Abstract
The vitamin D receptor (VDR) ligand, 1,25(OH)2D3, reduces proliferation and enhances differentiation and thus has been investigated for a role in preventing or treating cancer. Mice deficient for the VDR display a hyperproliferative response in the hair follicle and epidermis and decreased epidermal differentiation. Unlike their wild type littermates, when treated with 7,12 dimethylbenzanthracene (DMBA) or UVB, they develop skin tumors, including some characteristic of over-expression of the hedgehog (Hh) pathway. Both the epidermis and utricles of the VDR null animals over-express elements of the Hh pathway [Sonic Hedgehog (Shh, 2.02 fold), Patched1 1.58 fold, Smoothened 3.54 fold, Gli1 1.17 fold, and Gli2 1.66 fold]. This over-expression occurs at an age (11 weeks) where epidermal hyperproliferation is most visible and is spatially controlled in the epidermis. DMBA or UVB induced tumors in the VDR null mice also over-express elements of this pathway. Moreover, 1,25(OH)2D3 down-regulates the expression of some members of the Hh pathway in an epidermal explants culture system, suggesting a direct regulation by 1,25(OH)2D3. Our results suggest that increased expression of Shh in the keratinocytes of the VDR null animal activates the Hh pathway, predisposing the skin to the development of both malignant and benign epidermal neoplasms.
Introduction
Over 1 million skin cancers occur annually in the United States, 80% of which are basal cell carcinomas (BCC) (16% squamous cell carcinomas (SCC), 4% melanomas), making it by far the most common cancer (Greenlee et al., 2001). 1,25 Dihydroxyvitamin D3 [1,25(OH)2D3] has been evaluated for its potential anticancer activity (Eisman et al., 1979), and the accepted basis for the promise of 1,25(OH)2D3 for the prevention and treatment of malignancy includes its antiproliferative, prodifferentiating effects on many cell types. Epidemiologic evidence supports the importance of adequate vitamin D nutrition for the prevention of a number of cancers including those of the colon and breast (Bostick et al., 1993; Garland et al., 1985; Garland et al., 1990; Hanchette and Schwartz, 1992; Kearney et al., 1996).
Recent studies indicate that vitamin D signaling plays a protective role in skin carcinogenesis. Zinser et al. (Zinser et al., 2002) treated vitamin D receptor (VDR) null mice (VDRKO) bearing medroxyprogesterone pellets with two oral administrations of 7,12 dimethylbenzanthracene (DMBA) at 5.5 and 7 weeks, a protocol designed to induce breast cancers. No breast tumors were observed within 2 months, however during this period, 85% of the VDRKO mice developed skin tumors, while no tumors were found in wildtype controls. The tumors were mostly sebaceous, squamous, and follicular papillomas, but several BCC were also observed, while no SCC were reported. These results have recently been confirmed using topical administration of DMBA/TPA, although only papillomas were seen in the VDRKO mice, unlike RXRαKO, mice which developed both BCC and SCC (Indra et al., 2007). The appearance of BCC in these studies is surprising, since the typical malignancy induced in mouse skin by UVR, ionizing radiation, or chemical carcinogens is SCC rather than BCC (Daya-Grosjean and Sarasin, 2005). The appearance of BCC is characteristic of tumors formed when the hedgehog (Hh) signaling is activated (Regl et al., 2004a) by mutations in the Hh signaling pathway, in particular in patched1 (Ptch1) or over expression of Shh, Gli1 and Gli2 (Hahn et al., 1996; Johnson et al., 1996; Aszterbaum et al., 1998; Aszterbaum et al., 1999). The predisposition of VDRKO mice to skin tumor formation has also been demonstrated using UVB (Ellison et al., 2008).
Essentially all BCCs, whether arising sporadically or in patients with basal cell nevus syndrome (BCNS) (Gorlin Syndrome), have mutations in Ptch1, the membrane receptor for Shh, or other alterations in Hh signaling (Hahn et al., 1996; Aszterbaum et al., 1998; Aszterbaum et al., 1999). This phenotype is also observed in the Ptch1+/- (Gorlin) mouse (Regl et al., 2004a), which, unlike the wild type mouse produces BCC as well as SCC after treatment with UVR or ionizing radiation (Regl et al., 2004a). In the absence of Shh, Ptch1 inhibits the function of another membrane protein, smoothened (Smoh). Shh reverses this inhibition, freeing Smoh to enable the activation of a family of transcription factors Gli1, Gli2, and Gli3. Gli1 and 2 over-expression in keratinocytes can increase the expression of each other, as well as Ptch1, the anti-apoptotic factor bcl2, cyclins D1 and D2, E2F1, cdc45 (all of which promote proliferation), while suppressing genes associated with keratinocyte differentiation such as K1, K10, involucrin, loricrin and the VDR (Grachtchouk et al., 2000; Nilsson et al., 2000; Regl et al., 2002; Regl et al., 2004a; Regl et al., 2004b). Mice over-expressing Gli1, Gli2, or Shh in their basal keratinocytes (Oro et al., 1997; Grachtchouk et al., 2000; Nilsson et al., 2000) or grafted with human keratinocytes over-expressing Shh (Fan et al., 1997) develop BCC like lesions. Furthermore, BCC show over-expression of Ptch1, Smoh, Gli1 and Gli2 (Tojo et al., 1999; Bonifas et al., 2001). Gli2 may be more critical than Gli1 in mediating Shh activity in that Gli1 null animals display no obvious phenotype unlike Gli2 null animals. Gli2 null mice resemble Shh null animals in phenotype, Gli2 deletion partially rescues Ptch1 null animals, and Gli2 but not Gli1 is required for Shh signaling in hair follicle development (review in (Eichberger et al., 2004)).
Most skin tumors induced by systemic DMBA in mice lacking the VDR contain hair follicle elements and/or are of basal cell origin, tumors characteristic of over-expression of the Hh pathway in keratinocytes. The VDR is found in the outer root sheath and basal layer of the interfollicular epidermis, a postulated source for BCC development (Bikle et al., 2006). Lack of VDR causes a marked hyperproliferative response in these cells of the hair follicle and epidermis, with disruption of normal hair follicle cycling and decreased epidermal differentiation (Bikle et al., 2006; Xie et al., 2002). We found that the epidermis and epidermal portion (utricles) of the hair follicles of VDRKO animals over-express elements of the Hh signaling pathway (Shh, Ptch1, Smoh, Gli1, and Gli2), suggesting that one of the causes of the increased susceptibility of the epidermis to malignant transformation is due to a loss of 1,25(OH)2D3 and/or VDR regulation of Hh signaling. When we examined tumors in VDRKO mice treated with DMBA or UVB, we found the over expression of the same elements of the Hh signaling pathway predominantly in the tumors compared with adjacent tissue. Using an epidermal explant culture, we found that 1,25(OH)2D3 represses the expression of at least some members of the Hh signaling pathway, suggesting a direct regulation by the VDR.
Results
Acute UVB exposure increased epidermal thickness and proliferation in VDRKO mice
To address the short term sensitivity of skin to UVB exposure we irradiated wild-type and VDRKO mice with a single dose of UVB (400 mJ/cm2). Wild-type mice displayed increased epidermal thickening 1h and 24h after exposure to UVB with no further increase by 48h (Fig. 1A). This was accompanied with increased PCNA staining, a marker of cellular proliferation, in the epidermis at 1h and 24h with no further increase by 48h (Fig. 1B). VDRKO mice displayed a more pronounced epidermal hyperplasia at all time points compared to wild-type mice (Fig 1A), showing an increased and more durable hyperplasia. Consistent with the increased thickening, VDRKO mice had increased numbers of PCNA positive cells compared to wild-type mice at 1h and 24h, and these continued to increase at 48h (Fig. 1B).
Figure 1. Increased hyperplasia and hyperproliferation in UVB exposed epidermis from VDRKO mice.
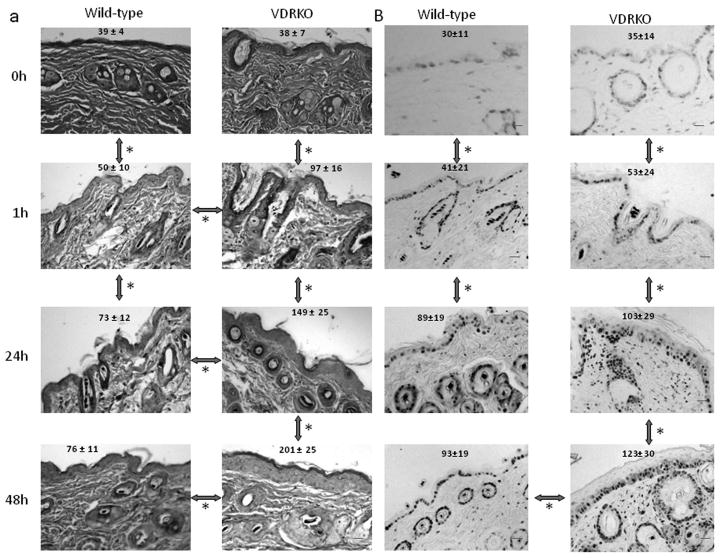
Wild-type mice exposed to 1 dose of UVB (477 mJ/cm2) show epidermal hyperplasia (A, H&E staining) and increased proliferation (B, PCNA staining), 1h and 24h after treatment with no further increase at 48h. VDRKO mice exposed to the same dose of UVB show significantly more pronounced epidermal hyperplasia (A) at 1h and 24h that continued to increase at 48h. VDRKO epidermis also show increased proliferation (B) comparable to that of wild-type mice at 1h and 24h that continued to increase at 48h. The bars denote 50 μm.
Over-expression of Hh pathway members in VDRKO mice
To address the long term sensitivity of VDRKO mice epidermis to tumor formation, we assessed the protein levels of multiple members of the Hh pathway in VDRKO mice and their wild type littermates by immunohistochemistry and western blotting. We examined 11 week old mice, the age matching the appearance of the skin papillomas in VDRKO mice treated with DMBA (Zinser et al., 2002). In wild-type mice Shh, Ptch1 and Smoh were expressed in both hair follicles and inter-follicular epidermis, whereas Gli1 was expressed mostly in hair follicles, and Gli2 expression was not detectable. Expression of all these proteins increased in VDRKO mice compared to their wild type littermates (Fig. 2A), and their spatial distribution was also affected by the lack of VDR. Shh expression increased in the epidermis of the VDRKO mice, but was markedly reduced in the utricles, the remaining portion of the hair follicle structures. Ptch1 was expressed throughout the epidermis and the hair follicle of the wild type mice, but primarily in the utricles and dermal cysts of the VDRKO mice. Smoh and Gli1 expression, found primarily in the outer root sheath of the hair follicles of wild-type mice, increased in both hair follicles and inter-follicular epidermis of the VDRKO mice. Gli2 expression, not detectable in wild type mice, was strongly expressed in utricles and dermal cysts of VDRKO mice. We confirmed quantitatively the increased expression of these proteins using Western blotting. When compared to the house-keeping gene beta-actin, we found an increased expression of Shh (2.02 fold), Ptch1 (1.58 fold), Smoh (3.54 fold), Gli1 (1.17 fold) and Gli2 (1.66 fold) in skin from VDRKO mice compared to their wild-type littermates (Fig. 2B).
Figure 2. Increased protein levels of Hh signaling pathway members in VDRKO mice.
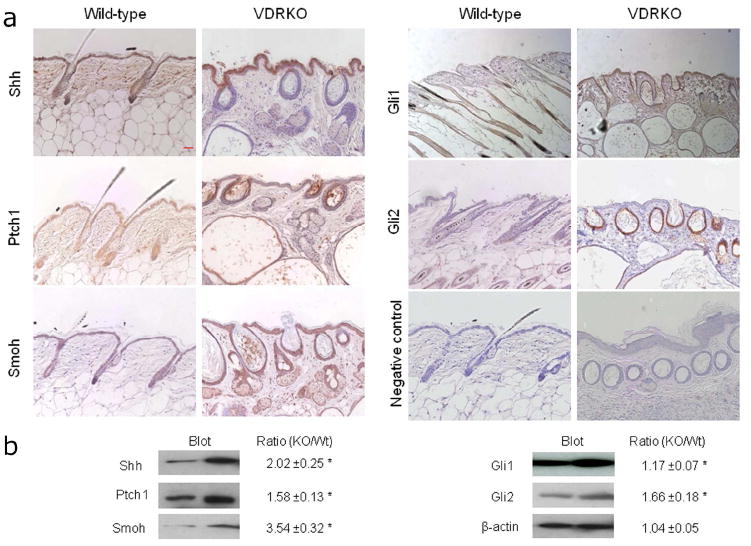
A. Shh, Ptch1, Smoh, Gli1 and Gli2 proteins as shown by the brown signal were detected in the epidermis and hair follicles from VDRKO mice and their wild-type littermates at 11 weeks after birth by immunocytochemistry. Slides were counterstained with hematoxylin (blue stain). The bar denotes 50 μm. B. Shh, Ptch1, Smoh, Gli1 and Gli2 protein levels were quantified by western blot in skin from VDRKO mice and their wild-type littermates and compared to beta-actin as a control. The numerical value represents the average ratio of VDRKO band intensity versus wild-type band intensity after subtraction of background level from three independent experiments. * p<0.05.
Different types of skin tumors are induced by UVB in VDRKO mice
VDRKO mice exposed to 40 weeks of UVB irradiation developed an average of 2.7 skin tumors greater than 1 mm in diameter per mouse, with at least 1 tumor per mouse. These tumors included papillomas (78%), BCC (11%), SCC (7.5%), and keratoacanthomas (3.5%). Representative sections of these tumors are presented in figure 3A. Furthermore, after 40 weeks of UVB no tumors were apparent in wild-type and CYP27B1KO mice (Fig. 3B and data not shown). No tumors developed in the VDRKO mice that were not exposed to DMBA or UVB (Fig. 3B and data not shown).
Figure 3. Skin structures induced by UVB in VDRKO mice.
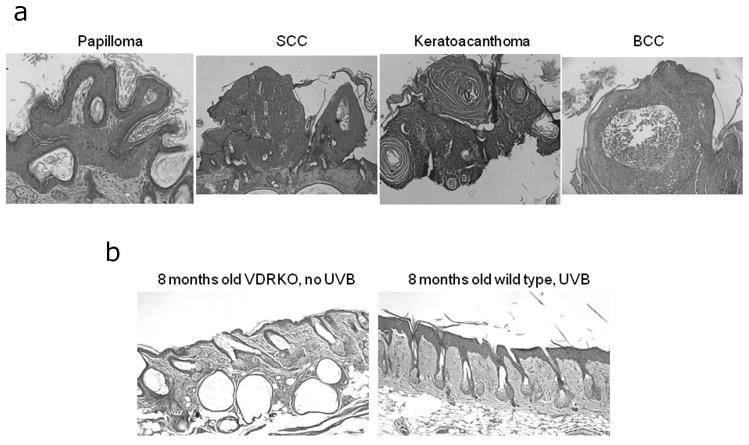
A. Representative structures from mice exposed to 40 weeks of UVB irradiation were collected and classified into papillomas, squamous cell carcinomas (SCC), keratoacanthomas and basal cell carcinomas (BCC). B. Representative structure of 8 months old VDRKO skin without UVB exposure and wild-type mice after 40 weeks of UVB exposure. The bars denote 50 μm.
DMBA and UVB induced skin tumors in VDRKO mice express members of the Hh pathway
We then examined the expression of Hh signaling pathway members by immunohistochemistry in tumors formed in VDRKO mice following DMBA and UVB treatment. In all these tumors we were able to find expression of Shh, Smoh, Ptch1, Gli1 and Gli2 (Fig. 4A and data not shown). Moreover, the expression of these markers was mostly localized to the tumor with little or no expression in adjacent tissues (Fig. 4A and data not shown). We confirmed the expression of these proteins specifically in the DMBA generated skin tumors using Western analysis of protein extracts from these tumors (Fig. 4B). When compared to the house-keeping gene beta-actin, we found an increased expression of Shh (3.08 fold), Ptch1 (1.53 fold), Smoh (3.65 fold), Gli1 (1.75 fold) and Gli2 (1.56 fold) in the tumors compared to tumor-free tissue (Fig. 4B).
Figure 4. Expression of members of the Hh signaling pathway in the tumors from VDRKO mice.
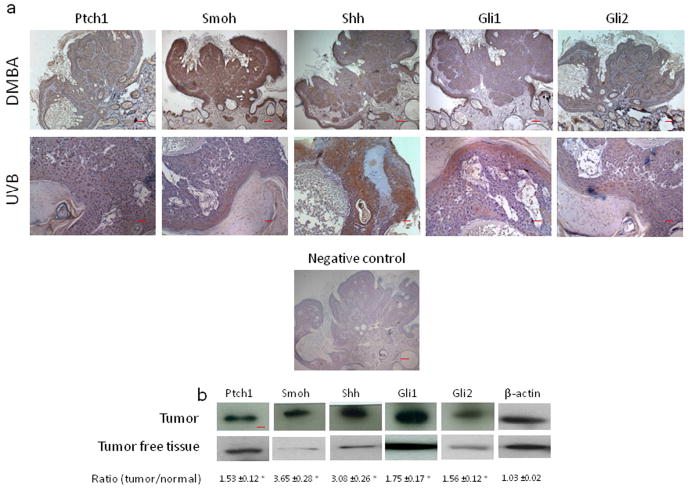
A. Shh, Ptch1, Smoh, Gli1 and Gli2 proteins as shown by the brown signal were detected by immunohistochemistry in a papilloma from a VDRKO mouse treated with DMBA and in a BCC from a VDRKO mouse treated with UVB. Slides were counterstained with hematoxylin (blue stain). The bars denote 50 mm. B. Shh, Ptch1, Smoh, Gli1 and Gli2 protein levels were measured by western blot in skin tumors and tumor free tissue from DMBA treated VDRKO mice. The numerical value represents the mean ratio of the tumor band intensity versus tumor free tissue band intensity after subtraction of background level from three independent experiments. * p<0.05.
1,25(OH)2D3 repression of the Hh pathway in the epidermis
To determine whether 1,25(OH)2D3 directly regulates the expression of components of the Hh pathway we measured their expression levels in epidermal explants from wild-type mice, following treatment with 1,25(OH)2D3 concentrations ranging from 10-10M to 10-7M. Shh was robustly expressed in neonatal epidermal explants but not in proliferating keratinocytes in culture (data not shown). After treatment with 1,25(OH)2D3, the epidermal explants displayed increased expression of Cyp24 and decreased expression of Shh, Gli1, Gli2 and Ptch1 in a dose dependant manner (Fig. 5A). To verify that 1,25(OH)2D3 regulation of the expression of those genes was dependent on the VDR, we measured their expression levels in epidermal explants from VDRKO mice compared to wild-type mice. Treatment with 10-8M of 1,25(OH)2D3 decreased the expression of Shh (3.5 fold), Gli1 (2.4 fold), Gli2 (2.3 fold) and Ptch1 (2 fold) in wild-type epidermis. Smoh expression was not affected. In contrast, 1,25(OH)2D3 inhibition of their expression was suppressed in VDRKO samples (Fig. 5B). Lack of VDR in VDRKO samples was verified by the lack of VDR expression and the inability of 1,25(OH)2D3 to induce Cyp24 expression, compared to wild-type samples (500 fold increase, Fig. 5B). Immunohistochemistry confirmed that Shh is expressed in the most differentiated layer of the epidermis but not in the basal layer of these explants (Fig. 5C).
Figure 5. 1,25(OH)2D3, through VDR action, represses Hh pathway expression in the epidermis.
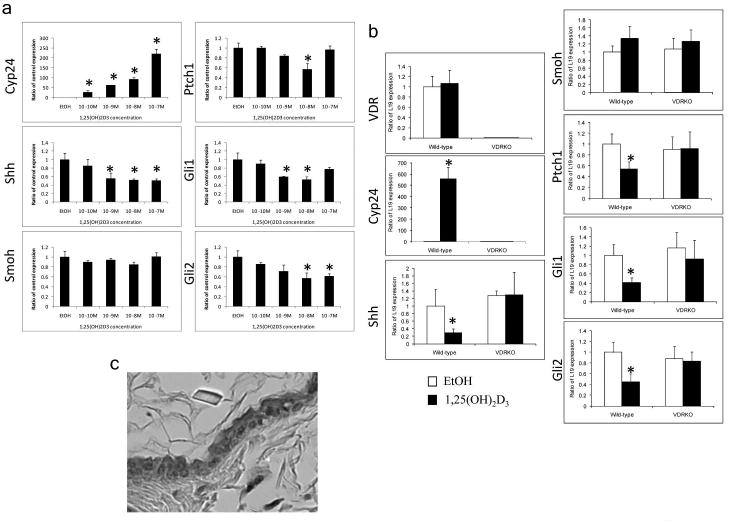
A. 1,25(OH)2D3 treatment of epidermal preparations from wild-type mice in culture for 24h induced Cyp24 expression and repressed Shh, Gli1, Gli2 and Ptch1 expression in a dose dependant manner. B. Epidermal preparations from wild-type and VDRKO mice in culture were treated with 1,25(OH)2D3 10-8M or EtOH for 24h. Absence of VDR expression was verified in VDRKO mice and their epidermis failed to respond to 1,25(OH)2D3 induction of Cyp24 expression unlike that in wild-type mice. 1,25(OH)2D3 treatment repressed Shh, Gli1, Gli2 and Ptch1 expression only in wild-type preparations. * p<0.05 C. Shh protein expression was localized in the upper layers of the epidermis by immunohistochemistry on a full skin section. The bar denotes 50 μm.
Discussion
Following recent studies showing the sensitivity of VDRKO mice to skin tumor formation upon exposure to carcinogen (Zinser et al., 2002) or to UVB (Ellison et al., 2008), we investigated potential mechanisms by which VDR could act as a tumor suppressor. VDRKO mice show increased sensitivity to acute UVB irradiation with respect to epidermal thickening and hyperproliferation. Hyperproliferation being a key element in tumor formation, our data indicate a predisposition of VDRKO epidermis to develop skin tumors, although no tumors were found in the VDRKO mice not exposed to UVB. Hyper-proliferation was also observed in wild-type, to a lesser extent, but no tumors arise in those mice following 40 weeks of UVB exposure. In a comparable study (Ellison et al., 2008), wild-type mice developed tumors after 45 weeks of exposure to UVB, with an incidence 85% lower than in VDRKO. As a potential mechanism for this hyperproliferative response we found that the epidermal portion (utricles) of the hair follicles and the dermal cysts of VDRKO animals over-express elements of the Hh signaling pathway, suggesting that one of the causes of the increased susceptibility of the VDRKO epidermis to malignant transformation is due to the loss of 1,25(OH)2D3 and/or VDR regulation of Hh signaling. This potential mechanism is further supported by the expression of elements of the Hh signaling pathway in the tumors arising in VDRKO mice treated with DMBA or UVB and the observation that 1,25(OH)2D3 suppresses the expression of the Hh signaling pathway in a VDR dependent fashion.
Lack of VDR increases the protein expression of the Hh pathway members Shh, Ptch1, Smoh, Gli1 and Gli2 in VDRKO mice. Our data show that these proteins are expressed in a spatially controlled way in the wild type animals and in the VDRKO even if the levels and areas of expression change. Since one of the main skin defects of the VDRKO mice is a blockage of their hair follicle cycling by the end of the first cycle and a progressive hair loss (Bikle et al., 2006; Li et al., 1997), one might consider that the hair follicle is the main target and regulator of the Hh signaling pathway. When this structure is missing or, as in VDRKO mice, altered to become utricles and dermal cysts, the Hh signaling pathway is over expressed in the epidermal and interfollicular epidermal parts of the skin, thus potentially predisposing it to develop tumors. Paradoxically loss of Hh signaling in the cycling portion of the hair follicle may contribute to its failure to cycle, as we recently show that VDRKO hair follicles have a decreased expression of Hh signaling and that treatment with an Hh signaling agonist restores partially hair follicle cycling (Teichert et al., 2010). Moreover, we found that Hh signaling pathway expression is spatially controlled in the epidermis. Shh, for example, is expressed in the most differentiated parts of the epidermis, whereas other members of the pathway have a different distribution, implying a paracrine action for Shh in the epidermis/hair follicle. The separation of the different components of the Hh pathway may explain why keratinocytes in culture express little or no Shh if various feedback/feedforward loops between the different layers of the skin are disrupted in monocultures.
The tumors observed in VDRKO mice treated by DMBA or UVB expressed all Hh signaling pathway members analyzed in this study, although as in the normal skin expression of these different pathway members varies in different cells of the tumor, suggesting that the role of the Hh pathway signaling components to the formation of these tumors results from the contribution of different cell types.
Thus we postulate that increased expression of Shh in the keratinocytes of the VDRKO mouse activates the Hh signaling pathway, predisposing the skin to the development of tumors in part by stimulating proliferation. Our data using epidermal preparations show that 1,25(OH)2D3 represses the expression of this pathway in a VDR dependent fashion, indicating a direct regulation of the Hh pathway by vitamin D. Others have observed that vitamin D may regulate this pathway not only via the genomic actions of 1,25(OH)2D3 acting through VDR, but also by direct inhibition by vitamin D independent of its receptor. The latter possibility stems from recent observations that vitamin D itself as well as its precursor 7-dehydrocholesterol can bind to and inhibit the actions of Smoh, a critical step in Hh signaling (Bijlsma et al., 2006). Thus both genomic and nongenomic actions of vitamin D may serve to block tumor formation in the skin.
Our data show that over expression of Hh signaling members in VDRKO mice arises when the utricles are well established and the epidermis is clearly hyperproliferative. This suggests that Hh signaling and the loss of the proximal portion of the hair follicle defect might be associated. One explanation might be that the subtle balance of expression of this pathway between the dermal and epidermal parts of the skin is impaired by the lack of VDR. Specifically, in VDRKO mice the dermal papilla, which plays an important role in hair follicle development and cycling, is dissociated from the hair follicle (Bikle et al., 2006). Thus, the over-expression of Hh signaling in the epidermis of the VDRKO mice may increase sensitivity toward carcinogenesis, whereas at the same time the loss of Hh signaling in the cycling portion of the hair follicle contributes to its failure to cycle. This duality of the role of Hh signaling in the skin between hair follicle and epidermis and its differential regulation by 1,25(OH)2D3/VDR is reminiscent of an analogous duality with respect to wnt/b-catenin signaling and its regulation by 1,25(OH)2D3/VDR (Palmer et al., 2008). Thus, although we have focused on the Hh signaling pathway in this study, these effects of vitamin D signaling on tumor predisposition in the epidermis will no doubt involve a number of pathways involving both the hair follicle and the epidermis that are yet to be fully explored.
Material and Methods
Animals
All animal experimentation described in the manuscript has been approved by the San Francisco VA Medical Center Animal Review Committee.
Mice heterozygous for the VDR null mutation, Vdrtm1Ska, bred into the C57BL/6 background, were provided by Dr. Shigeaki Kato (Molecular and Cellular Biosciences, University of Tokyo, Japan). These mice were bred to provide wild type and homozygous mutant VDRKO littermates. Genotyping was performed by PCR with different primers designed to amplify the mutant VDR DNA and the wild type VDR DNA (Table 1a). VDRKO DMBA treated mouse samples were provided from experiments described previously (Zinser et al., 2002).
For UVB exposure, dorsal skin was shaved 24 hours before UVB exposure. Mice (5 weeks old at the start of the study) were irradiated three times per week, with 2–3 days between treatments, and re-shaved as needed. The dorsal skin was exposed to UV irradiation from a band of six FS-40 fluorescent lamps; UVB and UVC wavelengths were filtered out from this UV light using Kodacel cellulose film. The dose of UVB varied to maximize UV exposure with minimal ulceration of the skin, using the following protocol: 120 mJ/cm2 for 2 weeks, the dose was then increased 25% per week for 5 weeks, up to 400 mJ/cm2 for 9 weeks, followed by 200 mJ/cm2 until week 40 (Ellison et al., 2008). Skin tumors were characterized with the help of the dermato-pathology department of the San Francisco VA medical center.
Immunohistochemistry
Affinity-purified antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) found to recognize mouse proteins following heat-induced antigen retrieval treatment (in 10 mM citrate buffer; pH 6.0, at 95°C, for 30 min) were used. The primary antibodies were used at a concentration of 4 ug per ml in 10 mM Tris buffer, pH 7.6, containing 4% bovine serum albumin, 1% teleostean skin gelatin, 0.1% Tween 20, and 500 mM NaCl. The binding of the primary antibody to the sections was detected by affinity-purified, biotinylated goat anti-rabbit IgG, followed by ABC-peroxidase reagent, both purchased from Vector (Burlingame, CA). Peroxidase activity was detected with diaminobenzidine substrate (QualTek Laboratories, Santa Barbara, CA) followed by counterstaining with methyl green or hematoxylin. Omitting the first antibody resulted in no signal, indicating the specificity of the immunodetection.
Protein extraction and Western Blot
Proteins were extracted from the back skin of mice or from the DMBA treated tumors after homogenization with a Polytron in RIPA buffer (150mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 50mM Tris). Protein concentrations were determined by the BCA Protein Assay Kit (Pierce Corp., Rockford, IL). Equal amounts of protein samples were electrophoresed through 4–15% gradient polyacrylamide gels and electroblotted onto polyvinylidene fluoride membranes (Immobilon-P, 0.45 mm; Millipore Corp., Bedford, MA). After incubation in blocking buffer (PBS, 5% nonfat milk, and 0.5% Tween 20), the blot was incubated overnight at 4°C with appropriate primary antibodies: polyclonal antibody against mouse Gli1 (Abcam, Cambridge, MA) at a dilution of 1:1000, Gli2, Patched, Shh or Smoh (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a dilution of 1:200. After washing 3 times with the blocking buffer, the membranes were incubated for 1 h with the appropriate anti-IgG secondary antibody conjugated to horseradish peroxidase (Amersham Biosciences) in the blocking buffer. After a second series of washes, bound antibody complexes were visualized using the SuperSignal ULTRA chemiluminescent kit (Pierce) and subsequent exposure to X-ray film. X-ray films were then scanned and the intensity of each band was quantified using Kodak 1D™ 3.6.4 (Kodak, Rochester, NY). The data presented are representative of three independent experiments.
Skin separation and cell culture
Skin separation was performed as described by Lichti et al (Lichti et al., 2008). Briefly, back skin from neonatal mice was collected and separated overnight in HBSS with 5U/ml dispase. Epidermal sheets (epidermal explants) were then peeled from the dermal fraction and maintained in culture in 154CF medium (Cascade Biologics, Portland, OR) with 1.2 mM CaCl2 and 10% chelexed fetal bovine serum. Epidermal explants were treated for 24h with 1,25(OH)2D3 (10-10 to 10-7M) or vehicle (100% Ethanol).
Quantitative real time PCR
Total RNA from back skin of mice, separated epidermis and dermis or cell culture was extracted using RNA-STAT 60 (Tel-Test, Inc., Friendwood, TX). Concentration and purity were determined by measuring the absorbance at OD 260/280 nm. 1ug of RNA was reversed transcribed using random hexamers with the TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA). The amount of cDNA was then quantified by quantitative real time PCR, performed on a PE Biosystems model 7900 HT sequence detector. The PCR amplification was done using TaqMan Universal PCR Master Mix (Applied Biosystems) and TaqMan Gene Expression Assays (Applied Biosystems) using their proprietary primer/probe sets or SYBRGreen Universal PCR Master Mix (Applied Biosystems) and primers from PrimerBank (Wang and Seed, 2003). Mitochondrial ribosomal protein L19 (Mrpl19) was used as the control gene to which the results were normalized.
Acknowledgments
We thank Dr. Geiss and his dermatopathology team at the San Francisco VA hospital for their help characterizing the skin tumors and Vadim Bul for his technical help.
Work supported by NIH grants AR050023, AR39448
Abbreviations
- 1,25(OH)2D3
1,25 Dihydroxyvitamin D3
- BCC
Basal cell carcinoma
- BCNS
basal cell nevus syndrome
- DMBA
7,12 dimethylbenzanthracene
- Hh
Hedgehog
- Ptch1
Patched
- Smoh
Smoothened
- Shh
Sonic Hedgehog
- SCC
Squamous cell carcinoma
- VDR
Vitamin D receptor
Footnotes
Conflict of Interest Authors state no conflict of interest.
References
- Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, et al. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5:1285–1291. doi: 10.1038/15242. [DOI] [PubMed] [Google Scholar]
- Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J, Bonifas JM, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885–888. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS biology. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Elalieh H, Chang S, Xie Z, Sundberg JP. Development and progression of alopecia in the vitamin D receptor null mouse. J cell Physiol. 2006;207:340–353. doi: 10.1002/jcp.20578. [DOI] [PubMed] [Google Scholar]
- Bonifas JM, Pennypacker S, Chuang PT, McMahon AP, Williams M, Rosenthal A, et al. Activation of expression of hedgehog target genes in basal cell carcinomas. J Invest Dermatol. 2001;116:739–742. doi: 10.1046/j.1523-1747.2001.01315.x. [DOI] [PubMed] [Google Scholar]
- Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women’s Health Study. Am J Epidemiol. 1993;137:1302–1317. doi: 10.1093/oxfordjournals.aje.a116640. [DOI] [PubMed] [Google Scholar]
- Daya-Grosjean L, Sarasin A. The role of UV induced lesions in skin carcinogenesis: an overview of oncogene and tumor suppressor gene modifications in xeroderma pigmentosum skin tumors. Mutat Res. 2005;571:43–56. doi: 10.1016/j.mrfmmm.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Eichberger T, Regl G, Ikram MS, Neill GW, Philpott MP, Aberger F, et al. FOXE1, a new transcriptional target of GLI2 is expressed in human epidermis and basal cell carcinoma. J Invest Dermatol. 2004;122:1180–1187. doi: 10.1111/j.0022-202X.2004.22505.x. [DOI] [PubMed] [Google Scholar]
- Eisman JA, Martin TJ, MacIntyre I, Moseley JM. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet. 1979;2:1335–1336. doi: 10.1016/s0140-6736(79)92816-2. [DOI] [PubMed] [Google Scholar]
- Ellison TI, Smith MK, Gilliam AC, MacDonald PN. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. Journal of Invest Dermatol. 2008;128:2508–2517. doi: 10.1038/jid.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Oro AE, Scott MP, Khavari PA. Induction of basal cell carcinoma features in transgenic human skin expressing Sonic Hedgehog. Nat Med. 1997;3:788–792. doi: 10.1038/nm0797-788. [DOI] [PubMed] [Google Scholar]
- Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985;1:307–309. doi: 10.1016/s0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19:614–622. doi: 10.1016/0091-7435(90)90058-r. [DOI] [PubMed] [Google Scholar]
- Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, et al. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet. 2000;24:216–217. doi: 10.1038/73417. [DOI] [PubMed] [Google Scholar]
- Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–2869. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Indra AK, Castaneda E, Antal MC, Jiang M, Messaddeq N, Meng X, et al. Malignant Transformation of DMBA/TPA-Induced Papillomas and Nevi in the Skin of Mice Selectively Lacking Retinoid-X-Receptor alpha in Epidermal Keratinocytes. J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5700672. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- Kearney J, Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, et al. Calcium, vitamin D, and dairy foods and the occurrence of colon cancer in men. Am J Epidemiol. 1996;143:907–917. doi: 10.1093/oxfordjournals.aje.a008834. [DOI] [PubMed] [Google Scholar]
- Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. PNAS. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Unden AB, Krause D, Malmqwist U, Raza K, Zaphiropoulos PG, et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci U S A. 2000;97:3438–3443. doi: 10.1073/pnas.050467397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH, Jr, Scott MP. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- Palmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM. The Vitamin D Receptor Is a Wnt Effector that Controls Hair Follicle Differentiation and Specifies Tumor Type in Adult Epidermis. PLoS ONE. 2008;3:e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Ikram MS, et al. The zinc-finger transcription factor GLI2 antagonizes contact inhibition and differentiation of human epidermal cells. Oncogene. 2004a;23:1263–1274. doi: 10.1038/sj.onc.1207240. [DOI] [PubMed] [Google Scholar]
- Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Philpott MP, et al. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004b;64:7724–7731. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- Regl G, Neill GW, Eichberger T, Kasper M, Ikram MS, Koller J, et al. Human GLI2 and GLI1 are part of a positive feedback mechanism in Basal Cell Carcinoma. Oncogene. 2002;21:5529–5539. doi: 10.1038/sj.onc.1205748. [DOI] [PubMed] [Google Scholar]
- Teichert A, Elalieh H, Bikle D. Disruption of the hedgehog signaling pathway contributes to the hair follicle cycling deficiency in Vdr knockout mice. Journal of cell Physiology. 2010;225:482–489. doi: 10.1002/jcp.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo M, Mori T, Kiyosawa H, Honma Y, Tanno Y, Kanazawa KY, et al. Expression of sonic hedgehog signal transducers, patched and smoothened, in human basal cell carcinoma. Pathol Int. 1999;49:687–694. doi: 10.1046/j.1440-1827.1999.00938.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31:e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11–16. doi: 10.1046/j.1523-1747.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- Zinser GM, Sundberg JP, Welsh J. Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis. 2002;23:2103–2109. doi: 10.1093/carcin/23.12.2103. [DOI] [PubMed] [Google Scholar]


