Abstract
Background.
Longevity clusters in families, and parental longevity may be associated with lower risk of chronic diseases in their children. It is unknown if diabetes risk is associated with parental longevity.
Methods.
We evaluated participants in the Diabetes Prevention Program with a parental history questionnaire at study entry. We classified them into five groups: premature death (parental death at age < 50 years), parental longevity (living to at least 80 years), and three intermediate groups (alive by age 49 but dying at age 50–59, 60–69, or 70–79). Those with alive parents and younger than 80 years were excluded. We analyzed separately effects of paternal (n = 2,165) and maternal (n = 1,739) longevity on diabetes incidence and risk after an average follow-up of 3.2 years.
Results.
At baseline, more diabetes risk factors (parental history of diabetes, coronary heart disease, higher body mass index, homeostasis model assessment for insulin resistance, and corrected insulin response) were found in participants whose parents died prematurely. Diabetes incidence was 9.5 cases/100 person-years in the 229 whose fathers died prematurely. In the 618 with paternal longevity, the rate was 6.6 cases/100 person-years (hazard ratio [95% confidence interval] = 0.68 [0.49–0.94]). The rates were 10.7 cases/100 person-years (n = 156) and 7.3 cases/100 person-years (n = 699, hazard ratio = 0.67 [95% confidence interval 0.47–0.95]) for those with maternal premature death or longevity, respectively. Associations with demographic and diabetes risk factors had minimal influence on the reduced risk found in those with paternal (adjusted hazard ratio = 0.78, 95% confidence interval 0.52–1.16) and maternal (adjusted hazard ratio = 0.64, 95% confidence interval 0.41–1.01) longevity.
Conclusion.
Parental longevity is associated with lower diabetes incidence in adults at high risk of type 2 diabetes.
Keywords: Parental longevity, Diabetes risk, Diabetes Prevention Program
LONGEVITY is a complex process resulting from genetic and environmental factors (including social, behavioral, and economic factors) and their interactions (1,2). Longevity involves biological processes that may protect individuals from age-related diseases (3). Longevity clusters in families, which could be explained by shared genetic or environmental risk factors (4,5). More than 100 genes are potentially involved in human longevity, including those in the insulin/insulin-like growth factor-1 pathway, FOXO3A, FOXO1A, lipoprotein metabolism, and cell cycle regulators (6).
Studies of parental longevity have shown that offspring of short-lived parents had higher mortality risk than those with long-lived parents (7–10). The offspring of centenarians have a more favorable cardiovascular profile compared with persons whose parents were born in the same year as the centenarians, but who died by age 73 years, the life expectancy for the centenarian birth cohort (11).
In the SUVIMAX Vascular Study, participants with premature paternal death had accelerated progression of systolic blood pressure and greater occurrence of hypertension compared with participants with parents living to age 80 or more (12). A recent report of this cohort also showed an inverse association of paternal longevity with the presence of carotid plaques and aortic arterial stiffness (13). To our knowledge, no study has evaluated prospectively the association of parental longevity with diabetes risk.
Diabetes and increased blood glucose are associated with premature death and decreased longevity (14–18). In response to the growing epidemic of diabetes, the Diabetes Prevention Program (DPP) demonstrated that intensive lifestyle modification or metformin could delay the development of type 2 diabetes (T2D) in a high-risk population who were overweight or obese, had impaired glucose tolerance, and elevated fasting plasma glucose (FPG) (19). The intensive lifestyle modification program, which included a goal of 7% weight loss and 150 minutes of moderate intensity physical activity per week, was exceptionally effective in older individuals (20). Participants were counseled to reduce dietary intake to 1,200–2,000 kcal/d based on their baseline weight and to reduce dietary fat to less than 25% of total calories (21).
In this paper, we have considered whether parental longevity is associated with decreased diabetes risk. Specifically, we tested the hypothesis that participants with parental longevity (father or mother alive at age 80) had lower incidence of diabetes than those whose parents died prematurely. The reduced risk for diabetes associated with parental longevity could be related to genetic, epigenetic, and/or environmental factors (22,23).
METHODS
Study Participants and Procedures
Detailed eligibility criteria, design, and methods of the DPP have been published (19,24). In brief, selection criteria included: age 25 years or older, body mass index 24 kg/m2 or higher (≥22 kg/m2 in Asian Americans), FPG levels between 95 and 125 mg/dL, and impaired glucose tolerance (2-hour post-load glucose of 140–199 mg/dL). Persons were excluded if they were taking medications known to alter glucose tolerance (including those for diabetes management) or if they had illnesses that could seriously reduce their life expectancy or their ability to participate in the trial. All participants provided written informed consent and signed documents approved by the institutional review board at each center. Participants received standard advice on healthy diet and physical activity and were randomized to one of three interventions: intensive lifestyle, metformin 850 mg bid, or matching placebo.
Recruitment was performed at 27 centers in the United States and by design included a diverse population in terms of age, sex, race, and geographic location. Compared with the general U.S. population, DPP participants had higher proportion of racial/ethnic minorities (25,26).
Parental history.—
A parental history questionnaire was administered at study entry. Participants were asked the year of birth of their mother and father, whether the parents were still alive, year of death if deceased, as well as parental history of diabetes and coronary heart disease (CHD) including their age at diagnosis.
Clinical and metabolic variables.—
Standardized interviewer-administered questionnaires were used to obtain self-reported demographic, socioeconomic, and clinical data. Height and weight were measured using standard techniques. All glucose and insulin measurements were performed at the Central Biochemistry Laboratory (University of Washington, Seattle). Insulin secretion was estimated with the corrected insulin response = (100 × 30-minute insulin)/(30-minute glucose × [30-minute glucose −70 mg/dL]) (27). Insulin resistance was estimated using the homeostasis model assessment (HOMAIR) = fasting insulin × fasting glucose/22.5 (28).
Outcomes.—
Development of incident diabetes was determined by an annual oral glucose tolerance test and semiannual FPG tests and required confirmation by a second test, using the 1997 criteria of the American Diabetes Association and the 1998 World Health Organization (ie, either FPG ≥ 126 mg/dL or 2-hour plasma glucose ≥ 2 00 mg/dL) (29,30).
Data Analysis
This analysis is based on data collected from the start of DPP (June 1996) through July 31, 2001, when the study results and treatment assignment were unmasked. Participants were followed for an average of 3.2 years. For the present analysis, we classified them into five groups according to their parents’ ages (at death or at study entry if living). Premature death was defined as parental death age younger than 50 years (31), parental longevity as lived to at least 80 years, and three intermediate status groups as alive by age 49 but dying at ages 50–59, 60–69, or 70–79.
We analyzed separately the effects of paternal (n = 2,165) and maternal (n = 1,739) longevity on diabetes risk. We excluded from this analysis participants whose parents were still alive and younger than 80 years (883 fathers and 1,402 mothers) at study entry because their parents may live 80 years or older and therefore cannot be classified (ie, their data are not informative). Because of missing data, multiple imputations were performed to confirm the modeling results and evaluate the simultaneous effects of paternal and maternal longevity (32). The Kruskal–Wallis test (33) was used to compare the five parental age groups for continuous baseline characteristics. Medians and interquartile ranges are reported. Chi-square test was used for categorical baseline characteristics. Cox proportional hazards models (34) were used to assess time to diabetes diagnosis. Stepwise adjustments were made for baseline demographic and socioeconomic factors, DPP treatment groups, parental history of diabetes and CHD, and known predictors of diabetes (body mass index, HOMAIR, and corrected insulin response). Because no interactions between treatment groups and parental longevity groups were found, models were not stratified by treatment groups. The SAS system was used for all analyses (version 8.2; SAS Institute Inc., Cary, NC).
RESULTS
Among the 3,234 DPP participants, 3,141 reported data on their mother’s age or age at death and 3,048 reported data on their father’s age or age at death. One thousand seven hundred and fifty-one (55.7%) of mothers and 1,122 (36.8%) of fathers were alive at study entry. Based on the Kaplan–Meier method with live parents treated as censored, the estimated median age at death (95% confidence interval [CI]) was 75 (74–76) for the fathers and 81 (81–82) for the mothers. The prevalences of premature death (<50 years) were 7.5% and 5% for fathers and mothers, respectively.
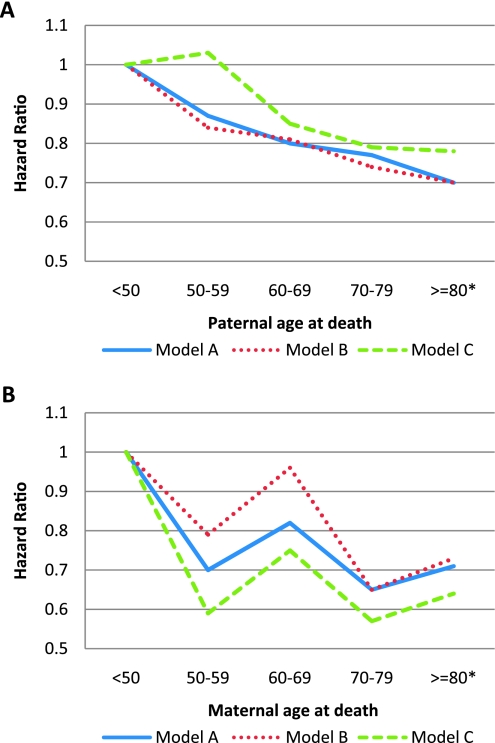
Baseline characteristics of the study population according to paternal (n = 2,165) and maternal (n = 1,739) longevity groups are shown in Tables 1 and 2. Compared with participants with paternal longevity, those with paternal premature death were younger and had higher mean values of body mass index and HOMAIR. The proportion of participants reporting paternal history of CHD before age 50 was lower in those with paternal longevity; a significant trend was also observed for paternal history of diabetes diagnosed before age 50. Similarly, participants with maternal premature death were also younger and had higher mean values of body mass index, HOMAIR, and corrected insulin response than in those with maternal longevity. The proportion of participants reporting a history of CHD or diabetes in their mothers was also lower in those with maternal longevity. No significant differences in baseline leisure physical activity, total caloric intake, or fasting or 2-hour plasma glucose were observed across paternal or maternal longevity groups. Overall, 444 and 364 incident diabetes cases were observed during follow-up for the analysis of paternal and maternal longevity, respectively. Diabetes incidence rates according to parental longevity groups are shown in Table 3. The diabetes risk was 32% (95% CI 6%–51%) lower in the paternal longevity group (father’s age ≥ 80 years) compared with the paternal premature death group (father’s age of death < 50 years; Table 4). A significant trend was observed for the protective effect of increasing paternal age at death on diabetes risk (p = .01). This trend remained significantly adjusted for baseline demographics and DPP treatment groups (Figure 1A, Model A, p = .03) but became less evident in subsequent models accounting for differences in socioeconomic status (income and education), paternal history of CHD and diabetes, and known predictors of diabetes.
Table 1.
Baseline Characteristics of DPP Study Participants According to Paternal Longevity Groups
| Participants’ Characteristics | Father’s Age at Death |
p Value |
||||
| <50 (n = 229) | 50–59 (n = 277) | 60–69 (n = 522) | 70–79 (n = 519) | 80+ (n = 618)* | ||
| Age, y | 49.8 (42.1–58.8) | 50.4 (44.6–59.4) | 51.2 (45.3–59.1) | 54.7 (49–61.1) | 55.4 (50–62.4) | <.0001† |
| % Female | 67.2 | 66.1 | 64.2 | 60.1 | 64.9 | .27 |
| % Caucasian | 46.3 | 53.8 | 56.1 | 61.8 | 59.2 | .003† |
| % Income ≥ $50,000 | 32.4 | 40.5 | 47.1 | 45.2 | 46.3 | .003‡ |
| % Education ≥ 17 y | 20.1 | 26.4 | 29.5 | 29.7 | 27.2 | .02‡ |
| BMI, kg/m² | 32.7 (29–37.6) | 32.1 (28.6–36.3) | 32.8 (28.9–37.5) | 31.6 (28.8–35.7) | 31.7 (28.2–35.9) | .01† |
| Physical activity, MET-h/wk | 9.7 (3.6–20.8) | 9.4 (4.1–21.7) | 9.5 (3.6–19.1) | 10.7 (4.5–20.1) | 10.8 (4.4–23.0) | .21 |
| Total, kcal/d | 1,788 (1,400–2,508) | 1,751 (1,396–2,383) | 1,886 (1,454–2,526) | 1,882 (1,414–2,480) | 1,843 (1,428–2,459) | .43 |
| Fasting PG, mg/dL | 106 (100–113) | 105 (101–111) | 105 (101–112) | 105 (101–112) | 105.5 (100–112) | .95 |
| 2-h PG, mg/dL | 166 (151–179) | 162 (150–175) | 164 (150–178) | 164 (150–180) | 161 (149–178) | .13 |
| Fasting insulin, IU/mL | 24 (16–35) | 23 (15–33) | 23 (16–34) | 22 (15–31) | 22 (15–30) | .01† |
| HOMA-IR | 6.4 (4–9.2) | 5.9 (3.9–8.5) | 6.0 (4.1–8.8) | 5.8 (3.8–8.1) | 5.7 (3.9–7.9) | .05† |
| CIR | 0.5 (0.3–0.8) | 0.5 (0.3–0.8) | 0.5 (0.4–0.8) | 0.5 (0.3–0.7) | 0.5 (0.3–0.8) | .79 |
| % Father having DM at age younger than 50 y | 11.2 | 19.6 | 16.4 | 8.1 | 3.8 | <.0001‡ |
| % Father having CHD at age younger than 50 y | 26.6 | 8.1 | 6.3 | 3.3 | 1.2 | <.0001‡ |
Notes: Data are median (interquartile range) unless otherwise specified. BMI = body mass index; CHD = coronary heart disease; CIR = corrected insulin response; DM = diabetes mellitus; DPP = Diabetes Prevention Program; HOMA-IR = homeostatic model assessment for insulin resistance; MET = metabolic equivalent; PG = plasma glucose. MET-hours represent the average amount of time engaged in specified physical activities multiplied by the MET value of each activity (data are based on responses to the Modifiable Activity Questionnaire).
The 80+ group includes people whose father was still alive and aged 80 years or older.
p Value < .05 for linear trend test across the five groups.
p Value < .05 comparing the 80+ group versus <50 group.
Table 2.
Baseline Characteristics of DPP Study Participants According to Maternal Longevity Groups
| Participants’ Characteristics | Mother’s Age at Death |
p Value | ||||
| <50 (n = 156) | 50–59 (n = 188) | 60–69 (n = 295) | 70–79 (n = 401) | 80+ (n = 699)* | ||
| Age, y | 54.7 (44.9–62.1) | 50.5 (43.3–58.2) | 51.6 (46.2–58.6) | 55.2 (50.6–62.5) | 59 (53.1–64.6) | <.0001† |
| % Female | 64.7 | 71.3 | 67.1 | 60.1 | 60.4 | .02† |
| % Caucasian | 43.6 | 53.2 | 54.6 | 61.8 | 67.7 | <.0001† |
| % Income ≥ $50,000 | 36.4 | 38.1 | 40.9 | 44.2 | 45.6 | .16† |
| % Education ≥ 17 y | 21.2 | 20.7 | 25.4 | 30.2 | 28.8 | .19 |
| BMI, kg/m² | 33 (29.3–36.1) | 34.3 (29.5–38.4) | 32.7 (29.2–37.5) | 32 (28.6–36) | 31.2 (28.1–35.3) | <.0001† |
| Physical activity, MET-hours/wk | 10.0 (3.0–20.6) | 8.3 (3.0–18.8) | 9.9 (3.5–22.8) | 9.9 (3.9–19.7) | 11.2 (4.7–22.6) | .06 |
| Total, kcal/day | 1,952 (1,381–2,412) | 1,900 (1,464–2,595) | 1,793 (1,385–2,472) | 1,790 (1,413–2,323) | 1,791 (1,373–2,399) | .46 |
| Fasting PG, mg/dL | 104 (100–113) | 105 (100–114) | 106 (100–112) | 105 (100–112) | 105 (101–112) | .87 |
| 2-h PG, mg/dL | 164.5 (149–179) | 161.5 (150.5–178) | 166 (150–179) | 161 (149–180) | 162 (149–179) | .84 |
| Fasting insulin, IU/mL | 25 (17–34) | 24 (17–32) | 24 (16–35) | 22 (15–30) | 21 (14–29) | .0004† |
| HOMA-IR | 6.4 (4.2–9) | 6.2 (4.6–8.5) | 6.2 (4.1–9.2) | 5.6 (3.8–8.2) | 5.4 (3.7–7.7) | <.0001† |
| CIR | 0.6 (0.4–0.8) | 0.5 (0.3–0.7) | 0.5 (0.4–0.8) | 0.5 (0.3–0.7) | 0.5 (0.3–0.7) | .0005† |
| % Mother having DM at age younger than 50 y | 13.6 | 24.7 | 20.6 | 15.2 | 4.8 | <.0001‡ |
| % Mother having CHD at age younger than 50 y | 9.8 | 5.0 | 3.1 | 1.8 | 0.7 | <.0001‡ |
Notes: Data are median (interquartile range) unless otherwise specified. BMI = body mass index; CHD = coronary heart disease; CIR = corrected insulin response; DM = diabetes mellitus; DPP = Diabetes Prevention Program; HOMA-IR = homeostatic model assessment for insulin resistance; MET = metabolic equivalent; PG = plasma glucose. MET-hours represent the average amount of time engaged in specified physical activities multiplied by the MET value of each activity (data are based on responses to the Modifiable Activity Questionnaire).
The 80+ group includes people whose father was still alive and aged 80 years or older.
p Value < .05 for linear trend test across the five groups.
p Value < .05 comparing the 80+ group versus <50 group.
Table 3.
Diabetes Incidence in Study Participants by Parents’ Age at Death or at Study Entry if Living
| Number of Participants | Number of Events (new diabetes cases) | Person-Years of Follow-up | Incidence Rate (95% CI) (per 100 person-y) | |
| Father’s age at death (y) | ||||
| <50 | 229 | 57 | 601.5 | 9.5 (7.4–12.2) |
| 50–59 | 277 | 61 | 737.0 | 8.3 (6.5–10.6) |
| 60–69 | 522 | 110 | 1420.5 | 7.7 (6.5–9.3) |
| 70–79 | 519 | 102 | 1416.5 | 7.2 (5.9–8.7) |
| ≥80* | 618 | 114 | 1740.0 | 6.6 (5.5–7.8) |
| Mother’s age at death (y) | ||||
| <50 | 156 | 45 | 422.5 | 10.7 (8.0–14.1) |
| 50–59 | 188 | 36 | 497.5 | 7.2 (5.3–10.0) |
| 60–69 | 295 | 66 | 788.0 | 8.4 (6.6–10.6) |
| 70–79 | 401 | 77 | 1106.5 | 7.0 (5.6–8.7) |
| ≥80* | 699 | 140 | 1921.0 | 7.3 (6.2–8.6) |
Note: *The ≥80 group includes people whose father (or mother) was still alive and aged 80 years or older.
Table 4.
Effect of Paternal or Maternal Longevity on Diabetes Risk
| Hazard Ratio (95% confidence interval) |
||
| Paternal Longevity | Maternal Longevity | |
| Unadjusted | 0.68 (0.49–0.94) | 0.67 (0.47–0.95) |
| Model A | 0.70 (0.50–0.97) | 0.71 (0.50–1.02) |
| Model B | 0.70 (0.48–1.03) | 0.73 (0.48–1.13) |
| Model C | 0.78 (0.52–1.16) | 0.64 (0.41–1.01) |
Notes: Model A: adjusted for baseline age, sex, race/ethnicity, and DPP treatment groups; Model B: Model A + baseline socioeconomic status (income and education), parental diabetes, and coronary heart disease (447 and 345 participants for paternal and maternal longevity analyses, respectively, were excluded due to missing data); Model C: Model B + fasting plasma glucose and insulin, body mass index, and corrected insulin response at baseline (43 and 35 additional participants for paternal and maternal longevity analyses, respectively, were excluded due to missing data).
Figure 1.
Diabetes risk according to paternal (A) or maternal (B) age of death. Those whose father or mother died prematurely (age younger than 50 years were used as the reference group). *The group aged 80 years or older includes participants whose father or mother was still alive and aged 80 years or older. Model A: adjusted for baseline age, sex, race/ethnicity, and treatment groups. Model B: Model A + income and education, parental diabetes, and coronary heart disease; Model C: Model B + fasting plasma glucose and insulin, body mass index, and corrected insulin response at baseline.
We also observed a reduction in diabetes risk (33%) in the maternal longevity group (mothers’ age ≥ 80 years) compared with the maternal premature death group. However, the trend for the protective effect of increasing maternal age at death was marginal (p = .06) and was less evident after adjusting for the same covariates listed above (Figure 1B).
Factors related to adherence to DPP interventions were examined. There were no differences in weight loss, calorie intake, or metformin adherence according to paternal or maternal longevity (data not shown). Higher physical activity in the paternal (15.0 [7.7–27.6] MET-hours/wk vs 12.5 [5.3–24.2] MET-hours/wk, p < .01) and maternal (15.5 [8.0–27.4] MET-hours/wk vs 11.3 [6.1–25.9] MET-hours/wk, p < .05) longevity groups were observed after 1 year of intervention, but they were not associated with diabetes risk when added to the multivariate models for paternal (p = .56) or maternal (p = .55) longevity analyses.
We used multiple imputations to assign age at death for the live parents of participants who were excluded in the original analyses. Models including the imputed data were compared with the main analyses excluding the live parents younger than 80 years. The risk reductions were 20% (95% CI −11% to 51%) in the paternal and 25% (−7% to 58%) in the maternal longevity groups. The same protective trend remained in the increasing parental age group and when both paternal and maternal longevity effects were considered simultaneously in the imputation models. For parental longevity (either mother or father with longevity), we used the larger of the two parental ages at death and found a similar pattern of lower diabetes risk in those with longer lived parents. The risk reduction was 34% (95% CI -32% to 67%) in the parental longevity group.
DISCUSSION
Parental longevity conferred a reduced diabetes risk in a population selected for their high risk for T2D. To our knowledge, this is the first study to prospectively evaluate the association of parental longevity with diabetes incidence assessed objectively. This protective association is comparable to the benefit reported from DPP and other clinical trials of pharmacological interventions in those at high risk (35).
The children of long-lived parents may age more successfully than those of short-lived parents. Analysis from cross-sectional studies showed that for every 10 additional years, the parents lived beyond age 54 their children had approximately a 20% reduction in the risk for chronic conditions (36). In our cohort, there was a significant trend of reduced diabetes risk related to paternal longevity, apparently independent of demographic, clinical, and metabolic risk factors. A similar pattern was seen for maternal longevity, but it was less evident when adjusting for the same confounding factors. Lower diabetes risk has been found in the offspring of long-lived parents. The offspring of centenarian Ashkenazi Jews had lower prevalence of diabetes than age-matched controls (4). In the Leiden Longevity Study, the offspring of nonagenarians siblings had a lower mean FPG and insulin levels and better glucose tolerance than their partners (37). This cohort also showed lower mortality and prevalence of myocardial infarction, hypertension, and diabetes (38) and more favorable lipoprotein particle profiles (39). Lower risk of Alzheimer’s disease and memory decline was found in the offspring of parents with exceptional longevity (40). Similarly data from the English Longitudinal Study of Aging showed that parental life span is positively associated with cognitive functioning at older age and with decreased likelihood of occurrence of chronic diseases (41).
Premature maternal or paternal death, relative to living past 80 years, had virtually the same effects on the offspring’s diabetes risk (adjusted hazard ratio 0.70 [95% CI = 0.50–0.97] for fathers and 0.71 [0.50–1.02] for mothers). The slightly wider CI for mothers is explained by the smaller size of the referent group (premature mortality) for mothers, 156, than for fathers, 229. The trend over all age groups was linear in fathers but less uniform in mothers (Figure 1), a difference attributed to the smaller size of the maternal referent group.
In the cross-sectional assessment of study participants, we found that CHD history at baseline was lower in fathers in the paternal longevity groups. This is consistent with results reported in a Northern Ireland and France study showing that family history and parental longevity, although related, may act independently in predicting 5-year incidence of coronary events in middle-aged men (42).
Studies on candidate gene polymorphisms in centenarians have shown a positive association with longevity, whereas other loci have been linked to the development of age-related diseases (43). Association with genes involved in inflammation, insulin/insulin-like growth factor-1 signaling pathway, lipid metabolism, and oxidative stress has been described (6,43,44). Although some have shown inconsistent results, which may be related to population-specific interactions between gene pools and environment (45), others have shown consistent association with the apolipoprotein E (46–48) and the forkhead box 03A (FOXO3A) (49–52). The Framingham Study showed that longevity and aging traits were associated with single-nucleotide polymorphisms, although in those analyses none of the associations achieved genome-wide significance (53). Recent targeted investigation showed three top-ranking markers located in the genes DUSP6, NALP1, and PERP involved in the induction of apoptosis and other diverse pathways linked to longevity or the aging process (54).
The advent of genome-wide association screening has uncovered many loci newly associated with T2D (55,56). A recent report from the Leiden Longevity Study found that well-established T2D gene variants were associated with higher glucose levels. However, no difference in the frequency of these polymorphisms was found between the offspring of long-lived siblings and their controls, suggesting that the better glucose tolerance reported in the offspring is not explained by a lower burden of these T2D risk alleles and rather protective alleles for longevity may be involved (57). Once longevity genes are better established, future analysis of these genes, their gene-to-gene interactions, shared good health habits, and gene-to-environment interactions (58) may provide further insights on the effect that parental longevity has on the risk for diabetes and other chronic diseases. Similarly, the assessment of the effect that changes in diet and physical activity have on biomarkers of longevity can provide insights to understand mechanisms of healthier aging in participants at high risk for T2D.
We acknowledge the limitations of our study. The reported ages of live and deceased parents on the parental history questionnaire were not validated, and we did not ascertain causes of death. Therefore, the association of diabetes risk with parental longevity/premature death, based on mortality related to chronic diseases (ie, cardiovascular disease, diabetes, and cancer), may have been diluted due to traumatic causes of death (ie, accidents, war, homicides, and suicides). In addition, self-selection of participants into the study led to inclusion of those with parental diabetes and potentially with history of parents with CHD and premature death. Finally, because the DPP participants were fairly young, many had parents who were still alive, not yet 80 years or older, and consequently could not be used for this analysis. If vital statistics on these younger fathers (n = 883) and mothers (n = 1,402) can be updated, then more will attain the age of 80 years or die before that age and thus be informative for our analysis.
In summary, we have shown that parental longevity is associated with lower diabetes incidence in adults at high risk for T2D. The assessment of parental longevity may provide information for risk stratification of these individuals, adding information beyond that suggested by glucose intolerance and associated cardiovascular risk factors. Future studies of candidate genes, epigenetic factors, and biomarkers for longevity may provide a better understanding of the mechanism whereby parental longevity may lead to reduction of diabetes risk.
FUNDING
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study; and collection, management, analysis, and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources supported data collection at many of the clinical centers. Funding for data collection and participant support was also provided by the Office of Research on Minority Health, the National Institute of Child Health and Human Development, the National Institute on Aging, the Centers for Disease Control and, Prevention, the Office of Research on Women’s Health, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided medication. This research was also supported, in part, by the intramural research program of the NIDDK. LifeScan Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. The opinions expressed are those of the investigators and do not necessarily reflect the views of the Indian Health Service or other funding agencies. A complete list of centers, investigators, and staff can be found in the Supplementary Appendix.
The work was also supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Miami CTSA-K12/GRECC program (H.F.). The General Clinical Research Center Program, National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers.
SUPPLEMENTARY DATA
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Acknowledgments
The investigators gratefully acknowledge the commitment and dedication of the participants of the DPP. The authors thank Dr. Bruce Troen from the University of Miami Geriatrics Institute/ Miami VAHS GRECC for his valuable input. Trial registration: DPP is registered in www.clinicaltrials.gov (NCT00004992).
References
- 1.Gallucci M, Ongaro F, Bresolin F, et al. The Treviso Longeva (Trelong) study: a biomedical, demographic, economic and social investigation on people 70 years and over in a typical town of North-East of Italy. Arch Gerontol Geriatr. 2007;44(suppl 1):173–192. doi: 10.1016/j.archger.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 2.Dupre ME, Liu G, Gu D. Predictors of longevity: evidence from the oldest old in China. Am J Public Health. 2008;98:1203–1208. doi: 10.2105/AJPH.2007.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perls TT, Wilmoth J, Levenson R, et al. Lifelong sustained mortality advantage of siblings of centenarians. Proc Natl Acad Sci U S A. 2002;99:8442–8447. doi: 10.1073/pnas.122587599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atzmon G, Schechter C, Greiner W, et al. Clinical phenotype of families with longevity. J Am Geriatr Soc. 2004;52:274–277. doi: 10.1111/j.1532-5415.2004.52068.x. [DOI] [PubMed] [Google Scholar]
- 5.Christensen K, Johnson TE, Vaupel JW. The quest for genetic determinants of human longevity: challenges and insights. Nat Rev Genet. 2006;7:436–448. doi: 10.1038/nrg1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung W-H, Dao RL, Chen LK, Hung SI. The role of genetic variants in human longevity. Aging Res Rev. 2010;9(suppl 1):S67–S78. doi: 10.1016/j.arr.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandenbroucke JP, Matroos AW, van der Heide-Wessel C, et al. Parental survival, an independent predictor of longevity in middle-aged persons. Am J Epidemiol. 1984;119:742–750. doi: 10.1093/oxfordjournals.aje.a113795. [DOI] [PubMed] [Google Scholar]
- 8.Petersen L, Nielsen GG, Andersen PK, Sørensen TI. Case-control study of genetic and environmental influences on premature death of adult adoptees. Genet Epidemiol. 2002;23:123–132. doi: 10.1002/gepi.1122. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda A, Iso H, Toyoshima H, et al. Parental longevity and mortality amongst Japanese men and women: the JACC Study. J Intern Med. 2006;259:285–295. doi: 10.1111/j.1365-2796.2005.01609.x. [DOI] [PubMed] [Google Scholar]
- 10.Perls T, Kohler IV, Andersen S, et al. Survival of parents and siblings of supercentenarians. J Gerontol A Biol Sci Med Sci. 2007;62:1028–1034. doi: 10.1093/gerona/62.9.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terry DF, Wilcox MA, McCormick MA, Perls TT. Cardiovascular disease delay in centenarian offspring. J Gerontol. 2004;59A:385–389. doi: 10.1093/gerona/59.4.m385. [DOI] [PubMed] [Google Scholar]
- 12.Zureik M, Galan P, Bertrais S, et al. Parental longevity and 7-year changes in blood pressures in adult offspring. Hypertension. 2005;46:287–294. doi: 10.1161/01.HYP.0000173068.13787.4d. [DOI] [PubMed] [Google Scholar]
- 13.Zureik M, Czernichow S, Courbon D, et al. Parental longevity, carotid atherosclerosis, and aortic arterial stiffness in adult offspring. Stroke. 2006;37:2702–2707. doi: 10.1161/01.STR.0000244759.62619.83. [DOI] [PubMed] [Google Scholar]
- 14.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 15.Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care. 1998;21:1167–1172. doi: 10.2337/diacare.21.7.1167. [DOI] [PubMed] [Google Scholar]
- 16.Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282:1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 17.Khaw KT, Wareham N, Bingham S, et al. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141:413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 18.Kramarow E, Lubitz J, Lentzner H, Gorina Y. Trends in the health of older Americans, 1970–2005. Health Aff. 2007;26:1417–1425. doi: 10.1377/hlthaff.26.5.1417. [DOI] [PubMed] [Google Scholar]
- 19.Knowler WC, Barrett-Connor E, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crandall J, Schade D, et al. Diabetes Prevention Program Research Group. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61:1075–1081. doi: 10.1093/gerona/61.10.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budovsky A, Muradian KK, Fraifeld VE. From disease-oriented to aging/longevity-oriented studies. Rejuvenation Res. 2006;9:207–210. doi: 10.1089/rej.2006.9.207. [DOI] [PubMed] [Google Scholar]
- 23.Kaati G, Bygren LO, Pembrey M, Sjöström M. Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genetics. 2007;15:784–790. doi: 10.1038/sj.ejhg.5201832. [DOI] [PubMed] [Google Scholar]
- 24.Diabetes Prevention Program Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimoto WY Diabetes Prevention Program Research Group. Background and recruitment data for the U.S. Diabetes Prevention Program. Diabetes Care. 2000;23(suppl 2):B11–B13. [PMC free article] [PubMed] [Google Scholar]
- 26.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care. 2000;23:1619–1629. doi: 10.2337/diacare.23.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 29.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report on the Expert Committee on Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 30.Alberti K, Zimmet P. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diab Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. for the WHO consultation. [DOI] [PubMed] [Google Scholar]
- 31.Sørensen TI, Nielsen GG, Andersen PK, Teasdale TW. Genetic and environmental influences on premature death in adult adoptees. N Engl J Med. 1988;318:727–732. doi: 10.1056/NEJM198803243181202. [DOI] [PubMed] [Google Scholar]
- 32.Little RJ, Rubin DB. Statistical Analysis with Missing Data. 2nd ed. New York: John Wiley & Sons; 2002. [Google Scholar]
- 33.Lehmann EL. Nonparametric Statistical Methods Based on Ranks. New York: McGraw-Hill; 1975. [Google Scholar]
- 34.Cox DR. Regression models and life tables. J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 35.Crandall JP, Knowler WC, Kahn SE, et al. The prevention of type 2 diabetes. Nat Clin Pract Endocrinol Metab. 2008;4:382–393. doi: 10.1038/ncpendmet0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frederiksen H, McGue M, Jeune B, et al. Do children of long-lived parents age more successfully? Epidemiology. 2002;13:334–339. doi: 10.1097/00001648-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Rozing MP, Westendorp RG, de Craen AJ, et al. Favorable glucose tolerance and lower prevalence of metabolic syndrome in offspring without diabetes mellitus of nonagenarian siblings: the Leiden longevity study. J Am Geriatr Soc. 2010;58:564–569. doi: 10.1111/j.1532-5415.2010.02725.x. [DOI] [PubMed] [Google Scholar]
- 38.Westendorp RG, van Heemst D, Rozing MP, et al. Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: the Leiden Longevity Study. J Am Geriatr Soc. 2009;57:1634–1637. doi: 10.1111/j.1532-5415.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- 39.Heijmans BT, Beekman M, Houwing-Duistermaat JJ, et al. Lipoprotein particle profiles mark familial and sporadic human longevity. PLoS Med. 2006;3:e495. doi: 10.1371/journal.pmed.0030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipton RB, Hirsch J, Katz MJ, et al. Exceptional parental longevity associated with lower risk of Alzheimer's disease and memory decline. J Am Geriatr Soc. 2010;58:1043–1049. doi: 10.1111/j.1532-5415.2010.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gjonça E, Zaninotto P. Blame the parents? The association between parental longevity and successful ageing. Demogr Res. 2008;19:1435–1450. [Google Scholar]
- 42.Yarnell J, Yu S, Patterson C, et al. Family history, longevity, and risk of coronary heart disease: the PRIME Study. Int J Epidemiol. 2003;32:71–77. doi: 10.1093/ije/dyg038. [DOI] [PubMed] [Google Scholar]
- 43.Capri M, Salvioli S, Sevini F, et al. The genetics of human longevity. Ann N Y Acad Sci. 2006;1067:252–263. doi: 10.1196/annals.1354.033. [DOI] [PubMed] [Google Scholar]
- 44.Cluett C, Melzer D. Human genetic variations: beacons on the pathways to successful ageing. Mech Ageing Dev. 2009;130:553–563. doi: 10.1016/j.mad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Salvioli S, Olivieri F, Marchegiani F, et al. Genes, ageing and longevity in humans: problems, advantages and perspectives. Free Radic Res. 2006;40:1303–1323. doi: 10.1080/10715760600917136. [DOI] [PubMed] [Google Scholar]
- 46.Eggertsen G, Tegelman R, Ericsson S, et al. Apolipoprotein E polymorphism in a healthy Swedish population: variation of allele frequency with age and relation to serum lipid concentrations. Clin Chem. 1993;39:2125–2129. [PubMed] [Google Scholar]
- 47.Schächter F, Faure-Delanef L, Guénot F, et al. Genetic associations with human longevity at the APOE and ACE loci. Nat Genet. 1994;6:29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- 48.Jazwinski SM, Kim S, Dai J, et al. HRAS1 and LASS1 with APOE are associated with human longevity and healthy aging. Aging Cell. 2010;9:698–708. doi: 10.1111/j.1474-9726.2010.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willcox BJ, Donlon TA, He Q, et al. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anselmi CV, Malovini A, Roncarati R, et al. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12:95–104. doi: 10.1089/rej.2008.0827. [DOI] [PubMed] [Google Scholar]
- 51.Flachsbart F, Caliebe A, Kleindorp R, et al. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pawlikowska L, Hu D, Huntsman S, et al. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lunetta KL, D’Agostino RB, Sr, Karasik D, et al. Genetic correlates of longevity and selected age-related phenotypes: a genome-wide association study in the Framingham Study. BMC Med Genes. 2007;8(suppl 1):S13. doi: 10.1186/1471-2350-8-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flachsbart F, Franke A, Kleindorp R, et al. Investigation of genetic susceptibility factors for human longevity—a targeted nonsynonymous SNP study. Mutat Res. 2010;694:13–19. doi: 10.1016/j.mrfmmm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Majithia AR, Florez JC. Clinical translation of genetic predictors for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2009;16:100–106. doi: 10.1097/med.0b013e3283292354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrie JR, Pearson ER, Sutherland C. Implications of genome wide association studies for the understanding of type 2 diabetes pathophysiology. Biochem Pharmacol. 2011;81:471–477. doi: 10.1016/j.bcp.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Mooijaart SP, van Heemst D, Noordam R, et al. Polymorphisms associated with type 2 diabetes in familial longevity: the Leiden Longevity Study. Aging. 2011;3:55–62. doi: 10.18632/aging.100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng Y, Cheng L, Chen H, et al. Effects of FOXO genotypes on longevity: a biodemographic analysis. J Gerontol A Biol Sci Med Sci. 2010;65:1285–1299. doi: 10.1093/gerona/glq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.