Abstract
Introduction
Acid maltase deficiency (AMD) (Pompe disease) is an inherited myopathic disorder of glycogen degradation. Diagnosis is often delayed. Muscle ultrasound could improve diagnosis.
Methods
We compared skeletal muscle ultrasound images from adults with AMD (n=10) to other myopathies (n=81) and, in AMD, compared qualitative (Heckmatt) and quantitative (backscatter) ultrasound measurements with strength and function.
Results
Qualitative ultrasound was abnormal in at least one muscle in all AMD subjects. Ultrasound patterns specific for AMD compared to other myopathies were: normal triceps brachii despite abnormalities in elbow flexors (89 vs. 17%, p<0.0001), focal abnormalities affecting deep more than superficial biceps brachii (40 vs. 4%, p=0.002), and more severe involvement of vastus intermedius than rectus femoris (40 vs. 11%, p=0.03). In AMD, both qualitative (Heckmatt) and quantitative (backscatter) ultrasound measures increased with decreasing strength and function.
Discussion
Muscle ultrasound identifies the presence and specific patterns of AMD pathology, measures disease severity, and could help diagnose AMD.
Keywords: ultrasound, muscle, acid maltase disease, Pompe, backscatter
Introduction
Acid maltase deficiency (AMD) (Pompe disease) is an autosomal recessive disorder of lysosomal glycogen degradation caused by a deficiency of alpha-glucosidase (GAA) activity. AMD incidence is about 1 in 45,000 live births and varies between different ethnic populations 1,2. Infantile onset AMD is characterized by cardiac, skeletal, and smooth muscle involvement. Later-onset AMD manifests in the first to sixth decades primarily as progressive, patchy weakness and respiratory insufficiency. Patterns of weakness are heterogeneous and can mimic other neuromuscular disorders, resulting in a delay in diagnosis 3,4. Treatment can modify the course of AMD 5, making a timely diagnosis important. Diagnostic evaluation, initiated by the presence of myopathic weakness and/or respiratory insufficiency, includes electromyography, GAA enzyme activity assay, and muscle biopsy 2. Although muscle imaging using magnetic resonance imaging (MRI) and computed tomography (CT) can demonstrate pathology in patients with adult-onset AMD 6–8, the specificity of any patterns of muscle involvement has not been evaluated. Muscle imaging is therefore not routinely included in the diagnostic evaluation of patients with suspected AMD 2.
Ultrasound imaging of muscle pathology can be used as an adjunct to the physical exam in evaluating neuromuscular disorders and offers some advantages over other imaging modalities. It is inexpensive and can rapidly evaluate multiple muscles in both the arm and leg at the bedside. Both qualitative and quantitative assessments of the degree of muscular pathology can be measured using ultrasound 9–15. We have developed a reproducible, quantitative technique for measuring skeletal muscle pathology using calibrated ultrasound images to estimate the level of acoustic energy reflected by tissue back to the transducer (calibrated muscle backscatter, cMB) 16. The ultrasound appearance and pattern of muscle pathology in adult AMD is unknown. In this study we used qualitative ultrasound to describe the appearance and pattern of muscle pathology in adults with AMD as compared to other myopathies and examined the utility of qualitative and quantitative ultrasound to measure disease severity.
Methods
We evaluated 6 men and 4 women ages 43 to 72 years with clinical presentations, alpha-glucosidase enzyme levels, and genotypes consistent with late-onset acid-maltase deficiency. Subjects with AMD had symptoms for 5 to 21 years. At the time of evaluation, 9 subjects with AMD were taking alglucosidase alfa (Myozyme ©) for an average of 91 (range: 12–134) weeks as part of a concurrent trial. We also reviewed the muscle ultrasound images of 37 men and 44 women ages 18–78 years referred for muscle ultrasound for evaluation of known or possible myopathy. These non-AMD subjects all had an established diagnosis or clinical presentation, electrophysiologic evaluation and/or muscle biopsy that were not suggestive of AMD 2 (Table 1).
Table 1.
Ultrasound of Patients with Known or Suspected Myopathy
| Type of Myopathy
|
# of Patients with Abnormal Ultrasound/Total (%)
|
|---|---|
| Acid maltase disease | 10/10 (100) |
| Immune myopathy/Myositis | 19/23 (83) |
| Limb girdle/facioscapulohumeral muscular dystrophy | 17/18 (94) |
| Myalgia, cramps, idiopathic elevated creatine kinase | 4/9 (44) |
| Metabolic/Mitochondrial | 3/7 (42) |
| Inclusion body Myositis (hereditary and idiopathic) | 6/7 (85) |
| Congenital Muscular Dystrophy | 5/5 (100) |
| Becker Muscular Dystrophy | 2/2 (100) |
| Other Myopathy | 8/11 (72) |
Ultrasound was performed on one unilateral arm and leg in all subjects using an L12-5 Hz linear array ultrasound probe and either the Philips HD11xe or IU22 ultrasound machine. The side examined was randomly determined unless one side was more affected, in which case that side was selected. Machine settings for image acquisition were saved as presets and held constant for all images and without adjusting the focal point, gain or time-gain compensation settings. Rarely, the depth setting was increased to accommodate for larger limbs/muscles. Each subject was seated with the arm supported by a pillow on a table at mid-thoracic height and the knee bent at ninety degrees. Ultrasound images were obtained of the elbow flexors (biceps brachii and brachialis) and triceps brachii approximately 2/3 the distance from the acromion to the lateral epicondyle of the humerus, for the extensor carpi radialis, approximately 3 cm distal to the lateral epicondyle of the humerus, for the rectus femoris, approximately 2/3 the distance from the superior iliac spine to the superior edge of the patella, and for the tibialis anterior, approximately 1/4 the distance from the lateral epicondyle of the femur to the lateral malleolus of the tibia. All acid-maltase subjects had ultrasound images of the elbow flexors, triceps brachii, extensor carpi radialis, rectus femoris, and tibialis anterior. All non-AMD subjects had ultrasound images of the elbow flexors (n=81) and most had images of the triceps brachii (n=67) and rectus femoris (n=75).
All ultrasound images were obtained and scored retrospectively by a single examiner (CMZ) based on Heckmatt’s rating scale as follows: 1-normal, 2-mildy increased muscle echoes with normal bone reflection, 3- moderately increased muscle echoes with reduced bone reflection, 4-severely increased muscle echoes with absent bone reflection (Figure 1). Images that were judged to be in- between two ratings were assigned an intermediate value (ie: a rating of 2.5 was borderline between 2 and 3). A muscle ultrasound image was considered normal if the Heckmatt rating scale was scored less than 2 and abnormal if it was scored 2 or higher 17.
Figure 1. Qualitative ultrasound assessment of skeletal muscle pathology using Heckmatt’s rating scale.
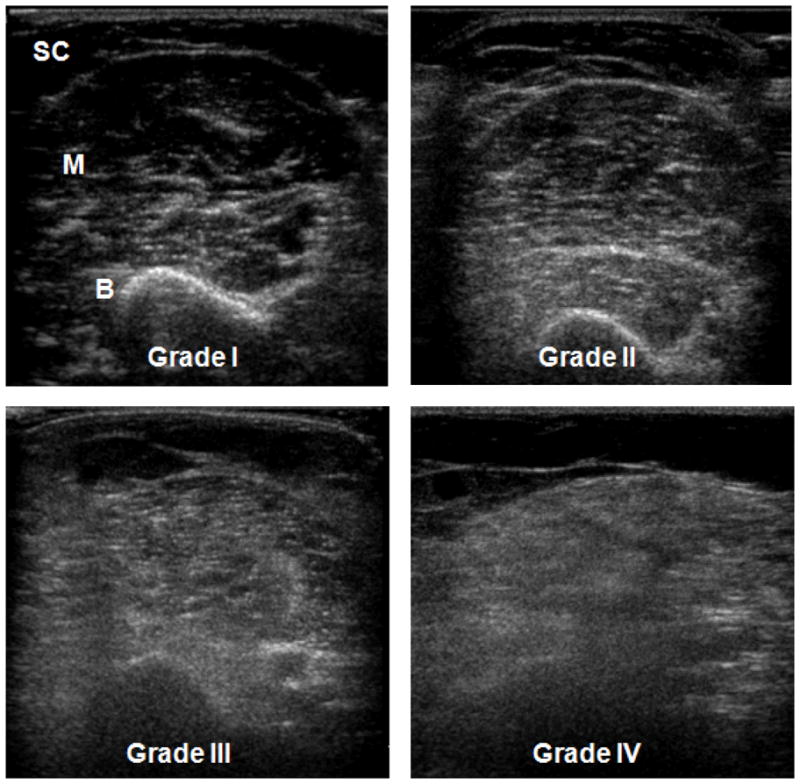
Normal (grade I) ultrasound appearance of the biceps brachii and brachialis shows predominantly dark muscle (M) bordered by subcutaneous fat (SC) and a bright, distinct bone reflection (B). Grade II: increased signal in the biceps brachii and brachialis with preserved bone reflection. Grade III: moderately increased signal and reduced bone reflection. Grade IV: markedly increased signal and absent bone reflection.
Quantitative ultrasound analysis was used to measure the amount of brightness of the ultrasound signal for comparison with strength and functional outcome measurements. Quantitative ultrasound image analysis was performed for 9 of 10 AMD subjects who had images obtained using the HD11xe imaging system and who underwent strength and functional analysis. An average quantitative (cMB) and qualitative (Heckmatt) muscle ultrasound score for each of these nine subjects was calculated by averaging the cMB or Heckmatt rating score of the elbow flexors, extensor carpi radialis, rectus femoris, and tibialis anterior.
The HD11XE ultrasound system was configured and calibrated for estimation of backscatter intensity (expressed in decibels, dB) and images of subjects with acid-maltase deficiency were analyzed and measured as described previously 16. Imaging parameters (gain, transmit focus, and time gain compensation settings) were uniform in all subjects. Eight-bit bitmap (BMP) images were exported and the mean grayscale values over the region-of-interest, defined as the entire depth of muscle between the subcutaneous tissue and humerus in the arm or the deep fascia (excluding the lateral margins of the imaged muscle), were determined using Philips™ quantitative software (QLAB™). Each BMP image was analyzed as exported from the ultrasound system without any adjustments or manipulations. All ultrasound measurements gave mean grayscale values in the linear range of the calibration curve for the ultrasound machine. Calibrated muscle backscatter values were calculated by dividing the average grayscale values by the slope of the best-fit line relating grayscale levels to backscatter (6.6 grayscale levels/dB) and then subtracting the backscatter of a reference phantom (13.7dB -obtained by imaging a CIRS Grey Scale Ultrasound Phantom, model 047, using the same imaging parameters as in human subjects). The calculation for cMB was (grayscale level/6.6) −13.7.
Disease severity, strength, degree of fatigue, and overall level of physical health was assessed in 9 of 10 subjects with acid-maltase deficiency as part of their participation in a concurrent clinical trial 5. These subjects completed the Rotterdam Handicap Scale 18, Short Form-36 Health Survey 19, and the Fatigue Severity Scale 20 an average of 73 days (range: 0–170 days) from the ultrasound evaluation. Strength was measured by specially trained neuromuscular physical therapists within one day of and on the same side as the ultrasound images using the modified MRC 10-point rating scale 21 and using quantitative muscle testing. An MRC score of 10 was considered normal. Quantitative muscle testing of strength was measured during maximal voluntary isometric contraction of elbow flexors and knee extensors and reported as a percent of the predicted normal score using the quantitative measurement system of the Cooperative International Neuromuscular Research Group 22. Dr. Zaidman was blinded to the results of the strength and disease severity assessments at the time of the ultrasound evaluation. Statistical tests include Fischer’s exact two-tailed probability (FET) and Spearman’s rank coefficient (rs). All variables were treated as independent samples for comparison. Written informed consent was obtained on all research subjects.
Results
The triceps brachii is relatively spared in AMD
Ultrasound showed at least one abnormal muscle (Heckmatt rating of 2 or higher) in all AMD subjects. Both proximal and distal muscles were often abnormal. Ultrasound was abnormal in 9 of 10 (90%) elbow flexor muscles, 7 of 10 (70%) rectus femoris muscles, 6 of 10 (60%) tibialis anterior muscles, and 7 of 10 (70%) extensor carpi radialis muscles. The triceps brachii muscle was usually relatively spared compared to other muscles, especially elbow flexors, and was abnormal in only 1 of 10 (10%) AMD subjects (p = 0.001 vs. elbow flexors) (Figure 2). Strength was also spared in elbow extensors compared to elbow flexors. The MRC rating was normal in 8 of 9 (89%) elbow-extensors (triceps brachii) but in only 3 of 9 (33%) elbow flexors of AMD subjects (p = 0.05). Pathologic evidence of sparing of the triceps brachii compared to the vastus lateralis was found in one subject with acid-maltase deficiency who had biopsies of both muscles performed six months before the ultrasound.
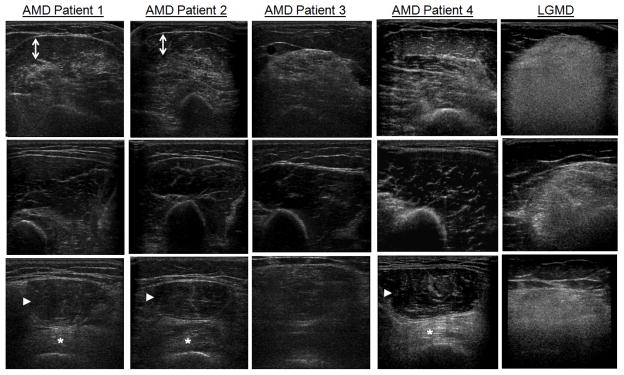
Figure 2. Patterns of Muscle Pathology on Ultrasound in Acid Maltase Disease.
Ultrasound of the biceps brachii and brachialis (Row 1), triceps brachii (Row 2) and rectus femoris and vastus intermedius (Row 3) is shown for four patients with acid-maltase disease (AMD) aged 45–63 years and a 49 year-old woman with limb-girdle muscular dystrophy (LGMD). In AMD patients, there is sparing of the triceps brachii compared to the biceps brachii (patients 1–4), sparing of the superficial portion of the biceps brachii (arrows, patients 1 and 2), and sparing of the rectus femoris (arrowhead) compared to the vastus intermedius (*, patients 1, 2, and 4). In contrast, in LGMD there is more diffuse, homogenous involvement of the proximal arm and leg muscles.
Patterns of muscle involvement on ultrasound have specificity for AMD
In patients with an abnormal ultrasound of the elbow flexors, a normal ultrasound of the triceps brachii was more common (p<0.0001, FET) in AMD (89%; 8 of 9) than in non-AMD myopathy patients (17%; 8 of 48). Sparing of the superficial biceps brachii with abnormal deep biceps brachii and brachialis (on both transverse and longitudinal images) was more common (p=0.002, FET; Figure 2) in AMD (40%; 4 of 10) than in other myopathies (4%; 3 of 81). Relative sparing of the rectus femoris (grade I–II) compared to more severe involvement of the vastus intermedius was more common (p=0.03, FET, Figure 2) in AMD (40%; 4 of 10) than in other myopathies (11%; 8 of 75). A combination of two of these ultrasound patterns (a normal triceps brachii but abnormal elbow flexors, sparing of the superficial biceps brachii, or relative sparing of the rectus femoris compared to the vastus intermedius) was seen in 60% (6 of 10) of patients with AMD but only 3% (2 of 67) of patients with other myopathies (p<0.0001, FET): one with inclusion body myositis and one with limb-girdle muscular dystrophy of unknown type.
In AMD, cMB and Heckmatt ratings are higher in patients with worse elbow flexion strength and overall function
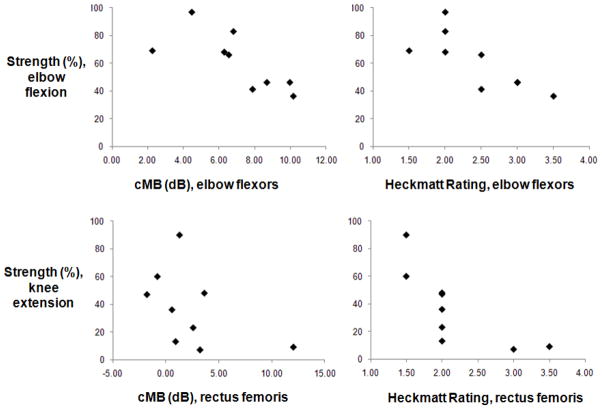
Quantitative elbow flexion strength decreased with both higher Heckmatt ratings (rs=−0.8, p=0.004) and higher cMB levels (rs=−0.8, p=0.008) of the elbow flexors (Figure 3). Quantitative knee extension strength decreased with higher Heckmatt ratings (rs=−0.9, p=0.001) but not cMB levels (rs =−0.4, p=0.2) of the rectus femoris. Compared to the elbow flexors, ultrasound measurements of the rectus femoris were relatively insensitive to changes in strength and in some patients did not vary despite large differences in knee extensor strength (Figure 3). Both average Heckmatt ratings (rs= −0.7, p=0.056) and average cMB levels (rs= −0.7, p=0.045) were higher in AMD patients with worse function on the Rotterdam Handicap Scale. Neither average cMB nor average Heckmatt ratings varied with overall physical health or level of fatigue, measured by the Short Form-36 Health Survey Physical Health Sum score and Fatigue Severity Scale, or with age, disease duration, or duration of treatment with alglucosidase alfa (all p>0.1). Average cMB levels and Heckmatt ratings were strongly correlated with each other (rs= 0.9, p=0.001).
Figure 3. Ultrasound in adults with AMD: Relations to Strength.
Elbow flexion strength (top, expressed as a percent of normal) decreased with both higher quantitative (cMB, rs=−0.8, p=0.008) and qualitative (Heckmatt rating, rs=−0.8, p=0.004) ultrasound measures of muscle pathology in the elbow flexors. Knee extension strength decreased with higher Heckmatt ratings (rs=−0.9, p=0.001) but not cMB levels (rs =−0.4, p=0.2) of the rectus femoris. Compared to the elbow flexors, ultrasound measurements of the rectus femoris were relatively insensitive to changes in strength. For example, five subjects had a Heckmatt rating of two despite differences in knee extension strength from 48 to 13% of normal.
Discussion
Diagnostic neuromuscular ultrasound commonly detects muscle pathology in symptomatic adults with AMD. Muscle ultrasound was qualitatively abnormal in at least one muscle in all subjects with AMD with pathology in both proximal and distal arm and leg muscles in most (70%). The degree of pathology in AMD is well known to vary between and even within muscles 23. An ultrasound pattern of normal elbow extensors (triceps brachii) but abnormal elbow flexors was common in AMD patients (89%) but not in other myopathies (17%). Strength testing showed a similar pattern of involvement. The differential diagnosis of weakness of elbow flexion out of proportion to elbow extension includes AMD, facioscapulohumeral dystrophy, limb-girdle muscular dystrophy 2B and 2L, and motor neuron disease 24. Additional radiologic and strength studies comparing elbow flexors to elbow extensors could identify other disorders with similar patterns.
In AMD, there is also heterogeneous pathology within muscle groups that is prominent on ultrasound images. There was relative sparing of the superficial compared to the deep portion of the elbow flexors or of the rectus femoris compared to the vastus intermedius in 40% of AMD patients. These patchy or focal ultrasound abnormalities within muscle groups are more common in patients with AMD than in other myopathies. Similar to our findings using ultrasound, MRI of anterior thigh muscles in AMD showed sparing of the rectus femoris and the superficial portion of the vastus lateralis 6. A pattern of medial and posterior thigh muscle pathology that can suggest AMD, early involvement of the leg adductors and hamstring muscles with sparing of the short head of the biceps femoris, has been detected using computer tomography 8. The appearance or pattern of muscle pathology may vary with differences in system settings, imaging modality, or disease severity. Experience with an imaging system is therefore required to distinguish neuromuscular pathology from healthy skeletal muscle. This study of ultrasound identifies patterns of muscle pathology specific for adults with late-onset AMD who were symptomatic for at least five years as compared to adults with other myopathies. Additional muscle imaging studies are needed in infant and child onset AMD and in early symptomatic adult AMD patients to determine if similar patterns of muscle pathology are present.
Quantitative and qualitative ultrasound measures can evaluate the presence, and measure the degree, of muscle pathology in myopathies 14–17. In this study both quantitative (cMB) and qualitative (Heckmatt) ultrasound measures increased with decreasing strength and function. Ultrasound measurements of the elbow flexors showed a strong relationship with strength. The variable association of ultrasound of the rectus femoris with motor function is likely due to relative sparing of the rectus femoris compared to other muscles in the quadriceps group that affect the strength of knee extension.
Both quantitative (cMB) and qualitative (Heckmatt) ultrasound measures can be used to measure the degree of pathlogy in AMD. Qualitative ratings can be assessed in different ultrasound systems without specific or fixed settings but are subjective and vary depending on examiner expertise 12. Quantitative ultrasound measurements such as cMB are more reliably measured than qualitative ratings but require a specific configuration and calibration of the ultrasound system if measurements are to be reproduced between systems 12,16. Our findings of increasing ultrasound signal with decreasing strength in AMD need to be confirmed in additional studies, as measurements of strength, function, or ultrasound images may vary between different machines, settings, or examiners.
The type of muscle pathology responsible for the abnormal ultrasound signal intensity remains to be determined. Abnormal signal on ultrasound 9,10,25, MRI 5 and CT 8 increases with greater amounts of fat or fibrous tissue. Other pathologies, including inflammation or muscle fiber atrophy from denervation, could also produce abnormal signal 26. There was no relationship between cMB or Heckmatt ratings with age or disease duration, similar to a prior study using MRI 6, or with measures of fatigue or physical health, which are both affected in AMD 19,20. This suggests that in AMD, ultrasound of skeletal muscle could specifically measure pathology affecting muscle strength and function but not patients’ overall health or stamina.
In conclusion, ultrasound of skeletal muscle is commonly abnormal in symptomatic adults with AMD and identifies patterns of muscle involvement that can suggest AMD. AMD myopathy patterns include normal elbow extensors despite abnormalities in the elbow flexors, focal abnormalities affecting the deep more than superficial biceps brachii, and more severe involvement of the vastus intermedius than rectus femoris. Ultrasound signal increases with decreasing strength and function in AMD. As ultrasound can be used to screen large areas of multiple muscles quickly and at the bedside, adding ultrasound to the routine evaluation of adults with neuromuscular disorders could improve the likelihood of identifying patients with AMD.
Acknowledgments
The study was supported by the Washington University Neuromuscular Research Fund and the National Institute of Health Neurological Sciences Academic Development Award Grant Number K12 NS00169009.
References
- 1.Martiniuk F, Chen A, Mack A, Arvanitopoulos E, Chen Y, Rom WN, Codd WJ, Hanna B, Alcabes P, Raben N, Plotz P. Carrier frequency for glycogen storage disease type II in New York and estimates of affected individuals born with the disease. Am J Med Genet. 1998;79:69–72. doi: 10.1002/(sici)1096-8628(19980827)79:1<69::aid-ajmg16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Diagnostic criteria for late-onset (childhood and adult) Pompe disease. Muscle Nerve. 2009;40:149–160. doi: 10.1002/mus.21393. [DOI] [PubMed] [Google Scholar]
- 3.Hagemans ML, Winkel LP, Van Doorn PA, Hop WJ, Loonen MC, Reuser AJ, Van der Ploeg AT. Clinical manifestation and natural course of late-onset Pompe’s disease in 54 Dutch patients. Brain. 2005;128:671–677. doi: 10.1093/brain/awh384. [DOI] [PubMed] [Google Scholar]
- 4.Winkel LP, Hagemans ML, van Doorn PA, Loonen MC, Hop WJ, Reuser AJ, van der Ploeg AT. The natural course of non-classic Pompe’s disease; a review of 225 published cases. J Neurol. 2005;252:875–884. doi: 10.1007/s00415-005-0922-9. [DOI] [PubMed] [Google Scholar]
- 5.van der Ploeg AT, Clemens PR, Corzo D, Escolar DM, Florence J, Groeneveld GJ, Herson S, Kishnani PS, Laforet P, Lake SL, Lange DJ, Leshner RT, Mayhew JE, Morgan C, Nozaki K, Park DJ, Pestronk A, Rosenbloom B, Skrinar A, van Capelle CI, van der Beek NA, Wasserstein M, Zivkovic SA. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 6.Pichiecchio A, Uggetti C, Ravaglia S, Egitto MG, Rossi M, Sandrini G, Danesino C. Muscle MRI in adult-onset acid maltase deficiency. Neuromuscul Disord. 2004;14:51–55. doi: 10.1016/j.nmd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Dlamini N, Jan W, Norwood F, Sheehan J, Spahr R, Al-Sarraj S, Anthony Hulse J, Hughes D, Champion MP, Jungbluth H. Muscle MRI findings in siblings with juvenile-onset acid maltase deficiency (Pompe disease) Neuromuscul Disord. 2008;18:408–409. doi: 10.1016/j.nmd.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 8.de Jager AE, van der Vliet TM, van der Ree TC, Oosterink BJ, Loonen MC. Muscle computed tomography in adult-onset acid maltase deficiency. Muscle Nerve. 1998;21:398–400. doi: 10.1002/(sici)1097-4598(199803)21:3<398::aid-mus15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.Heckmatt JZ, Leeman S, Dubowitz V. Ultrasound imaging in the diagnosis of muscle disease. J Pediatr. 1982;101:656–660. doi: 10.1016/s0022-3476(82)80286-2. [DOI] [PubMed] [Google Scholar]
- 10.Heckmatt J, Rodillo E, Doherty M, Willson K, Leeman S. Quantitative sonography of muscle. J Child Neurol. 1989;4 (Suppl):S101–106. doi: 10.1177/0883073889004001s15. [DOI] [PubMed] [Google Scholar]
- 11.Pillen S, Verrips A, van Alfen N, Arts IM, Sie LT, Zwarts MJ. Quantitative skeletal muscle ultrasound: diagnostic value in childhood neuromuscular disease. Neuromuscul Disord. 2007;17:509–516. doi: 10.1016/j.nmd.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Pillen S, van Keimpema M, Nievelstein RA, Verrips A, van Kruijsbergen-Raijmann W, Zwarts MJ. Skeletal muscle ultrasonography: Visual versus quantitative evaluation. Ultrasound Med Biol. 2006;32:1315–1321. doi: 10.1016/j.ultrasmedbio.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Maurits NM, Bollen AE, Windhausen A, De Jager AE, Van Der Hoeven JH. Muscle ultrasound analysis: normal values and differentiation between myopathies and neuropathies. Ultrasound Med Biol. 2003;29:215–225. doi: 10.1016/s0301-5629(02)00758-5. [DOI] [PubMed] [Google Scholar]
- 14.Zaidman CM, Connolly AM, Malkus EC, Florence JM, Pestronk A. Quantitative ultrasound using backscatter analysis in Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2010 doi: 10.1016/j.nmd.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brockmann K, Becker P, Schreiber G, Neubert K, Brunner E, Bonnemann C. Sensitivity and specificity of qualitative muscle ultrasound in assessment of suspected neuromuscular disease in childhood. Neuromuscul Disord. 2007;17:517–523. doi: 10.1016/j.nmd.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Zaidman CM, Holland MR, Anderson CC, Pestronk A. Calibrated quantitative ultrasound imaging of skeletal muscle using backscatter analysis. Muscle Nerve. 2008;38:893–898. doi: 10.1002/mus.21052. [DOI] [PubMed] [Google Scholar]
- 17.Zuberi SM, Matta N, Nawaz S, Stephenson JB, McWilliam RC, Hollman A. Muscle ultrasound in the assessment of suspected neuromuscular disease in childhood. Neuromuscul Disord. 1999;9:203–207. doi: 10.1016/s0960-8966(99)00002-4. [DOI] [PubMed] [Google Scholar]
- 18.Hagemans ML, Laforet P, Hop WJ, Merkies IS, Van Doorn PA, Reuser AJ, Van der Ploeg AT. Impact of late-onset Pompe disease on participation in daily life activities: evaluation of the Rotterdam Handicap Scale. Neuromuscul Disord. 2007;17:537–543. doi: 10.1016/j.nmd.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Hagemans ML, Janssens AC, Winkel LP, Sieradzan KA, Reuser AJ, Van Doorn PA, Van der Ploeg AT. Late-onset Pompe disease primarily affects quality of life in physical health domains. Neurology. 2004;63:1688–1692. doi: 10.1212/01.wnl.0000142597.69707.78. [DOI] [PubMed] [Google Scholar]
- 20.Hagemans ML, van Schie SP, Janssens AC, van Doorn PA, Reuser AJ, van der Ploeg AT. Fatigue: an important feature of late-onset Pompe disease. J Neurol. 2007;254:941–945. doi: 10.1007/s00415-006-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Florence JM, Pandya S, King WM, Robison JD, Baty J, Miller JP, Schierbecker J, Signore LC. Intrarater reliability of manual muscle test (Medical Research Council scale) grades in Duchenne’s muscular dystrophy. Phys Ther. 1992;72:115–122. doi: 10.1093/ptj/72.2.115. discussion 122–116. [DOI] [PubMed] [Google Scholar]
- 22.Muscular weakness assessment: use of normal isometric strength data. The National Isometric Muscle Strength (NIMS) Database Consortium. Arch Phys Med Rehabil. 1996;77:1251–1255. doi: 10.1016/s0003-9993(96)90188-4. [DOI] [PubMed] [Google Scholar]
- 23.Engel AG, Gomez MR, Seybold ME, Lambert EH. The spectrum and diagnosis of acid maltase deficiency. Neurology. 1973;23:95–106. doi: 10.1212/wnl.23.1.95. [DOI] [PubMed] [Google Scholar]
- 24.Pestronk A. Myopathy & Neuromuscular Junction Disorders: Differential Diagnosis. Neuromuscular Disease Center; [(accessed November 2010)]. http://neuromuscularwustledu/musdist/proxarmhtml#efweak. [Google Scholar]
- 25.Pillen S, Tak RO, Zwarts MJ, Lammens MM, Verrijp KN, Arts IM, van der Laak JA, Hoogerbrugge PM, van Engelen BG, Verrips A. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol. 2009;35:443–446. doi: 10.1016/j.ultrasmedbio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle Nerve. 2008;37:679–693. doi: 10.1002/mus.21015. [DOI] [PubMed] [Google Scholar]