Abstract
Practical radical cyclizations using organoboronic acids and trifluoroborates take place in water, open to air, and in a scalable fashion employing catalytic silver nitrate and stoichiometric potassium persulfate. Both Pschorr-type cyclizations and tandem radical cyclization/trap cascades are described, illustrating the utility of these mild conditions for the generation of polycyclic scaffolds.
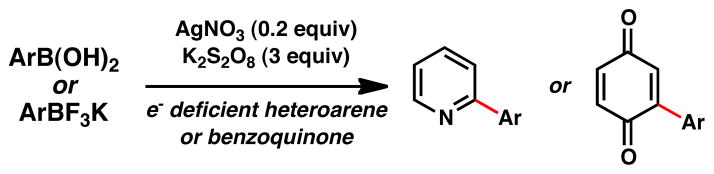
Radical cyclizations have proven to be a valuable tactic widely employed in organic synthesis.1 Often, however, the appeal they exhibit in generating molecular complexity is attenuated by the drawback of using toxic tin species and inert (oxygen-free) reaction conditions. In the case of aryl-centered radicals, diazonium salts serve as one means for entry into radical processes, although their preparation and handling offsets their synthetic value. Among the recent developments2 aimed at circumventing such drawbacks, the recently discovered Minisci-type reactivity of organoboronic acids3 and trifluoroborates3b,4 (Figure 1) addresses many of these. Indeed, the conditions involve the use of ubiquitous boronic acids, cheap inorganic salts (silver nitrate and potassium persulfate), can be performed in an open-flask without recourse to high temperatures, and can be safely conducted on gram-scale. In this letter, the chemistry of aryl radicals derived from boronic acids and trifluoroborates is explored in an intramolecular setting.
Figure 1.
Previously Reported Intermolecular C–H Functionalization of Heteroarenes3a and Benzoquinones3b
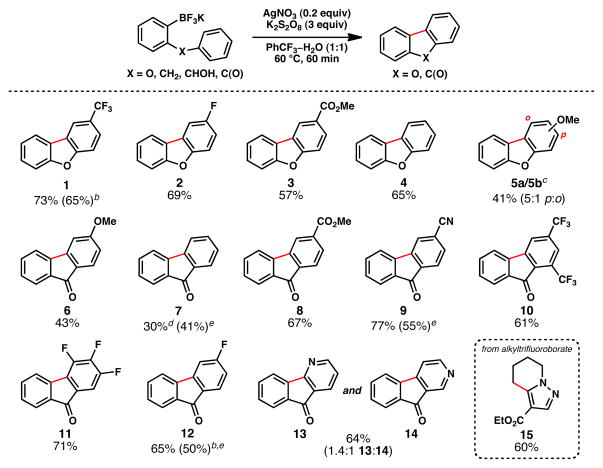
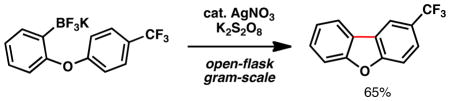
The preparation of tricyclic scaffolds such as dibenzofurans and fluorenones has received significant attention, owing to their presence in natural products and compounds of medicinal interest.5 One of the earliest means for access to such molecules involves the radical-based method known as the Pschorr cyclization,6 which dates back to 1896. This powerful transformation relies upon an arenediazonium salt as a radical precursor, and typically employs superstoichiometric iron or copper salts for radical generation. Figure 2 illustrates the invention and scope of a “borono-Pschorr” reaction that obviates the need for potentially dangerous arenediazonium salt preparation. Additionally, this method complements Pd-mediated processes recently described by Harvey,7a Fagnou,7b and Glorius.7c A variety of functional groups are tolerated, such as nitriles, esters, Lewis-basic heteroatoms, and products are obtained in synthetically useful yields. Concomitant benzylic oxidation is observed, and this can be exploited to increase step-economy. For instance, fluorenone (7) was obtained from a diarylmethane precursor under the standard conditions.
Figure 2.
Pschorr-Type Cyclization using Organotrifluoroborates as Radical Precursorsa
aAryltrifluoroborate (0.1 mmol), AgNO3 (0.02 mmol), K2S2O8 (0.3 mmol), PhCF3–H2O (1:1 v/v, 1.0 mL), 60 °C, 60 min; yields for chromatographically and spectroscopically pure products. bYield of reaction performed on gram-scale. cInseparable mixture. dYield from X = CH2. eYield from X = CHOH.
The boronic acid is installed onto the substrate through lithium-halogen exchange followed by borate trap,8 or by transition metal-mediated borylation.9 For a substrate bearing a pinacol boronate ester, conversion to the trifluoroborate salt is accomplished by treatment with aqueous KHF2 followed by repeated evaporation with MeOH–H2O (1:1 v/v) to remove pinacol.10
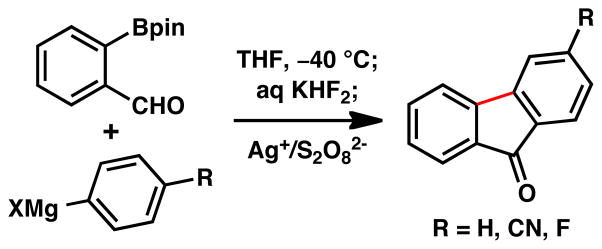
Given the durability of pinacol boronate esters under a variety of conditions, we secured (as proof-of-principle) three examples of fluorenone synthesis utilizing a bifunctional reagent, 2-formylphenylboronic acid pinacol ester (Figure 3). Direct addition of an aryl Grignard, followed by treatment with aqueous KHF2, provided radical cyclization precursors which, upon exposure to Ag+/S2O82−, furnished the corresponding fluorenones. Such a strategy can prove useful in situations where a masked radical precursor is carried through subsequent steps and then unmasked for the radical cyclization step.
Figure 3.
Three-Step Sequence to Fluorenones
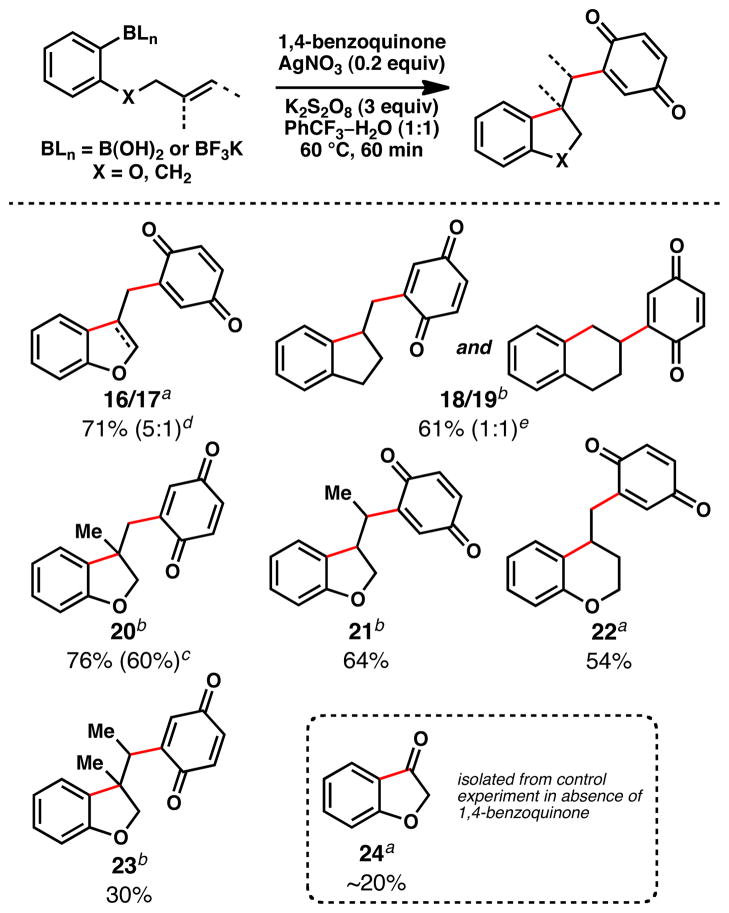
Vicinal olefin difunctionalization, in which multiple carbon–carbon bonds are forged in concert, is a valuable tactic for complexity generation in synthesis.11 Such a process is exemplified by the results in Figure 4. In this tandem radical cyclization/benzoquinone trap protocol, 5-exo, 6-exo, and 6-endo radical cyclizations are combined with an intermolecular radical capture.12 The trend in isolated yields (for products 16–23) suggests that increasing degrees of olefin substitution attenuate the efficiency of the tandem process. Importantly, this protocol stands in contradistinction to the more classic tributyltin hydride-based radical cyclization and the palladium-catalyzed Heck-type process, both of which were found to be incompatible with the use of benzoquinone in this context.13
Figure 4.
Tandem Radical Cyclization/Trap using 1,4-Benzoquinone as the Terminating Radicophile
aPotassium aryltrifluoroborate or barylboronic acid was employed; isolated yields of chromatographically and spectroscopically pure products displayed, unless otherwise noted. cYield of reaction performed on gram-scale. dMajor product is dihydrobenzofuran. eInseparable mixture.
Incidentally, we have isolated benzofuranone (24) in a control experiment (in the absence of 1,4-benzoquinone). In lieu of a competent radical trapping agent, oxygen from the air intercepts the radical intermediate. This, accompanied by benzylic C–H oxidation of the substrate, leads to oxidative carbon–carbon bond cleavage.14,15
While the results described in this letter demonstrate a capacity for performing open-flask radical cyclizations, it is important to note its limitations. Although high chemoselectivity has been observed under these biphasic conditions,3 persulfate (S2O82−) is a strong oxidizing agent and therefore motifs such as benzylic C–H bonds and vicinal diols may not be compatible. Radical ring closure and intermolecular radicophile capture must outpace the capture of oxygen (O2) or H-abstraction (either from an intramolecular donor or from solvent). Such considerations must be taken into account when planning a reaction using the Ag+/S2O82− system.16
The mechanism by which an organoboronic acid gives rise to a reactive radical species upon exposure to Ag+/S2O82− will be the subject of future work. The working hypothesis involves 1) Ag+-mediated decomposition of S2O82− to give SO4•−, 2) attack at boron by SO4•−, and 3) carbon–boron bond homolysis.17
We emphasize that this open-air18 radical chemistry does not involve high temperatures and avoids the use of toxic and/or expensive metals. Additionally, this method circumvents recourse to potentially hazardous entities such as arenediazonium salts,19 and can be safely conducted on gram-scale.20,21 With the emergence of non-cryogenic22 and C–H borylation23 protocols to complement classic methods for preparing organoboron species, we anticipate increased use of boronic acids and trifluoroborates as radical precursors in synthesis.
Supplementary Material
Acknowledgments
We thank Dr. D.-H. Huang (TSRI) and Dr. L. Pasternack (TSRI) for NMR spectroscopic assistance, Dr. G. Siuzdak (TSRI) for assistance with mass spectrometry, and Paul T. Hernandez (TSRI) for valuable technical assistance. Financial support for this work was provided by Amgen, Bristol-Myers Squibb, Leo Pharma, University of Copenhagen Faculty of Pharmaceutical Sciences, Glutarget, and the NIH/NIGMS (GM-073949).
Footnotes
Supporting Information Available Experimental procedures, copies of all spectral data, and full characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Giese B. Radicals in Organic Synthesis: Formation of Carbon-Carbon Bonds. Pergamon Press; New York: 1986. [Google Scholar]; (b) Abeywickrema AN, Beckwith ALJ. Tetrahedron Lett. 1986;27:109–112. [Google Scholar]; (c) Barton DHR, da Silva E, Zard SZ. J Chem Soc, Chem Commun. 1988:285–287. [Google Scholar]; (d) Jasperse CP, Curran DP, Fevig TL. Chem Rev. 1991;91:1237–1286. [Google Scholar]; (e) Thebtaranonth C, Thebtaranonth Y. Cyclization Reactions. CRC Press; Boca Raton: 1994. pp. 77–167. [Google Scholar]; (f) Renaud P, Sibi MP, editors. Radicals in Organic Synthesis. Wiley-VCH; Weinheim: 2001. [Google Scholar]; (g) Gansauer A, editor. Topics in Current Chemistry: Radicals in Synthesis I. Vol. 263 Springer; Berlin: 2006. [Google Scholar]; (h) Gansauer A, editor. Topics in Current Chemistry: Radicals in Synthesis II. Vol. 264 Springer; Berlin: 2006. [Google Scholar]
- 2.(a) Devin P, Fensterbank L, Malacria M. Tetrahedron Lett. 1999;40:5511–5514. [Google Scholar]; (b) Heinrich MR, Kirchstein MD. Tetrahedron Lett. 2000;47:2115–2118. [Google Scholar]; (c) Kita Y, Nambu H, Ramesh NG, Anilkumar G, Matsugi M. Org Lett. 2001;3:1157–1160. doi: 10.1021/ol010014p. [DOI] [PubMed] [Google Scholar]; (d) Gagosz F, Zard SZ. Org Lett. 2002;4:4345–4348. doi: 10.1021/ol0270024. [DOI] [PubMed] [Google Scholar]; (e) Khan TA, Tripoli R, Crawford JJ, Martin CG, Murphy JA. Org Lett. 2003;5:2971–2974. doi: 10.1021/ol035173i. [DOI] [PubMed] [Google Scholar]; (f) Bowman WR, Storey JMD. Chem Soc Rev. 2007;36:1803–1822. doi: 10.1039/b605183a. [DOI] [PubMed] [Google Scholar]; (g) Biechy A, Zard SZ. Org Lett. 2009;11:2800–2803. doi: 10.1021/ol900996k. [DOI] [PubMed] [Google Scholar]; (h) Wang B, Ramirez AP, Slade JJ, Morken JP. J Am Chem Soc. 2010;132:16380–16382. doi: 10.1021/ja108185z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Sorin G, Martinez Mallorquin R, Contie Y, Baralle A, Malacria M, Goddard J-P, Fensterbank L. Angew Chem Int Ed. 2010;49:8721–8723. doi: 10.1002/anie.201004513. [DOI] [PubMed] [Google Scholar]; (j) Braun MG, Zard SZ. Org Lett. 2011;13:1230–1233. doi: 10.1021/ol2001182. [DOI] [PubMed] [Google Scholar]
- 3.(a) Seiple IB, Su S, Rodriguez RA, Gianatassio R, Fujiwara Y, Sobel AL, Baran PS. J Am Chem Soc. 2010;132:13194–13196. doi: 10.1021/ja1066459. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fujiwara Y, Domingo V, Seiple IB, Gianatassio R, Del Bel M, Baran PS. J Am Chem Soc. 2011;133:3292–3295. doi: 10.1021/ja111152z. [DOI] [PMC free article] [PubMed] [Google Scholar]; For the direct arylation of caffeine, dihydroquinine, and ChantixTM, see: Ji Y, Brueckl T, Baxter RD, Fujiwara Y, Seiple IB, Su S, Blackmond DG, Baran PS. Proc Natl Acad Sci USA. 2011;108:14411–14415. doi: 10.1073/pnas.1109059108.
- 4.Molander GA, Colombel V, Braz VA. Org Lett. 2011;13:1852–1855. doi: 10.1021/ol2003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Wang W, Snieckus V. J Org Chem. 1992;57:424–426. [Google Scholar]; (b) Cone MC, Melville CR, Gore MP, Gould SJ. J Org Chem. Vol. 58. 1993. pp. 1058–1061. [Google Scholar]; (c) Jones WD, Jr, Ciske FL. J Org Chem. 1996;61:3920–3922. doi: 10.1021/jo9601369. [DOI] [PubMed] [Google Scholar]; (d) Kaniwa K, Ohtsuki T, Yamamoto Y, Ishibashi M. Tetrahedron Lett. 2006;47:1505–1508. [Google Scholar]; (e) Yu M, Danishefsky SJ. J Am Chem Soc. 2008;130:2783–2785. doi: 10.1021/ja7113757. [DOI] [PubMed] [Google Scholar]; (f) Ye YQ, Koshino H, Onose J, Yoshikawa K, Abe N, Takahashi S. Org Lett. 2009;11:5074–5077. doi: 10.1021/ol9020833. [DOI] [PubMed] [Google Scholar]; (g) Kemnitzer W, Sirisoma N, Jiang S, Kasibhatla S, Crogan-Grundy C, Tseng B, Drewe J, Cai SX. Bioorg Med Chem Lett. 2010;20:1288–1292. doi: 10.1016/j.bmcl.2009.11.025. [DOI] [PubMed] [Google Scholar]; (h) Teng H, Thakur GA, Makriyannis A. Bioorg Med Chem Lett. 2011;21:5999–6002. doi: 10.1016/j.bmcl.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Pschorr R. Ber. 1896;29:496–501. [Google Scholar]; (b) Leake PH. Chem Rev. 1956;56:27–48. [Google Scholar]; (c) Cano-Yelo H, Deronzier A. J Chem Soc, Perkin Trans 2. 1984:1093–1098. [Google Scholar]; (d) Wassmundt FW, Kiesman WF. J Org Chem. 1995;60:196–201. doi: 10.1021/jo962128y. [DOI] [PubMed] [Google Scholar]; (e) Yasuda N, Huffman MA, Ho GJ, Xavier LC, Yang C, Emerson KM, Tsay FR, Li Y, Kress MH, Rieger DL, Karady S, Sohar P, Abramson NL, DeCamp AE, Mathre DJ, Douglas AW, Dolling UH, Grabowski EJJ, Reider PJ. J Org Chem. 1998;63:5438–5346. [Google Scholar]; (f) Moorthy JN, Samanta S. J Org Chem. 2007;72:9786–9789. doi: 10.1021/jo7017872. [DOI] [PubMed] [Google Scholar]
- 7.ArOTf, cat. PdCl2(Ph3P)2, 145 °C: Wang J-Q, Harvey RG. Tetrahedron. 2002;58:5927–5931.ArH, cat. Pd(OAc)2, PivOH, 120 °C: Liegault B, Lee D, Huestis MP, Stuart DR, Fagnou K. J Org Chem. 2008;73:5022–5028. doi: 10.1021/jo800596m.ArCO2H, cat. Pd(TFA)2, Ag2CO3, 150 °C: Wang C, Piel I, Glorius F. J Am Chem Soc. 2009;131:4194–4195. doi: 10.1021/ja8100598.
- 8.Li W, Nelson DP, Jensen MS, Hoerrner RS, Cai D, Larsen RD, Reider PJ. J Org Chem. 2002;67:5394–5397. doi: 10.1021/jo025792p. [DOI] [PubMed] [Google Scholar]
- 9.Billingsley KL, Buchwald SL. J Org Chem. 2008;73:5589–5591. doi: 10.1021/jo800727s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Darses S, Michaud G, Genet JP. Eur J Org Chem. 1999:1875–1883. [Google Scholar]; (b) Bagutski V, Ros A, Aggarwal VK. Tetrahedron. 2009;65:9956–9960. [Google Scholar]
- 11.Ho T-L. Tandem Organic Reactions. Wiley-Interscience; New York: 1992. pp. 398–415. [Google Scholar]
- 12.Other examples of quinones in radical capture: Demchuk OM, Pietrusiewicz KM. Synlett. 2009;7:1149–1153.Luthy M, Darmency V, Renaud P. Eur J Org Chem. 2011:547–552.
- 13.A standard Bu3SnH reaction was performed using 1-(allyloxy)-2-bromobenzene in the presence of 1,4-benzoquinone. Analysis by TLC and 1H NMR showed only recovered bromide, and no cyclization product. Similar results were obtained upon performing a Pd-catalyzed Heck cyclization reaction using 1-(allyloxy)-2-bromobenzene in the presence of 1,4-benzoquinone.
- 14.Benzofuranone was not observed in reactions containing 1,4-benzoquinone.
- 15.It is known that persulfate can cleave vicinal diols: Kumar A. J Am Chem Soc. 1981;103:5179–5182.
- 16.(a) Snyder HR, Kuck JA, Johnson JR. J Am Chem Soc. 1938;60:105–111. [Google Scholar]; (b) Lewin AH, Cohen T. J Org Chem. 1967;32:3844–3850. [Google Scholar]; (c) Minisci F, Citterio A, Giordano C. Acc Chem Res. 1983;16:27–32. [Google Scholar]; (d) Karady S, Cummins JM, Dannenberg JJ, del Rio E, Dormer PG, Marcune BF, Reamer RA, Sordo TL. Org Lett. 2003;5:1175–1178. doi: 10.1021/ol027301t. [DOI] [PubMed] [Google Scholar]
- 17.A similar proposal has previously been put forth in which phenylcarbonyloxy radical (generated in situ) attack at boron leads to carbon-boron bond homolysis: Cadot C, Cossy J, Dalko PI. Chem Commun. 2000:1017–1018.
- 18.It was found that conducting these Ag+/S2O82− reactions under nitrogen atmosphere did not result in an appreciable difference in yield.
- 19.Arenediazonium salts are also utilized in Meerwein arylation of electron-deficient olefins: Heinrich MR, Wetzel A, Kirchstein M. Org Lett. 2007;9:3833–3835. doi: 10.1021/ol701622d.We have performed a vicinal difunctionalization of acrylonitrile employing 4-methoxyphenylboronic acid and TEMPO, obtaining the oxyarylation product in 50% yield. See Supporting Information, and see Dickschat A, Studer A. Org Lett. 2010;12:3972–3974. doi: 10.1021/ol101818k.
- 20.Representative Experimental Procedure for Pschorr-type cyclization: To a solution of aryltrifluoroborate (0.1 mmol, 1.0 equiv) in trifluorotoluene (0.5 mL) and water (0.5 mL) was added silver(I) nitrate (0.02 mmol, 0.2 equiv). Potassium persulfate (0.3 mmol, 3.0 equiv) was added in one portion and the reaction mixture was stirred vigorously at 60 °C for 60 min. The mixture was extracted with EtOAc (5 × 3 mL). The organic layers were combined, dried over Na2SO4, filtered, and concentrated in vacuo. Purification by silica gel chromatography (hexanes/EtOAc) afforded the product.
- 21.Representative Experimental Procedure for tandem radical cyclization/trap: To a solution of 1,4-benzoquinone (0.1 mmol, 1.0 equiv) in trifluorotoluene (0.5 mL) and water (0.5 mL) were added arylboronic acid (0.15 mmol, 1.5 equiv), silver(I) nitrate (0.02 mmol, 0.2 equiv), and potassium persulfate (0.3 mmol, 3.0 equiv). The reaction mixture was stirred vigorously at 60 °C for 60 min, diluted with EtOAc (3 mL), and washed with 5% aqueous NaHCO3 (3 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (3 × 3 mL). The organic layers were combined, dried over Na2SO4, filtered, and concentrated in vacuo. Purification by silica gel chromatography (hexanes/Et2O) afforded the product.
- 22.Molander GA, Trice SLJ, Dreher SD. J Am Chem Soc. 2010;132:17701–17703. doi: 10.1021/ja1089759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Liskey CW, Wei CS, Pahls DR, Hartwig JF. Chem Commun. 2009;37:5603–5605. doi: 10.1039/b913949d. [DOI] [PubMed] [Google Scholar]; (b) Chotana GA, Vanchura BA, II, Tse MK, Staples RK, Maleczka RE, Jr, Smith MR., III Chem Commun. 2009;37:5731–5733. doi: 10.1039/b914736e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem Rev. 2010;110:890–931. doi: 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.