Abstract
Objectives
To describe the interrelationships of glycemic control measures: HbA1c, glycated albumin, fructosamine, 1,5-anhydroglucitrol (1,5-AG), and continuous glucose monitoring (CGM) in children and adolescents with type 1 diabetes (T1D).
Study Design
26 subjects 4–17 years old had HbA1c measurement followed within 14 days by 3 laboratory measures of glycemia and the collection of CGM glucose data (N=21).
Results
Glycated albumin and fructosamine levels had a higher correlation with each other than with HbA1c. The correlation of 1,5-AG with HbA1c was lower (absolute r value = 0.25). All four measures had a similar degree of correlation with CGM-measured mean glucose (absolute r value=0.50 to 0.56), and with hyperglycemic area under the curve (AUC) at 180 mg/dL (0.50 to 0.60).
Conclusion
Each of the four measures (i.e., HbA1c, glycated albumin, fructosamine and 1,5-AG) had a similar correlation with mean glucose and hyperglycemic AUC-180. 1,5 AG did not correlate with hyperglycemic AUC-180 better than did HbA1c.
Keywords: Type 1 diabetes mellitus; 1,5-anhydroglucitol; Hemoglobin A1c
Introduction
HbA1c has been the mainstay of the determination of glycemic control in the management of diabetes since the 1980s. Other measures have been purported to also provide contrasting measures of glycemic control: glycated albumin, fructosamine, and 1,5-anhydroglucitrol (1,5-AG). HbA1c yields an estimate of glycemia for the previous 2–3 months (1–4) whereas glycated albumin and fructosamine represent glycemia for 2–3 weeks (4–6) and 1,5-AG summarizes the glycemic excursions occurring over a few days (1, 5, 7–11). The relevance of 1,5-A,G to diabetes stems from the fact that normally almost all filtered 1,5-AG is reabsorbed by the renal tubules. When glucose levels exceed approximately 180 mg/dL, reabsorption of 1,5-AG declines, and as a consequence plasma levels of 1,5-AG decrease. As a result, the greater the extent and duration of the blood glucose above 180 mg/dL, the lower will be the 1,5-AG level. Among nondiabetic individuals, females tend to have lower 1,5-AG levels than males (1, 12). Some studies have suggested that 1,5-AG reflects hyperglycemia better than HbA1c and may be useful in conjunction with HbA1c to assess glycemic control in patients with moderate or good control (1, 7).
As part of a study conducted by the Diabetes Research in Children Network (DirecNet) to evaluate the counter-regulatory response to hypoglycemia, blood samples were collected and continuous glucose monitoring (CGM) data were obtained that provided an opportunity to determine the correlation of these measures with each other and their relationship to glucose data collected with CGM.
Methods
A parent or guardian and each subject >7 years of age signed informed consent and assent forms, respectively, approved by an Institutional Review Boards and in accordance with the Declaration of Helsinki at the 5 DirecNet centers. The cohort included 26 children and adolescents with T1D who had a HbA1c value measured within 14 days (median 8 days, range 6 to 13 days) prior to the collection of blood samples that were used to measure 1,5-AG, glycated albumin, and fructosamine. Study eligibility criteria have been reported (13). HbA1c was measured using the DCA2000+ analyzer (Siemens Healthcare Diagnostics, Indianapolis). Blood samples were collected in CAPIJECT™ SST tubes and allowed to clot for 30 minutes prior to centrifugation. The serum was promptly transferred to transport tubes and stored frozen before shipping to the DirecNet Central Laboratory at the University of Minnesota where they remained frozen until the time of analysis.
Between the time of HbA1c measurement and the collection of the blood samples, each subject wore a Guardian® RT (Medtronic MiniMed, Northridge, CA) CGM device. Only the 21 subjects with at least 96 hours of CGM glucose data were included in the analysis involving CGM data (median amount of glucose data = 121 hours, interquartile range 114 to 134 hours, range 96 to 150 hours; 24% of data between 12 midnight and 6 a.m. and 76% between 6 a.m. and 12 midnight).
Laboratory Procedures
Laboratory procedures were as follows:
Glycated albumin
The glycated albumin assay included separate measurements of total albumin (bromocresol purple) and glycated albumin (enzymatic method utilizing ketoamine oxidase and an albumin-specific protease) with the glycated albumin result expressed as a percentage of total albumin (Method by Asahi Kasei Pharma (Tokyo Japan) adapted to the Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation)). For glycated albumin (as %), interassay CVs were 1.8% (at a mean of 56.0%) and 2.1% (at a mean of 22.7%). All materials needed for the glycated albumin assay were supplied by Asahi Kasei.
Fructosamine
This colorimetric assay from Roche on the Roche Mod P was based on the ability of ketoamines to reduce nitrotetrazolium-blue (NBT) to formazan in an alkaline solution. The rate of formation of formazan (measured photometrically at 546 nm) is directly proportional to the concentration of fructosamine. Uricase served to eliminate uric acid interference, and detergent eliminated matrix effects. The interassay CV was <3%.
1,5-anhydroglucitol
The 1,5-AG monosaccharide was measured using an enzymatic colorimetric method implemented on the Roche ModP with materials from GlycoMark, Inc. Reagents were provided by GlycoMark Inc., the distributor of the only FDA-approved assay of 1,5-anhydroglucitol. The interassay CV was <5%.
Statistical Methods
The associations of the measures with each other were evaluated with Spearman rank correlation coefficients (The absolute values of correlation coefficient were presented in Results section for consistency of interpretation. The directions of correlation were noted in Table 1). Separate multivariate least squares regression models were used to assess the additive effect of the various laboratory measures for predicting HbA1c, CGM mean glucose and hyperglycemic area under the curve for glucose above 180 mg/dL (AUC-180). The regression model for hyperglycemic AUC-180 was based on the van der Waerden normal scores due to the skewness of data.
Table 1.
Distribution of HbA1c, Glycated Albumin, Fructosamine, 1,5-AG and CGM-measured Glucose Indices
| Mean±SD | Median (interquartile range) | Range | |
|---|---|---|---|
| N=26 | |||
| HbA1c (%) 1 | 7.7 ± 0.7 | 7.8(7.4, 8.2) | 6.1 to 9.4 |
| Glycated albumin (%) 1 | 22 ± 4 | 23(20, 25) | 16 to 30 |
| Fructosamine (umol/L) 1 | 318 ± 42 | 313 (293, 344) | 241 to 427 |
| 1,5-AG (ug/mL) 1,2 | 4.7 ± 2.2 | 4.2 (3.4, 6.1) | 1.2 to 11.6 |
| N=21 | |||
| Mean glucose (mg/dL) | 168 ± 26 | 170 (143, 185) | 126 to 218 |
| % in range (71 to 180 mg/dL) | 58% ± 13% | 56% (48%, 70%) | 38% to 81% |
| % below 70 mg/dL | 3.7 ± 4.9% | 1.8% (0.6%, 5.0%) | 0.0% to 19% |
| % below 60 mg/dL | 1.3% ± 2.5% | 0.4% (0.0%, 1.4%) | 0.0% to 11% |
| % below 50 mg/dL | 0.2% ± 0.4% | 0.0% (0.0%, 0.1%) | 0.0% to 1.3% |
| Area under curve 70 mg/dL 3 | 0.3 ± 0.5 | 0.1 (0.0, 0.3) | 0.0 to 2.0 |
| Low blood glucose index (LBGI) 4 | 0.9 ± 0.9 | 0.6 (0.2, 1.1) | 0.1 to 3.6 |
| % above 180 mg/dL | 38% ± 15% | 40% (24%, 51%) | 10% to 62% |
| % above 200 mg/dL | 30% ± 14% | 32% (17%, 41%) | 4% to 55% |
| % above 250 mg/dL | 13% ± 9% | 10% (6%, 18%) | 0% to 34% |
| Area under curve 180 mg/dL 5 | 23.9 ± 13.8 | 20.6 (13.3, 30.8) | 1.9 to 54.0 |
| High blood glucose index (HBGI) 6 | 9.0 ± 4.1 | 9.1 (5.6, 11.0) | 2.4 to 17.4 |
| Coefficient variation (%) 7 | 0.39 ± 0.07 | 0.37 (0.35, 0.42) | 0.30 to 0.60 |
| Mean amplitude glucose excursion (MAGE) (mg/dL) 8 | 131 ± 28 | 132 (112, 153) | 79 to 185 |
| Absolute rate of change (mg/dL/min) | 0.77 ± 0.15 | 0.75 (0.70, 0.85) | 0.46 to 1.03 |
Abnormal values are as follows: HbA1c >6.0%, glycated albumin >16%, fructosamine >285 umol/L, and 1,5-AG <10.7 ug/dL for males and <6.8 ug/dL for females. Data were provided by the DirecNet Central Laboratory.
Mean± SD = 4.7±2.4 for males and for 4.8±1.7 females in this study
Total area below 70 mg/dL; reflects both percentage and severity of glucose values in the hypoglycemic range
Low blood glucose index (15)
Total area above 180 mg/dL.
High blood glucose index (15)
Standard deviation divided by mean glucose
Mean amplitude glucose excursion (16)
Results
The 26 subjects had a mean ± SD age of 10.6 ± 5.1 years (range 4.6 to 17.7 years), with 8 (31%) female and 24 (92%) white. Median duration of T1D was 4.5 years (interquartile range 2.6 to 6.6 years) and median BMI percentile was 81 (interquartile range 67 to 89). For the four measures of glycemic control, mean (SD) was 7.7 (0.7) % for HbA1c, 4.7 (2.2) ug/mL for 1,5-AG, 22% (4) for glycated albumin, and 318 (42) umol/L for fructosamine (Table 1). With CGM, the median of the subjects’ mean glucose levels was 170 mg/dL, with the median percentage of values in the range of 71 to 180 mg/dL being 56%, median ≤70 mg/dL being 1.8%, and median >180 mg/dL being 40% (see Table 1 for additional indices and see supplemental website table 1 for the full distribution of each measure).
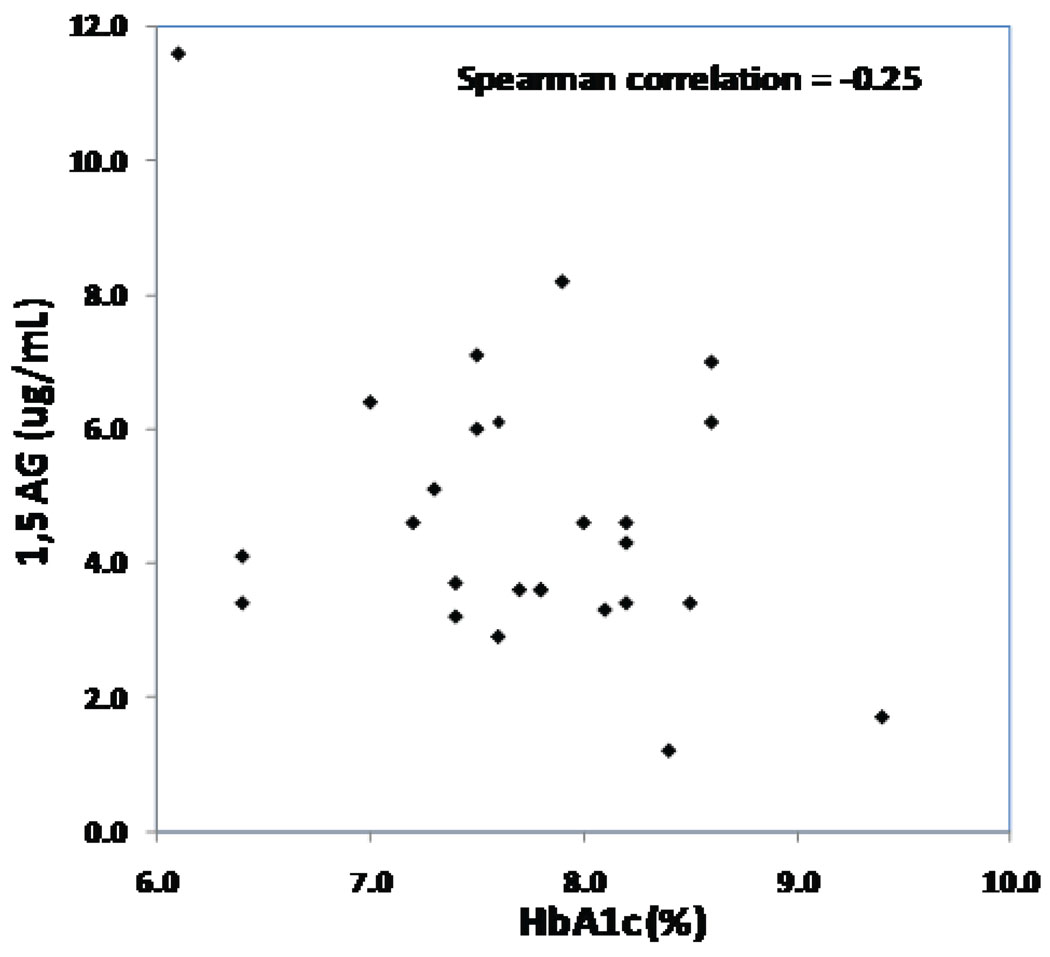
The intercorrelations of HbA1c, 1,5-AG, glycated albumin, and fructosamine are shown in Table 2. Glycated albumin and fructosamine levels had a higher correlation with each other (absolute r value = 0.86) than with HbA1c (absolute r value =0.57 and 0.56, respectively). The correlation of 1,5-AG with HbA1c was lower (absolute r value =0.25, Figure 1). All four measures had a similar degree of correlation with CGM-measured mean glucose (absolute r value =0.50 to 0.56), with hyperglycemic AUC-180 (0.50 to 0.60) and with CV (0.10 to 0.22). Limiting the analyses to subjects with HbA1c levels ≤8.0% did not improve the correlation of 1,5-AG with mean glucose and hyperglycemic AUC-180.
Table 2.
Spearman Correlation Matrix of Lab Results and Glucose Indices Measured with CGM 1
| Glycated Albumin (%) |
Fructos- amine (umol/L) |
1,5-AG 2 (ug/mL) |
Mean glucose (mg/dL) |
Hyperglycemic AUC-180 |
CV | |
|---|---|---|---|---|---|---|
| HbA1c (%) | 0.57 | 0.56 | 0.25 2 | 0.56 | 0.50 | 0.13 2 |
| Glycated Albumin (%) | 1 | 0.86 | 0.45 2 | 0.52 | 0.56 | 0.22 |
| Fructosamine (umol/L) | 0.86 | 1 | 0.41 2 | 0.50 | 0.50 | 0.14 |
| 1,5-AG (ug/mL) 2 | 0.45 2 | 0.41 2 | 1 | 0.54 2 | 0.60 2 | 0.10 2 |
| Mean glucose (mg/dL) | 0.52 | 0.50 | 0.54 2 | 1 | 0.93 | 0.28 2 |
| Hyperglycemic AUC-180 | 0.56 | 0.50 | 0.60 2 | 0.93 | 1 | 0.04 2 |
| CV | 0.22 | 0.14 | 0.10 2 | 0.28 2 | 0.04 2 | 1 |
N=26 for the correlations of HbA1c, glycated albumin, fructosamine and 1,5-AG with each other. N=21 for correlations involving CGM glucose indices.
Negative correlation. For simplicity of viewing, the negative signs have been omitted. All correlations with 1,5AG are negative because lower is more abnormal for 1,5-AG and higher is more abnormal for the other measures.
Figure 1. 1,5 AG versus HbA1c (N=26).
Below is the scatterplot of 1,5-AG versus HbA1c. The analysis included 26 subjects who had both 1,5-AG and HbA1c measures.
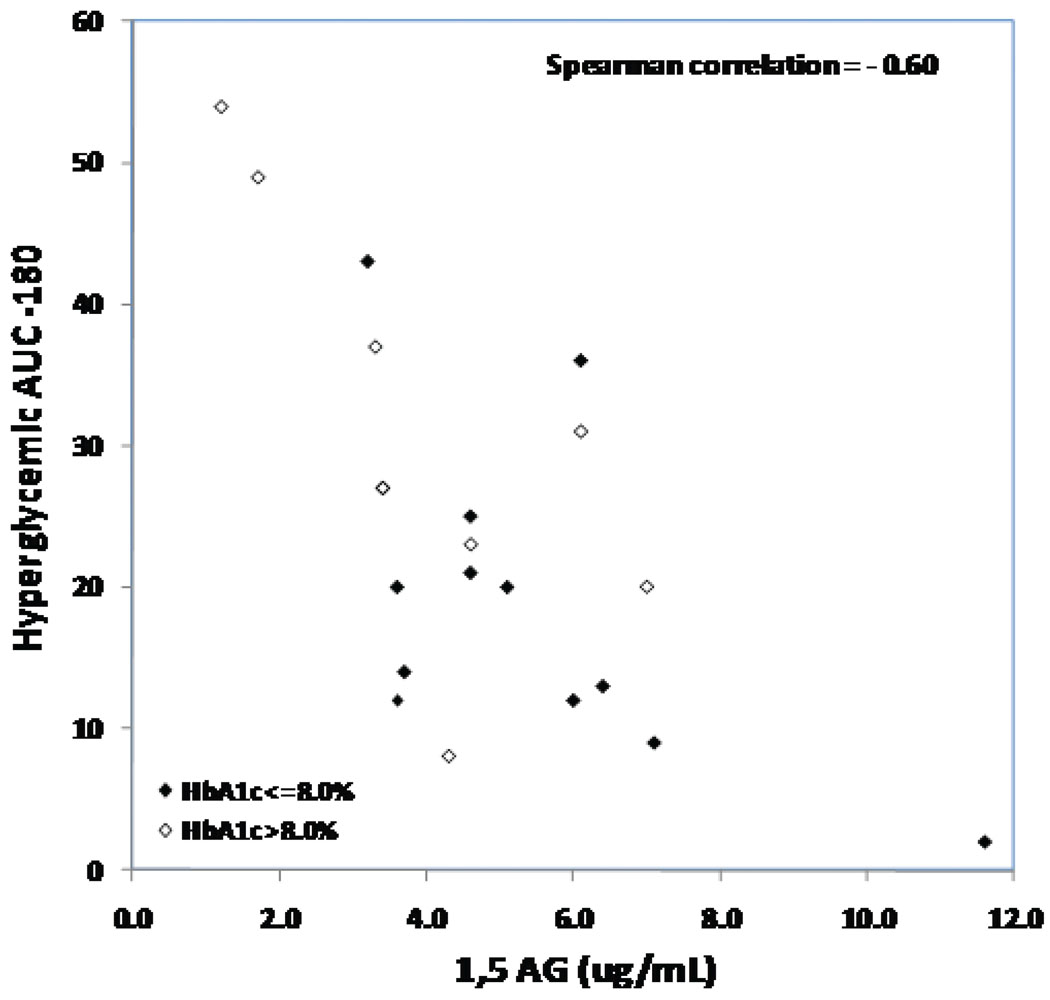
In multivariate analyses, fructosamine was the laboratory measure most strongly associated with HbA1c (r-squared = 0.44), while glycated albumin provided a similar result (r-squared = 0.38). Because fructosamine and glycated albumin were highly correlated, a model with both of these as covariates did not improve the r-square meaningfully compared with either factor by itself. Adding 1,5-AG to the model also did not increase the r-squared meaningfully. In the multivariate models for CGM indices, HbA1c was most strongly associated with mean glucose (r-squared = 0.37) and 1,5-AG was most strongly associated with hyperglycemic AUC-180 (r-squared=0.50, Figure 2); in both models, no additional factors increased the r-squared a meaningful amount.
Figure 2. Hyperglycemic AUC-180 versus 1,5 AG (N=21).
Below is the scatterplot of Hyperglycemic AUC −180 for glucose above 180 mg/dL versus 1,5-AG with different symbols for subjects with HbA1c ≤8.0% and >8.0%. The analysis included 21 subjects who had both lab measures and CGM data.
Discussion
We had hoped in performing these analyses to define a set of laboratory measures that would provide a better indicator of a T1D patient’s glycemic control than any one measure alone. Using CGM-measured mean glucose to define an individual’s level of glycemic control, we found that each of the laboratory measures (HbA1c, 1,5-AG, glycated albumin, and fructosamine) had a similar correlation with mean glucose which was not enhanced when other measures were included in the model. Similar results were found for predicting CGM-measured hyperglycemic AUC-180, which is not surprising since mean glucose and hyperglycemic AUC-180 are highly correlated.
We found a correlation of -0.60 between 1,5-AG and hyperglycemic AUC-180 measured by CGM in our study compared with -0.48 in the study of Dungan et al (7). However, in contrast to their study, we did not find that 1,5-AG levels had a substantially higher correlation with hyperglycemia than did HbA1c, glycated albumin, or fructosamine. In contrast with other studies, we found a lower correlation between 1,5-AG and HbA1c than reported by McGill et al and Moses et al (−0.25 versus approximately −0.60) (5, 14). This might be explained by small sample size and the narrow range of HbA1c in our study. In addition, our study was limited to patients with type 1 diabetes while other studies included either patients with both type 1 and type 2 diabetes (5) or solely type 2 diabetes (14). Another limitation of our study is the fact that HbA1c was measured before CGM data were obtained whereas the other laboratory measures were obtained following collection of CGM data. However, it is not likely that this was a meaningful cause of bias.
As far as we are aware, the current study is the first study on the association of 1,5-AG, HbA1c and CGM measured glucose in patients with diabetes less than 18 years old. Although we did not find enough evidence to support the use of 1,5-AG as a better marker of long-term glycemic control than HbA1c in this population, there may be an important role for 1,5-AG measurements in assessing short-term control. McGill et al reported in adults principally with type 2 diabetes that after a therapeutic change, 1,5-A,G levels showed a significant increase within 14 days (5, 14). In type 1 diabetes, monitoring of 1,5-A,G levels could be quite useful to quickly assess the effect of insulin dosing adjustments. Larger studies addressing this role for 1,5-AG in children with type 1 diabetes are warranted.
Supplementary Material
Acknowledgements
Asahi Kasei Pharma provided the materials needed for the glycated albumin assay. GlycoMark Inc. provided the materials needed for the 1,5-anhydroglucitol assay.
Appreciation is expressed for the work performed by the CRC Nurses at the five clinical centers.
Appendix
Writing Committee
Roy Beck, MD, PhD; Michael Steffes, MD, PhD; Dongyuan Xing, MPH; Katrina Ruedy, MSPH; Nelly Mauras, MD; Darrell M. Wilson, MD; Craig Kollman, PhD and the Diabetes Research in Children Network (DirecNet) Study Group
The DirecNet Study Group
Clinical Centers: (Listed in alphabetical order with clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator and (C) for Coordinators.) (1) Barbara Davis Center for Childhood Diabetes, University of Colorado, Denver, CO: H. Peter Chase, MD (PI); Rosanna Fiallo-Scharer, MD (I); Laurel Messer, RN (C); (2) Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA: Eva Tsalikian, MD (PI); Michael J. Tansey, MD (I); Julie Coffey, MSN (C); Joanne Cabbage (C); (3) Nemours Children’s Clinic, Jacksonville, FL: Tim Wysocki, PhD, ABPP (PI); Nelly Mauras, MD (I); Larry A. Fox, MD (I); Keisha Bird, MSN (C); Kim Englert, RN (C); (4) Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce A. Buckingham, MD (PI); Darrell M. Wilson, MD (I); Paula Clinton, RD, CDE (C); Kimberly Caswell, APRN (C); (5) Department of Pediatrics, Yale University School of Medicine, New Haven, CT: Stuart A. Weinzimer, MD (PI); William V. Tamborlane, MD (I); Amy Steffen (C); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; Dongyuan Xing, MPH; Alyssa Baptista; University of Minnesota Central Laboratory: Michael W. Steffes, MD, PhD; Jean M. Bucksa, CLS; Maren L. Nowicki, CLS; Carol A. Van Hale, CLS; Vicky Makky, CLS; National Institutes of Health: Gilman D. Grave, MD; Mary Horlick, PhD, Karen Teff, PhD; Karen K. Winer, MD;
Footnotes
Financial Disclosures
Financial support: This research was supported by the following NIH/NICHD Grants: HD041919-01; HD041915-01; HD041890; HD041918-01; HD041908-01; and HD041906-01. Clinical Centers also received funding through the following GCRC Grant Numbers M01 RR00069; RR00059; RR 06022 and RR00070-41.
Contributor Information
Roy Beck, Jaeb Center for Health Research, Tampa, FL, US, 33647.
Michael Steffes, University of Minnesota, Minneapolis, MN 55455.
Dongyuan Xing, Jaeb Center for Health Research, Tampa, FL, US, 33647.
Katrina Ruedy, Jaeb Center for Health Research, Tampa, FL, US, 33647.
Nelly Mauras, Nemours Children’s Clinic, Jacksonville, FL 32256.
Darrell M. Wilson, Stanford University, Stanford, CA, 94305.
Craig Kollman, Jaeb Center for Health Research, Tampa, FL, US, 33647, and the Diabetes Research in Children Network (DirecNet) Study Group*.
References
- 1.Buse JB, Freeman JLR, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-Anhydroglucitol (GlycoMark™): A Short-Term Glycemic Marker. Diabetes Technol Ther. 2003;5:355–363. doi: 10.1089/152091503765691839. [DOI] [PubMed] [Google Scholar]
- 2.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976;295:417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 3.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 4.Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18:440–447. doi: 10.2337/diacare.18.4.440. [DOI] [PubMed] [Google Scholar]
- 5.McGill JB, Cole TG, Nowatzke W, Houghton S, Ammirati EB, Gautille T, et al. Circulating 1,5-Anhydroglucitol Levels in Adult Patients With Diabetes Reflect Longitudinal Changes of Glycemia. Diabetes Care. 2004;27:1859–1865. doi: 10.2337/diacare.27.8.1859. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi S, Uchino H, Shimizu T, Kanazawa A, Tamura Y, Sakai K, et al. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J. 2007;54:139–144. doi: 10.1507/endocrj.k06-103. [DOI] [PubMed] [Google Scholar]
- 7.Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, et al. 1,5-Anhydroglucitol and Postprandial Hyperglycemia as Measured by Continuous Glucose Monitoring System in Moderately Controlled Patients With Diabetes. Diabetes Care. 2006;29:1214–1219. doi: 10.2337/dc06-1910. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto M, Yamasaki Y, Kubota M, Arai K, Morishima T, Kawamori R, et al. 1,5-Anhydro-D-glucitol evaluates daily glycemic excursions in well-controlled NIDDM. Diabetes Care. 1995;18:1156–1159. doi: 10.2337/diacare.18.8.1156. [DOI] [PubMed] [Google Scholar]
- 9.Yamanouchi T, Akanuma Y. Serum 1,5-anhydroglucitol (1,5 AG): New clinical marker for glycemic control. Diabetes Res Clin Pract. 1994;24:S261–S268. doi: 10.1016/0168-8227(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 10.Yamanouchi T, Minoda S, Yabuuchi M, Akanuma Y, Akanuma H, Miyashita H, et al. Plasma 1,5-anhydro-D-glucitol as new clinical marker of glycemic control in NIDDM patients. Diabetes. 1989;38:723–729. doi: 10.2337/diab.38.6.723. [DOI] [PubMed] [Google Scholar]
- 11.Yamanouchi T, Ogata N, Tagaya T, Kawasaki T, Sekino N, Funato H, et al. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. The Lancet. 1996;347:1514–1518. doi: 10.1016/s0140-6736(96)90672-8. [DOI] [PubMed] [Google Scholar]
- 12.GlycoMark®. GlycoMark Assay Overview[Internet]. c2008. [cited 2010 Nov 15] Available from: http://www.glycomark.com/lab/index.asp.
- 13.Diabetes Research in Children Network (DirecNet) Study Group. Blunted Counterregulatory Hormone Responses to Hypoglycemia in Young Children and Adolescents With Well-Controlled Type 1 Diabetes. Diabetes Care. 2009;32:1954–1959. doi: 10.2337/dc08-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moses AC, Raskin P, Khutoryansky N. Does serum 1,5-anhydroglucitol establish a relationship between improvements in HbA1c and postprandial glucose excursions? Supportive evidence utilizing the differential effects between biphasic insulin aspart 30 and insulin glargine. Diabet Med. 2008;25:200–205. doi: 10.1111/j.1464-5491.2008.02384.x. [DOI] [PubMed] [Google Scholar]
- 15.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke W. Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care. 1997;20:1655–1658. doi: 10.2337/diacare.20.11.1655. [DOI] [PubMed] [Google Scholar]
- 16.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.