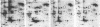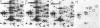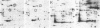Abstract
A set of at least 30 proteins disappears from the two-dimensional electrophoretic pattern of human lymphoid cells treated with various antimitochondrial agents. This set is similar to the set of proteins found in isolated mitochondria (except for the presence of action in the latter group), indicating that the inhibitor effect stops production of a majority of mature mitochondrial proteins. Several proteins having the characteristics of precursors to the major cytoplasmically synthesized mitochondrial proteins can be observed in cells during fast-pulse experiments and in a reticulocyte lysate system fed with total lymphoid cell RNA. In the three major instances of mitochondrial precursor--product processing, the removed peptide is quite basic in each case, suggesting that a lysine- or arginine-rich terminal sequence may be necessary for initial recognition by the mitochondrial protein uptake apparatus. The inhibitor effect allows easy identification of a large set of mitochondrial proteins in two-dimensional maps of various cells, thereby specifying a particularly tractable and functionally distinctive subset of the cellular proteins. The nature and wide scope of the effect support the concept of energy-dependent "vectorial processing" [Schatz, G. (1979) FEBS Lett. 103, 203--211] and indicate that such a mechanism is generally applicable to the major class of cytoplasmically synthesized mitochondrial proteins in mammalian cells.
Full text
PDF
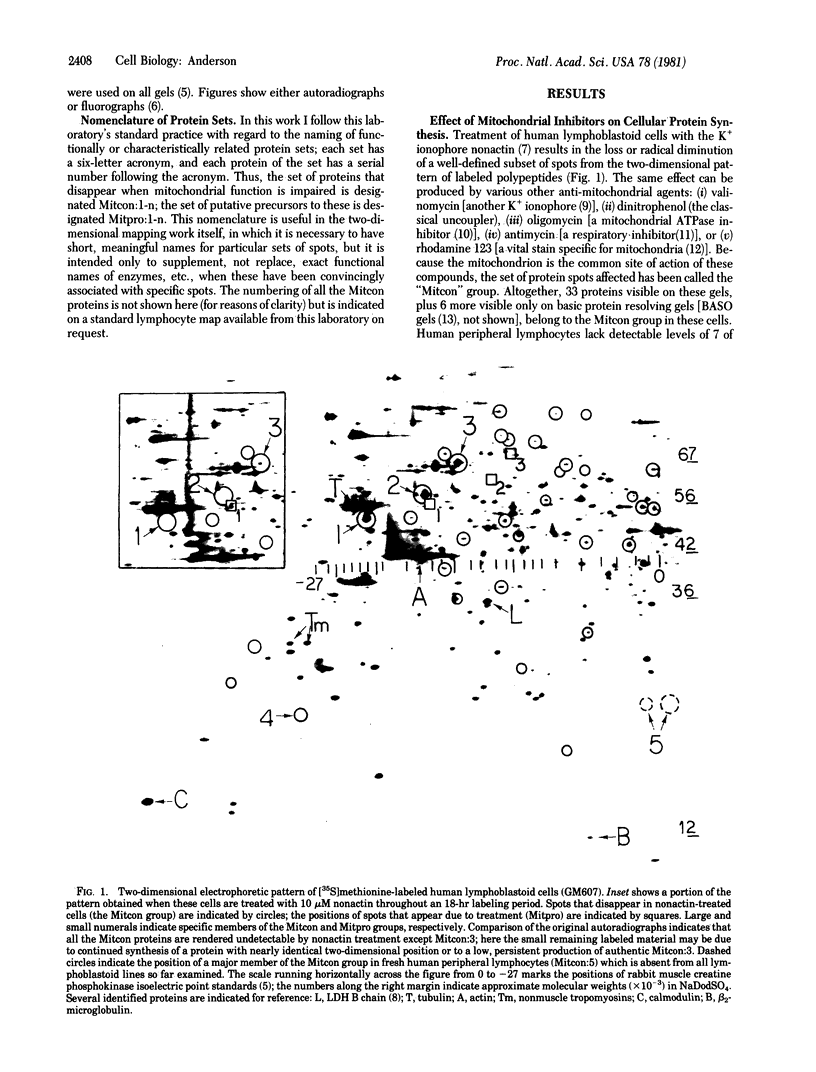

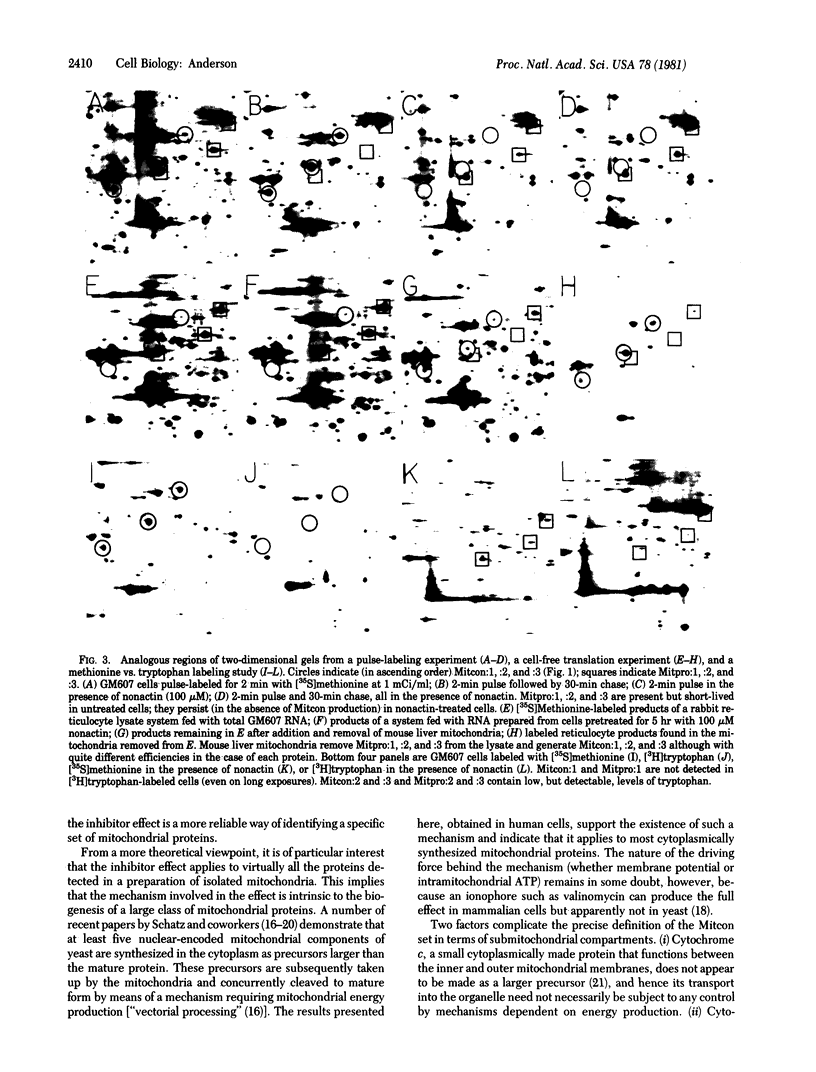

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMAD K., SCHNEIDER H. G., STRONG F. M. Studies on the biological action of antimycin A. Arch Biochem. 1950 Sep;28(2):281–294. [PubMed] [Google Scholar]
- Anderson N. L., Anderson N. G. Analytical techniques for cell fractions. XXII. Two-dimensional analysis of serum and tissue proteins: multiple gradient-slab gel electrophoresis. Anal Biochem. 1978 Apr;85(2):341–354. doi: 10.1016/0003-2697(78)90230-0. [DOI] [PubMed] [Google Scholar]
- Anderson N. L., Hickman B. J. Analytical techniques for cell fractions. XXIV. Isoelectric point stadnards for two-dimensional electrophoresis. Anal Biochem. 1979 Mar;93(2):312–320. doi: 10.1016/s0003-2697(79)80157-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Campbell P. N., Blobel G. The role of organelles in the chemical modification of the primary translation products of secretory proteins. FEBS Lett. 1976 Dec 31;72(2):215–226. doi: 10.1016/0014-5793(76)80973-8. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Côté C., Solioz M., Schatz G. Biogenesis of the cytochrome bc1 complex of yeast mitochondria. A precursor form of the cytoplasmically made subunit V. J Biol Chem. 1979 Mar 10;254(5):1437–1439. [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Schierlein W., Gerlach H. Makrotetrolide. Fortschr Chem Org Naturst. 1968;26:161–189. [PubMed] [Google Scholar]
- Korb H., Neupert W. Biogenesis of cytochrome c in Neurospora crassa. Synthesis of apocytochrome c, transfer to mitochondria and conversion to Holocytochrome c. Eur J Biochem. 1978 Nov 15;91(2):609–620. doi: 10.1111/j.1432-1033.1978.tb12714.x. [DOI] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Schatz G. Transport of proteins across the mitochondrial outer membrane. A precursor form of the cytoplasmically made intermembrane enzyme cytochrome c peroxidase. J Biol Chem. 1979 Aug 25;254(16):7468–7471. [PubMed] [Google Scholar]
- McConkey E. H. Identification of human gene products from hybrid cells: a new approach. Somatic Cell Genet. 1980 Jan;6(1):139–147. doi: 10.1007/BF01538702. [DOI] [PubMed] [Google Scholar]
- Nelson N., Schatz G. Energy-dependent processing of cytoplasmically made precursors to mitochondrial proteins. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4365–4369. doi: 10.1073/pnas.76.9.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S. Mitochondrial ATPase. Adv Enzymol Relat Areas Mol Biol. 1979;49:223–280. doi: 10.1002/9780470122945.ch6. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Schatz G. How mitochondria import proteins from the cytoplasm. FEBS Lett. 1979 Jul 15;103(2):203–211. doi: 10.1016/0014-5793(79)81328-9. [DOI] [PubMed] [Google Scholar]
- Willard K. E., Giometti C. S., Anderson N. L., O'Connor T. E., Anderson N. G. Analytical techniques for cell fractions. XXVI. A two-dimentional electrophoretic analysis of basic proteins using phosphatidyl choline/urea solubilization. Anal Biochem. 1979 Dec;100(2):289–298. doi: 10.1016/0003-2697(79)90232-x. [DOI] [PubMed] [Google Scholar]