cause a significant change in overall neogenesis pattern of pancreatic islets in aging. This observation was further supported by a lack of group difference in neogenesis of the β-cells from ducts and incorporation of single β-cells into the ductal epithelium or ductal-like tissue. Representative images of β-cell neogenesis from both groups are shown in Figure 5C and D (arrows).
Figure 5.
Pancreatic islet morphological configurations. The sizes of pancreatic islets per tissue block were measured at a 200× magnification using AxioVision software. Distributions of islet size (A and B) were obtained from individual data of islet sizes. After logarithmical transformation, the distribution of islet sizes was statistically compared by Kolmogorov–Smirnov test. For practical reason, the raw data of islet size are given and expressed as means ± standard error from 1,022 islets in CB rats and 1,056 islets in N rats (G). p Value was determined by unpaired t test based on logarithmically transformed variables. **p < .01 versus N rats. Newly formed islets by neogenesis were similarly frequent noted in ductal epithelia and duct-like tissue in both groups of rats. Representative images of β-cell neogenesis are shown in CB rats (arrows in C) and N rats (arrows in D). Numerous enlarged islets (>40,000 μm2) were present in the pancreatic tissue of CB rats in which tightly packed β-cells positioned centrally (E) in comparison of the islet from N rats (F). Original magnification of images, 200×.
We extended our analysis to large islets defined as having an area greater than 40,000 μm2. Representative images of islets were shown in Figure 5E and F. The large islets in both groups of animals were morphologically normal with tightly packed and centrally positioned β-cells. The percentages of β-cell mass per islet mass were not significantly different between the two groups of animals (63 ± 1.5% for rats fed CB diet vs 62 ± 1.8% for controls). However, the median percentage of large islets in pancreas was significantly increased in rats fed CB diet than in controls (6.2% vs 3.5%, p = .005 by Mann–Whitney U test; Figure 5G).
We further investigated the effect of cranberry supplementation on molecular changes in pancreas. The β cells in rats fed CB diet contained higher levels of insulin (Figure 6A, p < .05), and there was a trend of increase in the glucagon level by cranberry supplementation when compared with control rats (Figure 6B, p = .051). The expression level of PDX-1, a homeodomain transcription factor, was significantly higher in the pancreatic β-cells of rats fed CB diet than in those fed N diet (Figure 6C, p < .05). Taken together, these findings further reveal the beneficial effects of cranberry supplementation on endocrine pancreas in aging.
Figure 6.
Expression of insulin, PDX-1, and glucagon within islet cells. Triple immunofluorescence and confocal analysis with antibodies against insulin (green), glucagon (blue), and PDX-1 (red; upper panel) revealed that expression levels of insulin (A), glucagon (B), and PDX-1 (C), expressed as mean density within islets. Values are means ± standard error of 62–106 islets. **p < .01 versus N rats according to unpaired t test.
Effects of Cranberry Powder on Insulin Secretion In Vitro
To test whether cranberry directly affects β-cell function, insulin secretion assay was performed after a 72-hour preculture with a water-soluble cranberry powder. It is worth noting that insulin secretion from INS-1 cells that we used here has no paracrine influence, that is, by secreted glucagon or somatostatin that also respond to glucose and major insulinotropic peptides as occurred in vivo (29). In the presence of 3.3 mM glucose, INS-1 cells precultured with 0.25 and 0.5 mg/mL cranberry powder for 72 hours showed concentration-dependent increases in basal insulin secretion by 1.7- and 2.5-fold, respectively, when compared with nontreated controls (Figure 7). Insulin secretion was further increased in the presence of a higher concentration of glucose (27.7 mM; Figure 7). These in vitro observations complement and extend the OGTT and HOMA-β results and are consistent with the conclusion that cranberry supplementation improves β-cell function.
Figure 7.
Insulin secretion in vitro. The insulin-producing β-cell line, INS-1 cells, was cultured in RPMI medium containing 10% heat-inactivated fetal calf serum, 5.5 mM glucose, 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin, 1 mM sodium pyruvate, and 50 μM 2-mercaptoethanol for 72 hours. After preincubation in oxygenized Krebs–Ringer bicarbonate HEPES buffer at 3.3 or 27.7 mmol/L glucose, cells were shifted to 1 mL of the same buffer, and insulin release was then measured by using static incubation for a 60-minute period. At the end of the incubation, supernatants were collected. The amount of insulin secretion into the medium was measured with a commercially available enzyme-linked immunosorbent assay kit, normalized to total DNA content, and represented as nanograms per microgram DNA per 60 minute. Values are means ± standard error of eight independent samples. *p < .05, **p < .01, and ***p < .001 according to unpaired t test.
DISCUSSION
Epidemiological studies indicate that a proper diet rich in flavonoids from vegetables and fruits can reduce both incidence and progression of age-related diseases (31). In the present study, we have demonstrated that long-term cranberry consumption can delay an age-related decline in basal insulin, improve pancreatic β-cell glucose responsiveness, and expand functional β-cell mass in normal aged rats. Rats fed a cranberry rich diet for 12 months exhibited significant increases in both basal insulin and the amount of insulin secreted in response to glucose challenge. After 16 months of treatment, there was a significant increase in pancreatic β-cell mass with a corresponding increase in portal insulin concentration in rats fed the cranberry-rich diet. Parallel histological evidence from pancreatic islets revealed that cranberry supplementation enhanced β-cell mass and increased the fraction of large islets in pancreas from 3.5% to 6.2%. The expression levels of insulin and PDX-1 in islet cells of rats fed CB diet were also increased when compared with those fed normal diet. Against this background of improved β-cell function and total β-cell mass, no sign of structural pathology was detected among pancreatic islets in both groups.
We noticed that cranberry supplementation increased food intake over the course of the experiment and produced more body weight gain in rats under the fed state. However, we did not observe any significant difference in the terminal body weight and abdominal fat between two groups of rats after overnight fasting at the end of the experiment, suggesting that cranberry supplementation has marginal effects on body weight and fat accumulation. The discrepancy in body weight under the fed and fasting states may be due to the effect of cranberry on nutrient absorption. We found that cranberry supplementation reduced feed efficiency as indicated by the conversion efficiency from ingested food to body weight. One possible explanation is that dietary fibers from cranberry may directly reduce energy absorption and consequently contribute to lower feed efficiency and no change in the terminal body weight in both groups of animals. Under the fed state, the higher body weight in rats fed CB diet may be due to increased food intake and increased content in the gastrointestinal tract (sediments) when compared with controls. Taken together, it is unlikely that the changes in endocrine pancreas observed in the present study are due to increased food intake associated with cranberry consumption. However, a pair-feeding study should be conducted in the future to definitively exclude this remote possibility.
There are reasons to suspect that the effects of cranberry-rich diet on various aspects of β-cell functions may be mediated by flavonoid polyphenols. Previous in vitro studies have shown that the exposure of isolated islets to flavonoid polyphenols sustains β-cell function and enhances insulin release (32,33). Consistent with those studies, we have demonstrated that exposure of INS-1 cells to a cranberry powder enhances insulin secretion in a concentration-dependent manner. Potential mechanisms positioned to mediate insulinotropic effects of flavonoid polyphenols include altering Ca+2 fluxes, changing cyclic nucleotide metabolism (33), and decreasing oxidative stress (34).
The elevated basal insulin concentrations and improved β-cell function observed in the rats fed cranberry-rich diet are likely associated with the insulinotropic effect of cranberry. Many studies have attempted to define the pathophysiology of glucose intolerance with aging by focusing on glucose-stimulated β-cell function (35–38). In fact, basal insulin release may have a profound impact on development of glucose intolerance in the old animals (39). Our results point to an age-related decline in basal plasma insulin concentrations in Fischer 344 rats. This finding differs from other rodent models of metabolic aging, such as Sprague–Dawley rats (40), Wistar rats (41), and OLETF rats (42). Rats of those strains display a progressive increase in basal insulin concentrations, coupled with rapid gains in adipose tissue during early development (3–5 weeks) and adolescence (5–16 weeks), and followed by a slight change in adipose tissue with further aging. One interpretation is that metabolic alterations in these models are a secondary consequence of increased adiposity, particularly abdominal fat, rather than a central feature of aging per se. In addition, in those rat strains, connective tissues tend to proliferate into enlarged islets during aging, which results in multinodular appearance in islets and reduced insulin content in β-cells (1,42,43). The Fischer 344 strain used here, which has served as a major model of mammalian aging (20), is metabolically different from other rat models and generally does not display an age-related increase in adiposity (44). Even in the advanced age, structure of pancreatic islets remains normal, and β-cell neogenesis is more frequent in Fischer 344 compared with other rat strains (1,42,43). Consequently, there are no changes in insulin responsiveness following the stabilization of the maturational increase in muscle mass in Fischer 344 rats (45).
Increased portal insulin observed in the present study may also be a result of the insulinotropic effect of cranberry. Insulin is secreted in a pulsatile and orderly fashion, including rapid and ultradian insulin pulses. Several studies have demonstrated that rapid insulin pulses are of considerably higher amplitude in the portal vein than in the peripheral circulation (46). However, these rapid insulin pulses are declined during normal aging (47). In a previous study, we have demonstrated that Fischer 344 rats exhibit an age-related decline in portal insulin concentration, consistent with the interpretation that functional β-cell mass is compromised (48). Increased portal insulin by cranberry supplementation may provide health benefits to animals. Several studies have demonstrated that when portal insulin increases to a certain level, net hepatic glucose output is suppressed due to an increase in hepatic sinusoidal insulin (49). Furthermore, when both portal and peripheral insulin concentrations increase, non-esterified fatty acid levels fall and net hepatic lactate output rises due to the diversion of glycogenolytically derived carbon to lactate and a temporal suppression of non-esterified fatty acid uptake (49). The fall in non-esterified fatty acid has multiple potentially beneficial effects, including changes in substrate levels and mitochondrial redox state. These changes can enhance glycolysis and drive conversion of pyruvate to lactate by lactate dehydrogenase (50). Increased lactate production serves to maximize the efficiency of aerobic glycolysis, which is required for glucose-induced insulin release (51).
Many experimental conditions that increase insulin production not only affect secretion itself (52) and the release of other counter-regulatory hormones, such as glucagon (53), but also involve a positive feedback mechanism whereby insulin promotes β-cell growth (54). Indeed, pancreatic β-cell mass is tightly regulated through balancing cell renewal and apoptosis (55). Our morphologic evidence shows that insulin-positive cells within ducts, that is, neogenesis, have similar frequencies in both groups of rats (Figure 5C and D). Thus, it seems likely that increased replication and hypertrophy among preexisting β cells may contribute to the β-cell mass expansion observed in rats fed CB diet when compared with controls. In support of this explanation, we have found that the percentage of large islets in pancreas was increased, and expression of insulin and the transcription factor PDX-1, a glucose-sensitive factor for the insulin gene (56), was enhanced in cranberry-fed rats when compared with controls. Another possibility not tested here is that cranberry may modulate the release of incretins in the gut, such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) released from enteroendocrine L and K cells, respectively. GLP-1 and GIP are key players in the modulation of insulin secretion and do so in a glucose-dependent manner (57). These hormones also have positive effects on insulin gene transcription and at least GLP-1, which is known to regulate modest β-cell turnover (58).
In conclusion, the data presented here clearly demonstrate that supplementation of a whole cranberry powder at 2% in the diet can attenuate an age-related decline in basal level of insulin and insulin release in response to oral glucose challenge, that is, β-cell glucose responsiveness, together with a positive feedback on functional β-cell mass expansion in aging. Additional benefits of cranberry consumption include attenuation of the age-related declines in food intake and body weight. These findings provide a foundation to investigate the health benefits of long-term cranberry consumption in human. The daily dose of the cranberry powder used in the present study is approximately 1,000 mg/kg body weight for rats assuming that the average body weight of a rat is 0.4 kg and daily food intake is 20 g. This rat dose is equivalent to approximately daily 180 mg/kg body weight for a human adult of 70 kg in body weight using an FDA-recommended allometric human equivalent dose (HED) conversion method based on body surface area (59). This HED can be reasonably achieved by consuming approximately 12 g cranberry powder daily. However, clinical trials are required to identify the effective and safe dose of cranberry for human consumption. Taken together, the findings in the present study support that interventions with cranberry could fight against age-related changes in pancreatic islets. Certainly, additional studies will be necessary to confirm the effects of cranberry on pancreatic islets in diabetic animal models. Defining the molecular triggers for increased insulin release and retaining the capacity of β-cell mass in response to cranberry will represent important challenges for future research.
FUNDING
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, and in part by a grant from Cranberry Institute (East Wareham, MA) to S.Z.
CONFLICTS OF INTEREST
R.G. is employed by Decas Cranberry Products, Inc., which provided the cranberry powder for this research. The rest of the authors declare no conflicts of interest.
Acknowledgments
We thank Drs. Peter R. Rapp, Josephine Egans, and Edward Spangler for their helpful review of this paper. M.Z. and S.Z. designed and conducted research as well as wrote the manuscript, J.H., E.P., D.P., W.K., and J.N. conducted research, and R.G. provided essential materials. S.Z. has primary responsibility for final content. All authors read and approved the final manuscript.
References
- 1.Reaven EP, Reaven GM. Structure and function changes in the endocrine pancreas of aging rats with reference to the modulating effects of exercise and caloric restriction. J Clin Invest. 1981;68:75–84. doi: 10.1172/JCI110256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szoke E, Shrayyef MZ, Messing S, et al. Effect of aging on glucose homeostasis: accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care. 2008;31:539–543. doi: 10.2337/dc07-1443. [DOI] [PubMed] [Google Scholar]
- 3.Ihm SH, Moon HJ, Kang JG, et al. Effect of aging on insulin secretory function and expression of beta cell function-related genes of islets. Diabetes Res Clin Pract. 2007;77(suppl 1):S150–S154. doi: 10.1016/j.diabres.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 4.Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284:E7–E12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- 5.Iozzo P, Beck-Nielsen H, Laakso M, Smith U, Yki-Jarvinen H, Ferrannini E. Independent influence of age on basal insulin secretion in nondiabetic humans. European Group for the Study of Insulin Resistance. J Clin Endocrinol Metab. 1999;84:863–868. doi: 10.1210/jcem.84.3.5542. [DOI] [PubMed] [Google Scholar]
- 6.Bertoli A, Caputo S, Marini MA, Santini SA, Ghirlanda G, Greco AV. Inhibition of basal insulin secretion induces insulin resistance in normal man: evidence for a tonic effect of insulin on carbohydrate metabolism. Horm Res. 1989;31:238–243. doi: 10.1159/000181124. [DOI] [PubMed] [Google Scholar]
- 7.Suh KI, Song YM, Murata C, Joyce M, Ditzler TM, Henry RR. Role of basal insulin in maintenance of intracellular glucose metabolic pathways in non-insulin-dependent diabetes mellitus. Metabolism. 1995;44:41–46. doi: 10.1016/0026-0495(95)90287-2. [DOI] [PubMed] [Google Scholar]
- 8.Turner RC, McCarthy ST, Holman RR, Harris E. Beta-cell function improved by supplementing basal insulin secretion in mild diabetes. Br Med J. 1976;1:1252–1254. doi: 10.1136/bmj.1.6020.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swenne I. Effects of aging on the regenerative capacity of the pancreatic B-cell of the rat. Diabetes. 1983;32:14–19. doi: 10.2337/diab.32.1.14. [DOI] [PubMed] [Google Scholar]
- 10.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 12.Kris-Etherton PM, Lefevre M, Beecher GR, Gross MD, Keen CL, Etherton TD. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr. 2004;24:511–538. doi: 10.1146/annurev.nutr.23.011702.073237. [DOI] [PubMed] [Google Scholar]
- 13.Vattem DA, Ghaedian R, Shetty K. Enhancing health benefits of berries through phenolic antioxidant enrichment: focus on cranberry. Asia Pac J Clin Nutr. 2005;14:120–130. [PubMed] [Google Scholar]
- 14.Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271:751–754. doi: 10.1001/jama.1994.03510340041031. [DOI] [PubMed] [Google Scholar]
- 15.Colic M, Pavelic K. Molecular mechanisms of anticancer activity of natural dietetic products. J Mol Med. 2000;78:333–336. doi: 10.1007/s001090000121. [DOI] [PubMed] [Google Scholar]
- 16.Neto CC. Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res. 2007;51:652–664. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- 17.Knekt P, Kumpulainen J, Jarvinen R, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 18.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Kotaru M, Yoshikawa H, Ikeuchi T, Saito K, Iwami K, Ibuki F. An alpha-amylase inhibitor from cranberry bean (Phaseolus vulgaris): its specificity in inhibition of mammalian pancreatic alpha-amylases and formation of a complex with the porcine enzyme. J Nutr Sci Vitaminol (Tokyo) 1987;33:359–367. doi: 10.3177/jnsv.33.359. [DOI] [PubMed] [Google Scholar]
- 20.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Boyd O, Weng P, Sun X, et al. Nectarine promotes longevity in Drosophila melanogaster. Free Radic Biol Med. 2011;50:1669–1678. doi: 10.1016/j.freeradbiomed.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph JA, Shukitt-Hale B, Denisova NA, et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X, Seeberger J, Alberico T, et al. Acai palm fruit (Euterpe oleracea Mart.) pulp improves survival of flies on a high fat diet. Exp Gerontol. 2010;45:243–251. doi: 10.1016/j.exger.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinson JA, Su X, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem. 2001;49:5315–5321. doi: 10.1021/jf0009293. [DOI] [PubMed] [Google Scholar]
- 25.Zou S, Carey JR, Liedo P, Ingram DK, Yu B. Prolongevity effects of a botanical with oregano and cranberry extracts in Mexican fruit flies: examining interactions of diet restriction and age. Age (Dordr) doi: 10.1007/s11357-011-9230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou S, Carey JR, Liedo P, Ingram DK, Yu B, Ghaedian R. Prolongevity effects of an oregano and cranberry extract are diet dependent in the Mexican fruit fly (Anastrepha ludens) J Gerontol A Biol Sci Med Sci. 2010;65:41–50. doi: 10.1093/gerona/glp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Pickavance LC, Brand CL, Wassermann K, Wilding JP. The dual PPARalpha/gamma agonist, ragaglitazar, improves insulin sensitivity and metabolic profile equally with pioglitazone in diabetic and dietary obese ZDF rats. Br J Pharmacol. 2005;144:308–316. doi: 10.1038/sj.bjp.0706041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 30.Zhu M, Noma Y, Mizuno A, Sano T, Shima K. Poor capacity for proliferation of pancreatic beta-cells in Otsuka-Long-Evans-Tokushima fatty rat: a model of spontaneous NIDDM. Diabetes. 1996;45:941–946. doi: 10.2337/diab.45.7.941. [DOI] [PubMed] [Google Scholar]
- 31.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 32.Hii CS, Howell SL. Effects of flavonoids on insulin secretion and 45Ca2+ handling in rat islets of Langerhans. J Endocrinol. 1985;107:1–8. doi: 10.1677/joe.0.1070001. [DOI] [PubMed] [Google Scholar]
- 33.Hii CS, Howell SL. Effects of epicatechin on rat islets of Langerhans. Diabetes. 1984;33:291–296. doi: 10.2337/diab.33.3.291. [DOI] [PubMed] [Google Scholar]
- 34.Bast A, Wolf G, Oberbaumer I, Walther R. Oxidative and nitrosative stress induces peroxiredoxins in pancreatic beta cells. Diabetologia. 2002;45:867–876. doi: 10.1007/s00125-002-0846-1. [DOI] [PubMed] [Google Scholar]
- 35.Chen M, Bergman RN, Pacini G, Porte D., Jr. Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab. 1985;60:13–20. doi: 10.1210/jcem-60-1-13. [DOI] [PubMed] [Google Scholar]
- 36.Mori Y, Ojima K, Fuujimori Y, et al. Effects of mitiglinide on glucose-induced insulin release into the portal vein and fat-induced triglyceride elevation in prediabetic and diabetic OLETF rats. Endocrine. 2006;29:309–315. doi: 10.1385/endo:29:2:309. [DOI] [PubMed] [Google Scholar]
- 37.Novelli M, De Tata V, Fierabracci V, Barbera M, Rossetti R, Masiello P. Comparative study on the preventing effects of oral vanadyl sulfate and dietary restriction on the age-related glucose intolerance in rats. Aging Clin Exp Res. 2005;17:351–357. doi: 10.1007/BF03324622. [DOI] [PubMed] [Google Scholar]
- 38.Vera ER, Battell ML, Bhanot S, McNeill JH. Effects of age and anesthetic on plasma glucose and insulin levels and insulin sensitivity in spontaneously hypertensive and Wistar rats. Can J Physiol Pharmacol. 2002;80:962–970. doi: 10.1139/y02-124. [DOI] [PubMed] [Google Scholar]
- 39.Cherrington AD, Chiasson JL, Liljenquist JE, Jennings AS, Keller U, Lacy WW. The role of insulin and glucagon in the regulation of basal glucose production in the postabsorptive dog. J Clin Invest. 1976;58:1407–1418. doi: 10.1172/JCI108596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodman MN, Dluz SM, McElaney MA, Belur E, Ruderman NB. Glucose uptake and insulin sensitivity in rat muscle: changes during 3–96 weeks of age. Am J Physiol. 1983;244:E93–E100. doi: 10.1152/ajpendo.1983.244.1.E93. [DOI] [PubMed] [Google Scholar]
- 41.Nishimura H, Kuzuya H, Okamoto M, et al. Change of insulin action with aging in conscious rats determined by euglycemic clamp. Am J Physiol. 1988;254:E92–E98. doi: 10.1152/ajpendo.1988.254.1.E92. [DOI] [PubMed] [Google Scholar]
- 42.Okauchi N, Mizuno A, Zhu M, et al. Effects of obesity and inheritance on the development of non-insulin-dependent diabetes mellitus in Otsuka-Long-Evans-Tokushima fatty rats. Diabetes Res Clin Pract. 1995;29:1–10. doi: 10.1016/0168-8227(95)01114-s. [DOI] [PubMed] [Google Scholar]
- 43.Ogino T, Zhu M, Murakami T, Kuwajima M, Shima K. Effect of partial pancreatectomy on beta-cell mass in the remnant pancreas of Wistar fatty rats. J Med Invest. 1998;45:103–110. [PubMed] [Google Scholar]
- 44.Gabaldon AM, Florez-Duquet ML, Hamilton JS, McDonald RB, Horwitz BA. Effects of age and gender on brown fat and skeletal muscle metabolic responses to cold in F344 rats. Am J Physiol. 1995;268:R931–R941. doi: 10.1152/ajpregu.1995.268.4.R931. [DOI] [PubMed] [Google Scholar]
- 45.Barnard RJ, Lawani LO, Martin DA, Youngren JF, Singh R, Scheck SH. Effects of maturation and aging on the skeletal muscle glucose transport system. Am J Physiol. 1992;262:E619–E626. doi: 10.1152/ajpendo.1992.262.5.E619. [DOI] [PubMed] [Google Scholar]
- 46.Paolisso G, Scheen AJ, Giugliano D, et al. Pulsatile insulin delivery has greater metabolic effects than continuous hormone administration in man: importance of pulse frequency. J Clin Endocrinol Metab. 1991;72:607–615. doi: 10.1210/jcem-72-3-607. [DOI] [PubMed] [Google Scholar]
- 47.Meneilly GS, Veldhuis JD, Elahi D. Disruption of the pulsatile and entropic modes of insulin release during an unvarying glucose stimulus in elderly individuals. J Clin Endocrinol Metab. 1999;84:1938–1943. doi: 10.1210/jcem.84.6.5753. [DOI] [PubMed] [Google Scholar]
- 48.Zhu M, de Cabo R, Anson RM, Ingram DK, Lane MA. Caloric restriction modulates insulin receptor signaling in liver and skeletal muscle of rat. Nutrition. 2005;21:378–388. doi: 10.1016/j.nut.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 49.Sindelar DK, Balcom JH, Chu CA, Neal DW, Cherrington AD. A comparison of the effects of selective increases in peripheral or portal insulin on hepatic glucose production in the conscious dog. Diabetes. 1996;45:1594–1604. doi: 10.2337/diab.45.11.1594. [DOI] [PubMed] [Google Scholar]
- 50.Sekine N, Cirulli V, Regazzi R, et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J Biol Chem. 1994;269:4895–4902. [PubMed] [Google Scholar]
- 51.Mertz RJ, Worley JF, Spencer B, Johnson JH, Dukes ID. Activation of stimulus-secretion coupling in pancreatic beta-cells by specific products of glucose metabolism. Evidence for privileged signaling by glycolysis. J Biol Chem. 1996;271:4838–4845. doi: 10.1074/jbc.271.9.4838. [DOI] [PubMed] [Google Scholar]
- 52.Fanelli C, Pampanelli S, Epifano L, et al. Relative roles of insulin and hypoglycaemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycaemia in male and female humans. Diabetologia. 1994;37:797–807. doi: 10.1007/BF00404337. [DOI] [PubMed] [Google Scholar]
- 53.Liljenquist JE, Horwitz DL, Jennings AS, Chiasson JL, Keller U, Rubenstein AH. Inhibition of insulin secretion by exogenous insulin in normal man as demonstrated by C-peptide assay. Diabetes. 1978;27:563–570. doi: 10.2337/diab.27.5.563. [DOI] [PubMed] [Google Scholar]
- 54.Movassat J, Saulnier C, Portha B. Insulin administration enhances growth of the beta-cell mass in streptozotocin-treated newborn rats. Diabetes. 1997;46:1445–1452. doi: 10.2337/diab.46.9.1445. [DOI] [PubMed] [Google Scholar]
- 55.Angulo P, Alba LM, Petrovic LM, Adams LA, Lindor KD, Jensen MD. Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J Hepatol. 2004;41:943–949. doi: 10.1016/j.jhep.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 56.Marshak S, Benshushan E, Shoshkes M, Havin L, Cerasi E, Melloul D. Functional conservation of regulatory elements in the pdx-1 gene: PDX-1 and hepatocyte nuclear factor 3beta transcription factors mediate beta-cell-specific expression. Mol Cell Biol. 2000;20:7583–7590. doi: 10.1128/mcb.20.20.7583-7590.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Theodorakis MJ, Carlson O, Michopoulos S, et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006;290:E550–E559. doi: 10.1152/ajpendo.00326.2004. [DOI] [PubMed] [Google Scholar]
- 58.Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000;141:4600–4605. doi: 10.1210/endo.141.12.7806. [DOI] [PubMed] [Google Scholar]
- 59.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]