Abstract
Background
Deficits in automatic sensory discrimination, as indexed by a reduction in the mismatch negativity (MMN) and P3a event-related potential amplitudes, are well documented in chronic schizophrenia. However, MMN and P3a have not been sufficiently studied early in the course of psychotic illness. The present study aimed to investigate MMN, P3a, and reorienting negativity (RON) across the course of schizophrenia, from prodrome to the chronic phase of illness.
Methods
MMN, P3a, and RON were assessed in 118 subjects across 4 groups 1) prodromal patients putatively at risk for psychosis (N=26), 2) recent-onset patients (N=31), 3) chronic patients (N=33), and 4) normal controls (N=28) during a duration-deviant auditory oddball paradigm.
Results
Frontocentral deficits in MMN and P3a were present in all patient groups. The at-risk group's MMN and P3a amplitudes were intermediate between those of the control and recent-onset groups. The recent-onset and chronic groups, but not the at-risk group, showed significant RON amplitude reductions, relative to the control group. Associations between MMN, P3a, RON and psychosocial functioning were present in the chronic group only. Impaired P3a and RON correlated with more severe negative symptoms in the at-risk group.
Conclusions
Abnormalities in the automatic processes of sensory discrimination, orienting and reorienting of attention are evident in the early phases of schizophrenia and raise the possibility of progressive worsening across stages of the illness. The finding that MMN and P3a, but not RON, were reduced before psychosis onset supports the continued examination of these components as potential early biomarkers of schizophrenia.
Introduction
It is well documented that cognitive impairment is a hallmark of schizophrenia (Heaton et al., 2001, Palmer et al., 2009) and a better predictor of global functioning than clinical symptomatology (Green, 1996, Green et al., 2000). Disturbances in multiple neurocognitive domains have been reported in the first episode of schizophrenia (Addington et al., 2005, Bilder et al., 2000, Mesholam-Gately et al., 2009) as well as in the prodrome (Eastvold et al., 2007, Jahshan et al., 2010, Lencz et al., 2006, Seidman et al., 2010). The prodrome is the period that precedes illness onset and is characterized by a marked deviation from a person's normal level of functioning (Yung and McGorry, 1996). An emerging view is that the commonly observed clinical and cognitive deficits of schizophrenia patients may arise, at least in part by dysfunction in the coordination of neural activity at the earliest stages of sensory and cognitive information processing (Green and Nuechterlein, 1999, Javitt, 2009, Light et al., 2006). Schizophrenia patients exhibit deficits in basic levels of sensory information processing (Leitman et al., 2010, Turetsky et al., 2009). Those general sensory processing abnormalities are present early in the course of the illness and even precede the emergence of psychotic symptoms (Cadenhead et al., 2005, Quednow et al., 2008). Event-related potentials (ERP) allow investigators to interrogate early sensory processes, including sensory discrimination and the orienting and subsequent reorienting of attention, which occur outside of an individual's awareness or conscious control (Callaway and Naghdi, 1982, Ford et al., 2010, Holig and Berti, 2010, Rissling et al., 2010).
In a passive auditory oddball paradigm, a duration-deviant stimulus elicits a mismatch negativity (MMN) response that peaks 100 to 200 ms after the onset of a stimulus deviance (Naatanen et al., 1978). As MMN can be elicited even when participants do not attend to the stimuli, it is assumed to reflect an automatic, sensory-based deviance detection process (Picton et al., 2000), although some studies have shown that MMN and deviance-related negative (DRN) components can be attenuated by strongly focused attention to some other stimulus sequence (Woldorff et al., 1991, 1998).
Deficits in MMN generation using a variety of stimulation parameters (e.g., oddball stimuli that differ in pitch or duration) represent a remarkably robust finding in chronic schizophrenia (Javitt et al., 2000a, Kiang et al., 2009, Light and Braff, 2005a, b, Mathalon et al., 2000, Shelley et al., 1991, Umbricht and Krljes, 2005). Nevertheless, the extant literature on MMN in the early stages of the disease is mixed, with some studies identifying abnormalities (Devrim-Ucok et al., 2008, Hermens et al., 2010, Javitt et al., 2000b) and others failing to detect any significant decrements in either duration or pitch MMN in patients with a psychotic illness duration of less than three years (Salisbury et al., 2002, Umbricht et al., 2006, Valkonen-Korhonen et al., 2003). In a prospective study of first-hospitalized patients with schizophrenia (Salisbury et al., 2007), a strong relationship was found between the progressive reductions of MMN amplitude and left hemisphere Heschl gyrus gray matter volume. To date, only one study has assessed MMN in individuals in the prodromal phase of schizophrenia (Brockhaus-Dumke et al., 2005). Duration MMN amplitudes were slightly lower in at-risk patients compared to normal controls but this difference did not reach statistical significance. The clarification of the extent of MMN deficits in the prodrome contributes to the overall efforts to identify potential markers of vulnerability to schizophrenia and to understand the underlying pathological processes leading to the development of the illness.
Recent studies have also demonstrated that MMN is highly associated with psychosocial functioning in schizophrenia patients (Kawakubo et al., 2007, Light and Braff, 2005b, Rasser et al., 2011) as well as healthy subjects (Light et al., 2007). Yet, only two studies have examined this relationship in first-episode patients; one showed that larger duration MMN is associated with a better score on a quality of life measure (Hermens et al., 2010) while the other did not find a relationship between pitch MMN and social functioning (Salisbury et al., 2007). Given the observation that the at-risk patients who do not convert to psychosis still show substantial impairment in social functioning at outcome (Ballon et al., 2007), it is important to determine whether MMN is associated with the degree of functional disability in the early stage of illness.
In the ERP paradigm, the MMN is often followed by the P3a, a frontocentral positivity peaking between 250–300 ms. The P3a component is assumed to reflect the covert orienting or shift in attention (Friedman et al., 2001). Several studies have found P3a amplitude in response to infrequent nontarget or distracter stimuli to be decreased in schizophrenia patients (Grillon et al., 1990a, 1990b, Grzella et al., 2001, Mathalon et al., 2000). Nonetheless, only one study has examined P3a in the early stages of the illness, finding impairment in first-episode psychosis patients (Valkonen-Korhonen et al., 2003). To our knowledge, P3a has yet to be examined in subjects at risk for developing psychosis.
Automatic sensory discrimination and covert shifting of attention are important to our understanding schizophrenia, as is the reorienting of attention or the automatic preparation for detecting subsequent stimulus changes (Naatanen et al., 1982). This attentional reorienting or automatic preparation is reflected in an automatically elicited negativity following the P3a, peaking at latencies between 400–600 ms, and centered on frontocentral electrodes (Otten et al., 2000, Schroger et al., 2000). Schröger and Wolff (1998) were the first to refer to this component as the reorienting negativity (RON). RON has been considered an automatically elicited response component during active auditory (Schroger et al., 2000) and visual (Escera et al., 1998, Escera et al., 2001) discrimination tasks (Escera and Corral, 2007). To date, there are no published reports of RON in schizophrenia spectrum populations.
The MMN, P3a and RON complex provides a serial, hierarchical neurophysiological index of the cascade of three main processes involved in involuntary attention control (automatic change detection, orienting of attention, and reorienting of attention) following deviant stimuli (Berti et al., 2004, Horvath et al., 2008). When examined separately, those components may index discrete processes with dissociable underlying neural and genomic substrates as well as relationships to symptoms and functional outcome (Braff and Light, 2004, Light and Braff, 2005a, Light et al., 2010).
The primary aim of the present study was to examine the MMN/P3a/RON response complex across different stages of schizophrenia by assessing the extent of MMN, P3a, and RON amplitude reductions in 1) at-risk, 2) recent-onset, and 3) chronic schizophrenia patients relative to normal controls using a cross-sectional design. We hypothesized that MMN, P3a, and RON amplitudes would be significantly reduced in recent-onset and chronic patients relative to normal controls and that the amplitudes of the at-risk subjects would lie in between those of the normal controls and those of the recent-onset patients. The secondary hypothesis was that MMN, P3a, and RON deficits would be associated with symptoms and social functioning impairment within the patient groups.
Method and Materials
Subjects
At-risk subjects (n=26), recent-onset patients (n=31), chronic patients (n=33), and normal control subjects (n=28) were enrolled in the study. At-risk and recent-onset patients were selected from a pool of individuals who were participating in the Cognitive Assessment and Risk Evaluation (CARE) program at UCSD and referred to the Schizophrenia Program laboratory for EEG testing. They were help-seeking and receiving treatment as usual (pharmacological or psychosocial) according to their presenting symptoms. Chronic patients were recruited from community residential facilities and via physician referral. Normal control subjects were recruited through newspaper advertisements and flyers posted at the UCSD medical center. Subjects over the age of 18 years were asked to give informed consent. Those below the age of 18 (N=12) provided assent, and their guardian was asked to sign a consent form for study participation. All participants were tested on a passive auditory oddball paradigm and scheduled for a short clinical evaluation on the day (if possible), or within a month, of their EEG recording session.
The Structured Interview for Prodromal Symptoms (SIPS; Miller et al., 2003) was used to identify subjects at risk for psychosis and measure the severity of prodromal symptoms. The majority of the at-risk subjects met criteria for at least one of the two most common prodromal syndromes (Seeber and Cadenhead, 2005, Yung et al., 2005) per the SIPS: “Attenuated Positive Symptom” (new onset of subsyndromal psychotic symptoms) and “Genetic Risk and Deterioration” (family history of schizophrenia in a first-degree relative or criteria for schizotypal personality disorder met in patient, associated with a decline in global functioning over the past year). The recent-onset and chronic patients had a DSM-IV diagnosis of schizophrenia and a mean duration of illness of 1.2 ± 0.82 and 10.7 ± 3.3 years respectively. Normal control subjects were not included if they had a history of mental illness or learning disability, Cluster A personality disorder or prodromal symptoms, a family history of psychotic illness, or a history of taking psychotropic medications.
Axis I and axis II diagnoses were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1995) and the Structured Interview for DSM-IV Personality Disorders (Pfohl et al., 1995) respectively. The Kiddie-Schedule for Affective Disorders and Schizophrenia (Chambers et al., 1985) was administered to patients under the age of 16 (N=6). Subjects with a history of head injury, seizures, neurological disorder, or an IQ below 80 were excluded from the study. Those who met DSM-IV criteria for lifetime substance abuse/dependence were included in the sample unless they endorsed having used substances during the month preceding neurophysiological testing or their urine toxicology test results were positive.
Clinical symptoms were evaluated using the Scales for the Assessment of Positive Symptoms (SAPS; Andreasen et al., 1977) and Negative Symptoms (SANS; Andreasen, 1984). Current level of functioning was assessed with the Modified Global Assessment of Functioning (Hall and Parks, 1995). Family history of psychiatric illness was assessed, after receiving consent to contact a relative, using the Family History Research Diagnostic Criteria (Andreasen et al., 1977). Six at-risk and 3 recent-onset patients had a first-degree relative with psychosis. Seven at-risk, 25 recent-onset, and 31 chronic patients were on at least one atypical antipsychotic with or without other psychotropic medications at the time of testing. These at-risk subjects were prescribed antipsychotics for their attenuated positive symptoms or other mood problems that required treatment. Two of the 26 at-risk subjects transitioned to psychosis; one converted to psychotic mania and one to schizophrenia 25 days and one year after their ERP testing, respectively.
Neurophysiological Testing
Electroencephalographic recordings were acquired with a Neuroscan NuAmp system. EEG was recorded from the scalp using 34 electrodes attached to an electrode cap. The following 34 equidistant electrode positions were used: Fp1, Fp2, Fz, F3, F4, F7, F8, FC1, FC2, FC5, FC6, Cz, C3, C4, CP1, CP2, CP5, CP6, Pz, P3, P4, P7, P8, O1, O2, PO9, PO10, Iz, T1, T2, T7, T8, TP9, and TP10. A reference electrode was placed at the nose tip, in addition to a ground electrode at Fpz. Four additional electrodes were placed above and below the left eye as well as at the outer canthi of both eyes in order to monitor blinks and eye movements. EEG was digitally referenced off-line to linked mastoids (TP9/TP10). All impedances were kept below 4 kΩ. Signals were digitized at a rate of 1 kHz with system acquisition filter settings at 0.5 – 100 Hz. Subjects were presented with binaural tones (1 kHz 85 dB sound pressure level, with 1 ms rise/fall) with a fixed stimulus onset-to-onset asynchrony of 500 ms using a San Diego Instruments ERP-Lab system. Standard (90% probability; 50 ms duration) and deviant (10% probability; 100 ms duration) tones were presented in pseudorandom order using foam insert earphones. During EEG recording, subjects were instructed to watch a silent cartoon video. EEG acquisition was terminated when a minimum of 225 artifact-free deviant trials were collected. Data processing was performed offline using automated procedures. Continuous recordings were mathematically corrected for eye movement artifact employing standard procedures (Semlitsch et al., 1986). Continuous data were divided into epochs relative to the onset of stimuli (−100 to 500 ms) and centered at the mean of the prestimulus baseline. Following blink correction, epochs containing more than ± 50 μV in frontal recording sites were automatically rejected. MMN waveforms were generated by subtracting the ERP waveforms in response to standard tones from the waveforms elicited by the deviant tones. The resultant difference waves were low-pass filtered at 20 Hz (zerophase shift, 24 dB/octave rolloff) to remove any residual high-frequency artifact consistent with established methods (Kiang et al., 2009, Light and Braff, 2005a, b, Light et al., 2007). ERP component peaks were manually identified using butterfly plots, mean global field power, topographical inspection, and comparison of individual frontocentral and mastoid electrodes. This allowed for the confirmation of polarity inversion at mastoid electrodes for MMN analyses. Search windows for peak MMN, P3a, and RON were 135–205, 250–350, and 350–500 ms, respectively. Mean amplitudes in the 25 ms surrounding the identified peaks were then automatically calculated for each electrode.
Statistical Analyses
In order to investigate group differences in MMN, P3a, and RON amplitudes, a repeated measures analysis of variance (ANOVA) with electrode site as the within-subject variable and diagnostic group as the between-subject variable was performed for each component. Greenhouse-Geisser adjustments were used in the repeated measures ANOVAs that contained more than one degree of freedom. Significant group × electrode interactions were decomposed using a series of oneway ANOVAs to assess group effects at each of the frontocentral electrodes. Cohen's d was also calculated to further characterize group differences and minimize the reliance on p-values for interpreting potential effects. Relationships between MMN, P3a, and RON amplitudes at frontocentral sites and GAF, SAPS, SANS, and SIPS ratings were investigated using Spearman rank correlations. Only significant correlations at contiguous electrodes were reported.
Results
Sample Characteristics
There were significant group differences in age (F[3,114]=47.75, p<.001) and education (F[3,113]=3.76, p=.01). As expected, the chronic schizophrenia group was significantly older than all the other groups (p<.001) and had significantly fewer years of education than the normal control group (p=.02). The at-risk group was also significantly younger than the recent-onset group (p=.04). There were no significant group differences in ethnicity (X2[12]=15.37, p=.22) and handedness (X2[6]=7.03, p=.32) but there were significantly more males than females in the patient groups relative to the normal control group (X2[3]=17.34, p=.001). As expected, there were significant differences in SAPS (F[2,81]=11.38, p<.001), SANS (F[2,80]=10.53, p<.001), and GAF (F[2,80]=5.47, p=.006) among the patient groups, with significantly less severe positive symptoms in the at-risk relative to the recent-onset (p=.006) and chronic (p<.001) groups. Similarly, the chronic group had significantly more severe negative symptoms (SANS) than the recent-onset (p=.006) and at-risk (p<.001) groups and more impairment in global functioning (GAF) than the at-risk group (p=.004) (Table 1).
Table 1.
Demographic and Clinical Characteristics
| Normal Controls (N=28) | At Risk for Psychosis (N=26) | Recent-Onset Schizophrenia (N=31) | Chronic Schizophrenia (N=33) | |
|---|---|---|---|---|
| Age (Mean/SD) | 21.04 (4.43) | 19.15 (3.39) | 21.90 (3.71) | 29.82 (3.56) |
| Range | 12 to 30 | 13 to 29 | 14 to 33 | 24 to 35 |
| Gender (% Male) | 39.3% | 65.4% | 83.9% | 81.8% |
| Ethnicity (% Caucasian) | 64.3% | 46.2% | 54.8% | 60.6% |
| Handedness (% Right) | 100% | 80.8% | 83.9% | 84.8% |
| SIPS Positive (Mean/SD) | 6.82 (5.40) | |||
| SIPS Negative (Mean/SD) | 11 (7.10) | |||
| SIPS Disorganized (Mean/SD) | 4.59 (3.43) | |||
| SIPS General (Mean/SD) | 6.50 (5.38) | |||
| SAPS (Mean/SD) | 3.81 (3.36) | 7.07 (4.27) | 8.55 (2.96) | |
| SANS (Mean/SD) | 7.20 (5.14) | 9.40 (5.88) | 13.73 (4.89) | |
| GAF (Mean/SD) | 51 (11.76) | 46.17 (10.0) | 42.45 (6.41) |
Given the significant group differences in age and results from our preliminary analyses showing that older age was associated with smaller MMN (r= .36 to .57; Fp1, Fp2, F7, F8, Fz, F3, F4, FC2), P3a (r= −.35 to −.53; Fp2, F8, F4, C4), and RON (r= .38 to .46; Fz, F3, F4, FC1, FC2, Cz) activity in the chronic group (p<.05), we included age as a covariate in each of the subsequent analyses. However, age was not a significant covariate for any of the components (main effect of age: F= .17 to .52, p>.45; electrode × age interaction: F=.70 to 1.99, p>.10) and did not affect the results. Therefore, we decided to exclude it from further analyses. Moreover, in order to control for possible gender confounds, we repeated the analyses below after including gender as another between-subject variable. No significant main effects or interactions with gender were present.
Group Differences in MMN Amplitudes
The repeated measures ANOVA revealed a significant effect of electrode (F[2.56, 291.48]=280.24, p<.001) indicating a frontal maxima in the MMN amplitude distribution, with the expected polarity inversion of responses at temporo-parietal and other posterior electrodes. There was a significant group × electrode interaction (F[7.67,291.48]=3.60, p=.001) indicating the presence of deficits at frontocentral but not temporo-parietal sites. The follow-up ANOVAs revealed significant group differences at p<.01 at all frontocentral electrodes, with the largest effect size differences at FC1. Relative to the normal control group, the chronic (d=−.78 to −1.78) and recent-onset (d=−.52 to −.92) groups had large effect-size MMN decrements at frontocentral recording sites (Fp1, F7, F8, Fz, F3, F4, FC1, FC2, FC5, FC6, C3, Cz, C4, CP1, CP2, CP5, and CP6; p<.01). Similarly, the at-risk group had significant medium effect-size (d= −.49 to −.64) MMN reductions at F3, FC1, FC2, FC5, FC6, CP5, and CP6 (p<.05) relative to the normal control group. There were no significant MMN amplitude differences between the at-risk and recent-onset groups. However, the at-risk group had significantly smaller MMN amplitudes at all frontocentral electrodes (p<.01) relative to the chronic group. There were no significant group differences in peak MMN latency (F[3, 114]=2.29, p=.08, M=182.52, SD=20.0). Mean MMN amplitudes, standard deviations, and effect sizes for each electrode site are presented in Table 2.
Table 2.
Descriptive Statistics and Effect Sizes of MMN Relative to Normal controls
| Group | Effect Size | ||||||
|---|---|---|---|---|---|---|---|
| Electrode | Normal Controls (N=31) | At Risk for Psychosis (N=26) | Recent-Onset Schizophrenia (N=28) | Chronic Schizophrenia (N=33) | AR | RO | SZ |
| Fp1 | −1.66 (0.99) | −1.26 (1.02) | −0.98 (0.75) | −0.83 (0.82) | −0.42 | −0.72 | −0.88 |
| Fp2 | −1.68 (1.07) | −1.46 (0.99) | −1.18 (0.83) | −0.93 (0.82) | −0.23 | −0.52 | −0.78 |
| F7 | −1.73 (1.05) | −1.35 (1.01) | −1.08 (0.90) | −0.68 (0.81) | −0.38 | −0.64 | −1.04 |
| F8 | −1.81 (1.28) | −1.34 (1.35) | −0.99 (0.96) | −0.65 (0.76) | −0.40 | −0.71 | −1.00 |
| Fz | −4.47 (2.19) | −3.54 (1.73) | −2.77 (1.72) | −2.04 (1.55) | −0.46 | −0.85 | −1.78 |
| F3 | −3.89 (1.98) | −2.95 (1.42) | −2.28 (1.54) | −1.74 (1.38) | −0.53 | −0.91 | −1.21 |
| F4 | −4.03 (2.02) | −3.19 (1.60) | −2.56 (1.62) | −1.83 (1.28) | −0.46 | −0.81 | −1.21 |
| FC1 | −4.71 (2.50) | −3.64 (1.88) | −2.69 (1.95) | −2.04 (1.48) | −0.49 | −0.92 | −1.22 |
| FC2 | −4.72 (2.48) | −3.64 (1.88) | −2.84 (1.95) | −1.98 (1.32) | −0.50 | −0.87 | −1.27 |
| FC5 | −3.15 (1.68) | −2.26 (1.56) | −1.75 (1.38) | −1.20 (1.16) | −0.56 | −0.87 | −1.22 |
| FC6 | −3.10 (1.95) | −2.19 (1.76) | −1.70 (1.48) | −1.10 (0.84) | −0.54 | −0.83 | −1.18 |
| C3 | −3.76 (2.11) | −2.90 (1.76) | −2.06 (1.77) | −1.40 (1.35) | −0.44 | −0.87 | −1.21 |
| Cz | −4.46 (2.68) | −3.53 (1.94) | −2.50 (2.10) | −1.64 (1.45) | −0.40 | −0.85 | −1.22 |
| C4 | −3.55 (2.16) | −2.90 (1.77) | −2.02 (1.74) | −1.25 (1.10) | −0.34 | −0.80 | −1.20 |
| CP1 | −3.19 (2.14) | −2.49 (1.66) | −1.63 (1.74) | −1.07 (1.19) | −0.37 | −0.83 | −1.13 |
| CP2 | −3.07 (2.02) | −2.45 (1.63) | −1.55 (1.67) | −0.91 (1.14) | −0.34 | −0.84 | −1.19 |
| CP5 | −1.94 (1.60) | −1.06 (1.35) | −0.65 (1.51) | −0.32 (1.11) | −0.58 | −0.85 | −1.07 |
| CP6 | −1.48 (1.75) | −0.72 (1.47) | −0.27 (1.33) | −.009 (0.88) | −0.52 | −0.82 | −1.00 |
| P7 | 0.10 (1.75) | 0.83 (1.27) | 0.87 (1.19) | 1.02 (1.18) | −0.53 | −0.56 | −0.67 |
| P3 | −1.89 (1.89) | −1.15 (1.29) | −0.63 (1.53) | −0.25 (1.09) | −0.47 | −0.80 | −1.04 |
| Pz | −2.10 (1.82) | −1.53 (1.37) | −0.83 (1.50) | −0.37 (1.07) | −0.36 | −0.80 | −1.09 |
| P4 | −1.44 (1.66) | −0.93 (1.30) | −0.40 (1.36) | −0.05 (1.00) | −0.36 | −0.73 | −0.98 |
| P8 | 0.32 (1.95) | 1.04 (1.30) | 1.27 (1.26) | 1.22 (1.13) | −0.49 | −0.65 | −0.62 |
| T7 | −0.93 (.97) | −0.15 (1.35) | −0.09 (1.25) | 0.28 (1.03) | −0.64 | −0.69 | −0.99 |
| T8 | −0.63 (1.49) | 0.09 (1.66) | 0.44 (1.00) | 0.44 (0.88) | −0.54 | −0.80 | −0.80 |
| TP9 | 1.06 (1.71) | 1.47 (1.19) | 1.42 (1.10) | 1.41 (1.08) | −0.32 | −0.28 | −0.27 |
| TP10 | 1.46 (1.55) | 2.07 (1.31) | 1.86 (1.10) | 1.77 (1.15) | −0.48 | −0.31 | −0.24 |
| T1 | 0.40 (0.86) | 0.81 (0.82) | 0.76 (0.78) | 0.86 (0.82) | −0.49 | −0.43 | −0.55 |
| T2 | 0.78 (0.95) | 1.34 (0.94) | 1.10 (0.74) | 1.19 (0.90) | −0.63 | −0.36 | −0.46 |
| PO9 | 0.90 (1.55) | 1.29 (1.26) | 1.17 (1.00) | 1.29 (1.08) | −0.32 | −0.22 | −0.32 |
| PO10 | 1.09 (1.44) | 1.46 (1.23) | 1.51 (1.07) | 1.52 (1.13) | −0.30 | −0.34 | −0.35 |
| O1 | 0.18 (1.49) | 0.60 (1.26) | 0.75 (1.13) | 0.93 (1.03) | −0.34 | −0.57 | −0.60 |
| O2 | 0.16 (1.67) | 0.59 (1.18) | 0.88 (1.05) | 1.05 (1.16) | −0.33 | −0.55 | −0.68 |
| Iz | 0.82 (1.32) | 1.21 (1.13) | 1.23 (1.01) | 1.45 (1.33) | −0.32 | −0.34 | −0.52 |
Group Differences in P3a Amplitudes
The repeated measures ANOVA revealed a significant effect of electrode (F[3.38, 385.84]=213.20, p<.001) indicating that the P3a response was maximal at frontocentral electrodes, as well as a significant group × electrode interaction (F[10.15, 385.84]=6.33, p<.001) indicating the presence of deficits at frontocentral but not temporo-parietal sites. The follow-up ANOVAs revealed significant group differences at p<.01 at all frontocentral electrodes, with the largest effect size differences at FC1. Both the chronic (d=.71 to 1.46) and recent-onset (d=.66 to 1.19) patients had significant P3a deficits at p<.01 at all frontocentral recording sites. The at-risk group had medium to large effect-size (d=.56 to .96) reductions in P3a amplitudes relative to the normal control group, which were significant at FC1, FC2, FC5, FC6, C3, Cz, CP1, CP2, CP5, and CP6 (p<.01) as well as F7, F8, Fz, F3, F4, and C4 (p<.05). Moreover, there were significant P3a amplitude differences between the at-risk and recent-onset groups at Fp1, F3, Fz, FC1, and FC2 (p<.05), as well as between the at-risk and chronic groups at Fz, FC1, FC2, C3, C4, CP1, CP2 (at p<.01) and F3, F4, FC5 (at p<.05). There were no significant group differences in peak P3a latency (F[3, 114]=.99, p=.40, M=276.53, SD=26.33). Mean P3a amplitudes, standard deviations, and effect sizes for each electrode site are presented in Table 3.
Table 3.
Descriptive Statistics and Effect Sizes of P3a Relative to Normal controls
| Group | Effect Size | ||||||
|---|---|---|---|---|---|---|---|
| Electrode | Normal Controls (N=31) | At Risk for Psychosis (N=26) | Recent-Onset Schizophrenia (N=28) | Chronic Schizophrenia (N=33) | AR | RO | SZ |
| Fp1 | 1.05 (0.92) | 0.75 (0.75) | 0.33 (0.53) | 0.51 (0.53) | 0.39 | 0.95 | 0.71 |
| Fp2 | 1.08 (1.08) | 0.76 (0.89) | 0.46 (0.73) | 0.51 (0.61) | 0.37 | 0.72 | 0.66 |
| F7 | 1.60 (0.82) | 1.01 (0.94) | 0.81 (0.91) | 0.62 (0.88) | 0.62 | 0.83 | 1.03 |
| F8 | 1.73 (1.24) | 1.00 (1.36) | 0.85 (1.02) | 0.60 (0.79) | 0.62 | 0.75 | 0.97 |
| Fz | 4.60 (1.95) | 3.38 (2.15) | 2.13 (1.58) | 2.02 (1.62) | 0.59 | 1.19 | 1.24 |
| F3 | 3.70 (1.53) | 2.66 (1.80) | 1.76 (1.31) | 1.70 (1.30) | 0.62 | 1.15 | 1.19 |
| F4 | 3.65 (1.72) | 2.60 (1.77) | 1.83 (1.40) | 1.69 (1.37) | 0.61 | 1.06 | 1.14 |
| FC1 | 5.54 (1.84) | 4.06 (2.61) | 2.81 (1.77) | 2.16 (1.82) | 0.62 | 1.15 | 1.43 |
| FC2 | 5.38 (1.91) | 3.86 (2.53) | 2.74 (1.83) | 2.24 (1.69) | 0.66 | 1.14 | 1.36 |
| FC5 | 3.36 (1.08) | 2.14 (1.61) | 1.75 (1.24) | 1.32 (1.28) | 0.81 | 1.07 | 1.36 |
| FC6 | 3.36 (1.74) | 1.89 (1.76) | 1.74 (1.44) | 1.19 (1.13) | 0.86 | 0.95 | 1.28 |
| C3 | 4.47 (1.57) | 2.91 (2.34) | 2.39 (1.52) | 1.55 (1.36) | 0.78 | 1.04 | 1.46 |
| Cz | 5.92 (2.10) | 4.14 (3.08) | 3.13 (2.01) | 2.27 (1.71) | 0.68 | 1.07 | 1.40 |
| C4 | 4.07 (1.82) | 2.91 (2.58) | 2.17 (1.59) | 1.41 (1.27) | 0.56 | 0.92 | 1.29 |
| CP1 | 4.34 (1.78) | 2.76 (2.76) | 2.34 (1.73) | 1.35 (1.25) | 0.72 | 0.92 | 1.37 |
| CP2 | 4.13 (1.83) | 2.72 (2.74) | 2.19 (1.75) | 1.30 (1.22) | 0.65 | 0.90 | 1.31 |
| CP5 | 2.65 (1.28) | 1.07 (2.15) | 1.21 (1.26) | 0.59 (1.08) | 0.96 | 0.88 | 1.26 |
| CP6 | 2.20 (1.51) | 0.99 (2.16) | 1.11 (1.46) | 0.44 (0.91) | 0.73 | 0.66 | 1.07 |
| P7 | 0.84 (1.24) | −0.18 (2.17) | 0.01 (1.12) | −0.28 (1.16) | 0.68 | 0.55 | 0.75 |
| P3 | 2.69 (1.47) | 1.26 (2.49) | 1.37 (1.55) | 0.60 (1.09) | 0.78 | 0.72 | 1.14 |
| Pz | 2.89 (1.68) | 1.68 (2.64) | 1.60 (1.70) | 0.79 (1.06) | 0.62 | 0.66 | 1.08 |
| P4 | 2.30 (1.59) | 1.06 (2.36) | 1.22 (1.52) | 0.50 (1.00) | 0.71 | 0.62 | 1.03 |
| P8 | 0.92 (1.42) | −0.25 (1.94) | 0.16 (1.33) | −0.23 (0.97) | 0.79 | 0.51 | 0.78 |
| T7 | 1.71 (1.10) | 0.54 (1.71) | 0.69 (1.22) | 0.20 (1.13) | 0.84 | 0.73 | 1.11 |
| T8 | 1.77 (1.64) | 0.63 (1.79) | 0.67 (1.46) | 0.16 (0.92) | 0.73 | 0.70 | 1.03 |
| TP9 | 0.06 (1.15) | −0.72 (1.70) | −0.36 (1.10) | −0.66 (1.05) | 0.61 | 0.33 | 0.56 |
| TP10 | 0.25 (1.31) | −0.59 (1.40) | −0.21 (1.22) | −0.46 (1.03) | 0.67 | 0.36 | 0.56 |
| T1 | 0.19 (0.79) | −0.44 (1.14) | −0.16 (0.85) | −0.43 (1.00) | 0.65 | 0.36 | 0.64 |
| T2 | 0.33 (1.13) | −0.44 (1.06) | −0.18 (0.93) | −0.46 (0.78) | 0.76 | 0.50 | 0.78 |
| PO9 | 0.07 (1.16) | −0.70 (1.72) | −0.25 (1.02) | −0.52 (0.94) | 0.62 | 0.26 | 0.48 |
| PO10 | 0.08 (1.20) | −0.63 (1.56) | −0.18 (1.20) | −0.42 (0.88) | 0.58 | 0.21 | 0.41 |
| O1 | 0.47 (1.34) | −0.37 (2.12) | 0.16 (1.27) | −0.21 (1.08) | 0.56 | 0.21 | 0.46 |
| O2 | 0.49 (1.32) | −0.40 (1.90) | 0.11 (1.35) | −0.19 (0.96) | 0.63 | 0.27 | 0.48 |
| Iz | 0.23 (1.46) | −0.72 (1.56) | −0.11 (1.13) | −0.51 (1.12) | 0.71 | 0.25 | 0.55 |
Group Differences in RON Amplitudes
The repeated measures ANOVA revealed a significant effect of electrode (F[3.21, 366.53]=44.17, p<.001) indicating a maximal RON response at frontocentral electrodes, as well as a significant group × electrode interaction (F[9.64, 366.53]=4.49, p<.001) indicating the presence of deficits at frontocentral but not temporo-parietal sites. The follow-up ANOVAs revealed significant group differences at p<.01 only at Fz, F3, F4, FC1, FC2, and Cz. As with MMN and P3a, the largest effect size differences were present at FC1. Both the chronic (d=−.34 to −.89) and recent-onset (d=−.60 to −.96) patients had significant RON reductions relative to the normal control subjects at Fz, F3, F4, FC1, FC2, and Cz (p<.05). However, unlike the recent-onset and chronic patients, the at-risk subjects did not have significant RON deficits at any of the electrodes. The at-risk group had significantly larger RON amplitudes relative to both the recent-onset group (at Fz, F4, FC1, FC2; p<.01 and Fp1, F3, FC6, Cz, C4; p<.05) and the chronic group (at Fz, FC1, FC2, and Cz; p<.05). There were no significant group differences in peak RON latency (F[3, 114]=.57, p=.64, M=414.50, SD=47.22). Mean RON amplitudes, standard deviations, and effect sizes for each electrode site are presented in Table 4.
Table 4.
Descriptive Statistics and Effect Sizes of RON Relative to Normal controls
| Group | Effect Size | ||||||
|---|---|---|---|---|---|---|---|
| Electrode | Normal Controls (N=31) | At Risk for Psychosis (N=26) | Recent-Onset Schizophrenia (N=28) | Chronic Schizophrenia (N=33) | AR | RO | SZ |
| Fp1 | −0.20 (0.45) | −0.06 (0.40) | 0.24 (0.62) | 0.02 (0.59) | −0.25 | −0.80 | −0.40 |
| Fp2 | −0.20 (0.54) | 0.04 (0.55) | 0.28 (0.57) | 0.04 (0.61) | −0.41 | −0.81 | −0.41 |
| F7 | 0.01 (0.51) | 0.09 (0.55) | 0.29 (0.70) | 0.10 (0.40) | −0.14 | −0.51 | −0.16 |
| F8 | 0.14 (0.55) | 0.23 (0.59) | 0.51 (0.60) | 0.16 (0.52) | −0.15 | −0.64 | −0.34 |
| Fz | −1.22 (1.40) | −0.86 (0.94) | 0.08 (1.57) | −0.16 (1.05) | −0.26 | −0.96 | −0.78 |
| F3 | −0.78 (1.07) | −0.49 (0.75) | 0.17 (1.24) | −0.10 (0.81) | −0.28 | −0.90 | −0.65 |
| F4 | −0.68 (1.07) | −0.48 (0.89) | 0.23 (1.04) | −0.08 (0.77) | −0.20 | −0.91 | −0.60 |
| FC1 | −1.43 (1.39) | −1.08 (0.96) | −0.20 (1.44) | −0.29 (0.89) | −0.27 | −0.95 | −0.88 |
| FC2 | −1.28 (1.36) | −1.03 (0.98) | −0.17 (1.33) | −0.25 (0.83) | −0.20 | −0.90 | −0.84 |
| FC5 | −0.46 (0.81) | −0.21 (0.66) | 0.18 (0.98) | 0.01 (0.58) | −0.31 | −0.80 | −0.59 |
| FC6 | −0.22 (0.83) | −0.04 (0.82) | −0.40 (0.81) | 0.09 (0.61) | −0.23 | 0.23 | −0.39 |
| C3 | −0.75 (1.08) | −0.48 (0.84) | −0.05 (1.04) | −0.12 (0.67) | −0.28 | −0.74 | −0.66 |
| Cz | −1.43 (1.43) | −1.09 (1.04) | −0.36 (1.30) | −0.33 (0.80) | −0.27 | −0.86 | −0.89 |
| C4 | −0.41 (1.05) | −0.44 (1.05) | 0.17 (0.99) | −0.04 (0.70) | 0.03 | −0.60 | −0.38 |
| CP1 | −0.67 (1.17) | −0.46 (0.92) | −0.13 (1.05) | −0.13 (0.69) | −0.21 | −0.55 | −0.55 |
| CP2 | −0.48 (1.19) | −0.42 (1.09) | 0 (1.07) | −0.03 (0.68) | −0.06 | −0.47 | −0.44 |
| CP5 | 0.01 (0.94) | 0.22 (0.87) | 0.24 (0.95) | 0.16 (0.67) | −0.25 | −0.27 | −0.18 |
| CP6 | 0.39 (0.90) | 0.33 (1.03) | 0.51 (0.86) | 0.27 (0.72) | 0.07 | −0.14 | 0.14 |
| P7 | 0.38 (0.87) | 0.46 (0.83) | 0.32 (0.81) | 0.29 (0.71) | −0.10 | 0.08 | 0.11 |
| P3 | 0.04 (1.05) | 0.14 (0.88) | 0.10 (0.93) | 0.10 (0.68) | −0.11 | −0.07 | −0.07 |
| Pz | −0.05 (1.17) | −0.05 (0.93) | 0.02 (1.00) | 0.03 (0.67) | 0 | −0.07 | −0.08 |
| P4 | 0.29 (1.09) | 0.18 (0.97) | 0.27 (0.96) | 0.11 (0.66) | 0.12 | 0.02 | 0.20 |
| P8 | 0.54 (0.83) | 0.51 (0.88) | 0.49 (0.70) | 0.31 (0.58) | 0.04 | 0.07 | 0.31 |
| T7 | 0.13 (0.73) | 0.29 (0.77) | 0.30 (0.97) | 0.27 (0.65) | −0.20 | −0.22 | −0.18 |
| T8 | 0.30 (0.67) | 0.46 (0.81) | 0.63 (0.74) | 0.34 (0.70) | −0.22 | −0.45 | −0.05 |
| TP9 | 0.24 (0.67) | 0.36 (0.64) | 0.23 (0.67) | 0.27 (0.64) | −0.18 | 0.15 | −0.05 |
| TP10 | 0.42 (0.74) | 0.42 (0.69) | 0.38 (0.53) | 0.29 (0.55) | 0 | 0.06 | 0.21 |
| T1 | 0.15 (0.53) | 0.23 (0.51) | 0.20 (0.64) | 0.22 (0.50) | −0.15 | −0.09 | −0.13 |
| T2 | 0.21 (0.51) | 0.39 (0.45) | 0.36 (0.46) | 0.29 (0.60) | −0.35 | −0.29 | −0.16 |
| PO9 | 0.27 (0.70) | 0.36 (0.61) | 0.12 (0.58) | 0.15 (0.58) | −0.14 | 0.24 | 0.19 |
| PO10 | 0.36 (0.78) | 0.36 (0.70) | 0.18 (0.55) | 0.20 (0.50) | 0 | 0.29 | 0.25 |
| O1 | 0.28 (0.94) | 0.39 (0.71) | 0.17 (0.77) | 0.15 (0.67) | −0.14 | 0.14 | 0.17 |
| O2 | 0.35 (0.91) | 0.37 (0.75) | 0.26 (0.77) | 0.24 (0.55) | −0.03 | 0.12 | 0.15 |
| Iz | 0.22 (0.73) | 0.31 (0.60) | 0.08 (0.60) | 0.19 (0.66) | −0.14 | 0.21 | 0.05 |
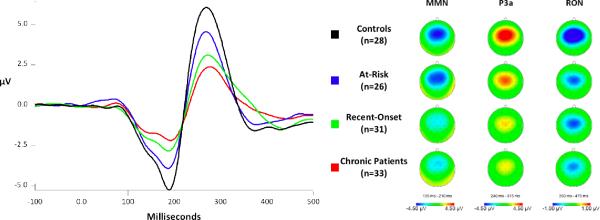
Figure 1 shows the grand average waveforms with MMN, P3a, and RON amplitudes at FC1 for each of the groups. The at-risk group's mean MMN and P3a amplitudes were intermediate between those of the normal control group and the recent-onset group. As noted above, two of the at-risk subjects transitioned to psychosis during the study period. These subjects qualitatively appeared to have a more pronounced reduction in MMN and P3a amplitudes relative to the remaining at-risk subjects, although this small N precludes further analyses.
Figure 1.
Grand average difference ERPs, at electrode FC1, and their corresponding scalp distributions, formed by subtracting the ERP elicited by standard tones from the ERP elicited by deviant tones, for each group.
Relationships of MMN, P3a, and RON with Social Functioning and Clinical Symptoms
Spearman rank correlation coefficients were generated in order to assess relationships between MMN, P3a, and RON responses at frontocentral electrodes and social functioning as measured by the GAF scale ratings in the patient groups. There were significant correlations (p<.05) between GAF and MMN (rs= −.35 to −.46; Fz, F3, F4, F7, F8, Fp1, Fp2, FC2, FC5, Cz, C4) at frontocentral electrodes only in the chronic patients. P3a and RON were not significantly associated with GAF in any of the patient groups.
We found no significant Spearman rank correlations between severity of positive and negative symptoms, as measured by the SAPS and SANS total scores, and MMN, P3a, and RON responses in the recent-onset and chronic patients. However, significant correlations (p<.05) between SANS and RON (rs = .46 to .52; Fp2, F4, F8), as well as between SIPS Negative and P3a at frontocentral electrodes (rs = −.43 to −.59; Fp1, Fp2, F8, Fz, F3, F4, FC6) were present in the at-risk group.
Discussion
To our knowledge, this is the first report of concurrently examining three ERP components (MMN, P3a, RON) of automatic, preattentive information processing across different stages of schizophrenia. In a between-groups design, results indicate that individuals identified as being at risk for psychosis have robust deficits in MMN and P3a, whereas patients early in the course of schizophrenia and chronic patients exhibit significant deficits in MMN, P3a, and RON relative to normal controls. Although this is a cross-sectional study, the findings suggest that MMN and P3a abnormalities may precede the onset of psychosis while RON deficits do not emerge until the full manifestation of the illness. Cross-sectionally, it appears as though MMN and P3a deficits progressively increased with illness chronicity. The at-risk group's P3a amplitudes at frontocentral electrodes were significantly different from both the normal control and recent-onset groups' amplitudes. The at-risk group also had MMN amplitudes that were intermediate between those of the normal control and recent-onset groups (although only significantly different from the normal control group's amplitudes). The recent-onset group tended to show less severe MMN, P3a, and RON deficits relative to the chronic group.
Our findings suggest that individuals at risk for developing a psychotic disorder, as well as those with manifest schizophrenia, have deficits in processing auditory input or detecting changes in their acoustic environment, failing to notice stimuli that are salient to most people (Michie et al., 2002). In other words, if someone has difficulty organizing sensory stimulation from their surrounding environment, they may also exhibit difficulty in mounting an appropriate response when necessary. It has been suggested that fundamental sensory processing abnormalities may result in inattentiveness and disorganization, causing significant disruption in everyday functioning (Braff and Light, 2004). Consistent with our previous results (Light and Braff, 2005a), we found significant associations between psychosocial functioning and MMN in the chronic group. P3a and RON, in contrast, were not significantly associated with functional ratings in any of the patient groups.
Previous reports of the association between MMN and symptom severity have yielded inconsistent results, providing more support for an association with negative rather than positive symptoms in both chronic (Umbricht and Krljes, 2005) and first-episode samples (Oades et al., 2006, Umbricht et al., 2006). We found no associations between MMN and negative symptoms in any the patient groups, which is consistent with previously reported findings in both first-episode (Salisbury et al., 2007) and chronic schizophrenia patients (Kasai et al., 1999, Light and Braff, 2005b, Shelley et al., 1991). Nonetheless, there were significant correlations between both P3a and RON and negative symptoms in the at-risk group, suggesting that further study of the relationships and timing of the emergence of symptoms and neurophysiological abnormalities is warranted.
Certain limitations of our study require appropriate caveats, especially the fact that our samples are relatively modest and unbalanced on key demographic features. We did, however, attempt to control for these potential demographic confounds. Based on our extensive analyses, we believe that the group differences in age and gender did not markedly influence our results. The low conversion rate in our at-risk sample did not yield sufficient statistical power to employ a longitudinal design and assess the potential role of MMN/P3a/RON as predictors of conversion to psychosis. However, the inclusion of newly diagnosed and chronic schizophrenia groups alleviates this problem to some degree. Furthermore, it must be acknowledged that the influence of antipsychotic medications cannot be completely excluded since 27% of the at-risk, 81% of the recent-onset, and 94% of the chronic patients were receiving antipsychotic medications at the time of testing. Independent samples t-tests within the at-risk group revealed no significant differences (all d's < .30) in MMN, P3a, or RON amplitudes between patients who were taking one or more atypical antipsychotics (N=7) and those who were antipsychotic-free (N=19). Although it is hard to make any conclusions regarding treatment effects given our small sample size, there is evidence showing that MMN is uninfluenced by Olanzapine (Korostenskaja et al., 2005), Risperidone (Umbricht et al., 1999), and Clozapine (Umbricht et al., 1998). Also, our patients show substantial deficits despite the possibility that Clozapine may exert some normalization of sensory ERP components (Horton et al., 2011, Light et al., 2000).
Our results support the utility of examining RON in the context of MMN and P3a in studies of at-risk populations. MMN and P3a appear to be deficient before the onset of full-blown psychosis and RON deficits do not develop until later in the disease process. To date, the literature regarding the utility of the MMN to duration deviants as an endophenotype (Gottesman and Gould, 2003) is inconclusive. Nonetheless, we may be able to argue that MMN and P3a are possible markers of vulnerability to schizophrenia that reflect premorbid neuropathology. Longitudinal designs are needed to determine whether the preattentive auditory processing abnormalities observed in the early course of schizophrenia are trait-related and/or whether they worsen with illness progression, as well as to delineate the rate of change in those risk indicators in subjects who convert to psychosis (Bodatsch et al., 2011). It will be valuable to determine whether the size of the MMN/P3a amplitude can differentiate between subjects at various stages of the prodrome and identify those for whom psychosis is imminent. Additionally, it will be useful to ascertain whether measures derived from the MMN/P3a/RON complex can be used to predict medication adherence, academic or vocational functioning, and other instrumental activities of daily living in affected subjects (Banati and Hickie, 2009).
It remains an option question as to whether impairment in basic auditory sensory information processing improves the sensitivity and predictive accuracy in conjunction with other known risk factors of psychosis (Cannon et al., 2008), including cannabis abuse (Kristensen and Cadenhead, 2007), severity of subsyndromal symptoms, and decline in social functioning (Haroun et al., 2006), working memory, and processing speed (Jahshan et al., 2010).
Acknowledgements
This work was supported by grants from the Department of Veteran Affairs (VISN 22 Mental Illness Research, Education, and Clinical Center) and National Institute of Mental Health (MH079777, MH042228, and MH065571). The authors wish to thank Jason Nunag, Steven Reding, Kathleen Shafer, Marlena Pela, Joyce Sprock, and Richard Sharp for their assistance. The authors do not have any interests that might be interpreted as influencing the research.
References
- Addington J, Saeedi H, Addington D. The course of cognitive functioning in first episode psychosis: changes over time and impact on outcome. Schizophrenia Research. 2005;78:35–43. doi: 10.1016/j.schres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City, Iowa: 1984. [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Archives of General Psychiatry. 1977;34:1229–35. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Ballon JS, Kaur T, Marks, Cadenhead KS. Social functioning in young people at risk for schizophrenia. Psychiatry Research. 2007;151:29–35. doi: 10.1016/j.psychres.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati R, Hickie IB. Therapeutic signposts: using biomarkers to guide better treatment of schizophrenia and other psychotic disorders. The Medical Journal of Australia. 2009;190:26–32. doi: 10.5694/j.1326-5377.2009.tb02371.x. [DOI] [PubMed] [Google Scholar]
- Berti S, Roeber U, Schroger E. Bottom-up influences on working memory: behavioral and electrophysiological distraction varies with distractor strength. Experimental Psychology. 2004;51:249–57. doi: 10.1027/1618-3169.51.4.249. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JM, Woerner MG, Geisler S, Kane JM, Lieberman JA. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. American Journal of Psychiatry. 2000;157:549–59. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Bodatsch M, Ruhrmann S, Wagner M, Muller R, Schultze-Lutter F, Frommann I, Brinkmeyer J, Gaebel. W, Maier W, Klosterkotter J, Brockhaus-Dumke A. Prediction of psychosis by mismatch negativity. Biological Psychiatry. 2011;69:959–66. doi: 10.1016/j.biopsych.2010.09.057. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology (Berl) 2004;174:75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Tendolkar I, Pukrop R, Schultze-Lutter F, Klosterkotter J, Ruhrmann S. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophrenia Research. 2005;73:297–310. doi: 10.1016/j.schres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Light GA, Shafer KM, Braff DL. P50 suppression in individuals at risk for schizophrenia: the convergence of clinical, familial, and vulnerability marker risk assessment. Biological Psychiatry. 2005;57:1504–9. doi: 10.1016/j.biopsych.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Callaway E, Naghdi S. An information processing model for schizophrenia. Archives of General Psychiatry. 1982;39:339–47. doi: 10.1001/archpsyc.1982.04290030069012. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Archives of General Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Archives of General Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Devrim-Ucok M, Keskin-Ergen HY, Ucok A. Mismatch negativity at acute and post-acute phases of first-episode schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2008;258:179–85. doi: 10.1007/s00406-007-0772-9. [DOI] [PubMed] [Google Scholar]
- Eastvold AD, Heaton RK, Cadenhead KS. Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophrenia Research. 2007;93:266–77. doi: 10.1016/j.schres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escera C, Alho K, Winkler I, Naatanen R. Neural mechanisms of involuntary attention to acoustic novelty and change. Journal of Cognitive Neuroscience. 1998;10:590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- Escera C, Corral M. Role of mismatch negativity and novelty-P3 in involuntary auditory attention. Journal of Psychophysiology. 2007;21:251–264. [Google Scholar]
- Escera C, Yago E, Alho K. Electrical responses reveal the temporal dynamics of brain events during involuntary attention switching. The European Journal of Neuroscience. 2001;14:877–83. doi: 10.1046/j.0953-816x.2001.01707.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSMIV Axis I Disorders. Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Ford JM, Roach BJ, Miller RM, Duncan CC, Hoffman RE, Mathalon DH. When it's time for a change: failures to track context in schizophrenia. International Journal of Psychophysiology. 2010;78:3–13. doi: 10.1016/j.ijpsycho.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain's evaluation of novelty. Neuroscience and Biobehavioral Reviews. 2001;25:355–73. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. Cortical oscillations and schizophrenia: timing is of the essence. Archives of General Psychiatry. 1999;56:1007–8. doi: 10.1001/archpsyc.56.11.1007. [DOI] [PubMed] [Google Scholar]
- Grillon C, Courchesne E, Ameli R, Elmasian R, Braff D. Effects of rare non-target stimuli on brain electrophysiological activity and performance. International Journal of Psychophysiology. 1990a;9:257–67. doi: 10.1016/0167-8760(90)90058-l. [DOI] [PubMed] [Google Scholar]
- Grillon C, Courchesne E, Ameli R, Geyer MA, Braff DL. Increased distractibility in schizophrenic patients. Electrophysiologic and behavioral evidence. Archives of General Psychiatry. 1990b;47:171–9. doi: 10.1001/archpsyc.1990.01810140071010. [DOI] [PubMed] [Google Scholar]
- Grzella I, Muller BW, Oades RD, Bender S, Schall U, Zerbin D, Wolstein J, Sartory G. Novelty-elicited mismatch negativity in patients with schizophrenia on admission and discharge. Journal of Psychiatry and Neuroscience. 2001;26:235–46. [PMC free article] [PubMed] [Google Scholar]
- Hall RC, Parks J. The modified global assessment of functioning scale: addendum. Psychosomatics. 1995;36:416–7. doi: 10.1016/S0033-3182(95)71656-5. [DOI] [PubMed] [Google Scholar]
- Haroun N, Dunn L, Haroun A, Cadenhead KS. Risk and protection in prodromal schizophrenia: ethical implications for clinical practice and future research. Schizophrenia Bulletin. 2006;32:166–78. doi: 10.1093/schbul/sbj007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Archives of General Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Progress in Neuropsychopharmacology and Biological Psychiatry. 2010;34:822–9. doi: 10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Holig C, Berti S. To switch or not to switch: brain potential indices of attentional control after task-relevant and task-irrelevant changes of stimulus features. Brain Research. 2010;1345:164–75. doi: 10.1016/j.brainres.2010.05.047. [DOI] [PubMed] [Google Scholar]
- Horton J, Millar A, Labelle A, Knott VJ. MMN responsivity to manipulations of frequency and duration deviants in chronic, clozapine-treated schizophrenia patients. Schizophrenia Research. 2011;126:202–11. doi: 10.1016/j.schres.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Horvath J, Winkler I, Bendixen A. Do N1/MMN, P3a, and RON form a strongly coupled chain reflecting the three stages of auditory distraction? Biological Psychology. 2008;79:139–47. doi: 10.1016/j.biopsycho.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Heaton RK, Golshan S, Cadenhead KS. Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology. 2010;24:109–20. doi: 10.1037/a0016791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Sensory processing in schizophrenia: neither simple nor intact. Schizophrenia Bulletin. 2009;35:1059–64. doi: 10.1093/schbul/sbp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clinical Neurophysiology. 2000a;111:1733–7. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Archives of General Psychiatry. 2000b;57:1131–7. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- Kasai K, Okazawa K, Nakagome K, Hiramatsu K, Hata A, Fukuda M, Honda M, Miyauchi M, Matsushita M. Mismatch negativity and N2b attenuation as an indicator for dysfunction of the preattentive and controlled processing for deviance detection in schizophrenia: a topographic event-related potential study. Schizophrenia Research. 1999;35:141–56. doi: 10.1016/s0920-9964(98)00116-9. [DOI] [PubMed] [Google Scholar]
- Kawakubo Y, Kamio S, Nose T, Iwanami A, Nakagome K, Fukuda M, Kato N, Rogers MA, Kasai K. Phonetic mismatch negativity predicts social skills acquisition in schizophrenia. Psychiatry Research. 2007;152:261–5. doi: 10.1016/j.psychres.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Kiang M, Braff DL, Sprock J, Light GA. The relationship between preattentive sensory processing deficits and age in schizophrenia patients. Clinical Neurophysiology. 2009;120:1949–57. doi: 10.1016/j.clinph.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen K, Cadenhead KS. Cannabis abuse and risk for psychosis in a prodromal sample. Psychiatry Research. 2007;151:151–4. doi: 10.1016/j.psychres.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. American Journal of Psychiatry. 2010;167:818–27. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, Cornblatt BA. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biological Psychiatry. 2006;59:863–71. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Archives of General Psychiatry. 2005a;62:127–36. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. American Journal of Psychiatry. 2005b;162:1741–3. doi: 10.1176/appi.ajp.162.9.1741. [DOI] [PubMed] [Google Scholar]
- Light GA, Geyer MA, Clementz BA, Cadenhead KS, Braff DL. Normal P50 suppression in schizophrenia patients trated with atypical antipsychotic medications. American Journal of Psychiatry. 2000;157:767–71. doi: 10.1176/appi.ajp.157.5.767. [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biological Psychiatry. 2006;60:1231–40. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. Journal of Cognitive Neuroscience. 2007;19:1624–32. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Williams LE, Minow F, Sprock J, Rissling A, Sharp R, Swerdlow NR, Braff DL. Electroencephalography (EEG) and event-related potentials (ERPs) with human participants. Current Protocols in Neuroscience. 2010;25:1–24. doi: 10.1002/0471142301.ns0625s52. Chapter 6, Unit 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM, Pfefferbaum A. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biological Psychiatry. 2000;47:434–49. doi: 10.1016/s0006-3223(99)00277-2. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–36. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Michie PT, Innes-Brown H, Todd J, Jablensky AV. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biological Psychiatry. 2002;52:749–58. doi: 10.1016/s0006-3223(02)01379-3. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophrenia Bulletin. 2003;29:703–15. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Gaillard AW, Mantysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychologica (Amst) 1978;42:313–29. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Simpson M, Loveless NE. Stimulus deviance and evoked potentials. Biological Psycholology. 1982;14:53–98. doi: 10.1016/0301-0511(82)90017-5. [DOI] [PubMed] [Google Scholar]
- Oades RD, Wild-Wall N, Juran SA, Sachsse J, Oknina LB, Ropcke B. Auditory change detection in schizophrenia: sources of activity, related neuropsychological function and symptoms in patients with a first episode in adolescence, and patients 14 years after an adolescent illness-onset. BMC Psychiatry. 2006;6:7. doi: 10.1186/1471-244X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Alain C, Picton TW. Effects of visual attentional load on auditory processing. Neuroreport. 2000;11:875–80. doi: 10.1097/00001756-200003200-00043. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychology Review. 2009;19:365–84. doi: 10.1007/s11065-009-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. The Structured Interview for DSM-IV Personality (SIDP-IV) Department of Psychiatry, University of Iowa; Iowa City, Iowa: 1995. [Google Scholar]
- Picton TW, Alain C, Otten L, Ritter W, Achim A. Mismatch negativity: different water in the same river. Audiology and Neurootology. 2000;5:111–39. doi: 10.1159/000013875. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Frommann I, Berning J, Kuhn KU, Maier W, Wagner M. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biological Psychiatry. 2008;64:766–73. doi: 10.1016/j.biopsych.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Rasser PE, Schall U, Todd J, Michie PT, Ward PB, Johnston P, Helmbold K, Case V, Soyland A, Tooney PA, Thompson PM. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophrenia Bulletin. 2011;37:131–40. doi: 10.1093/schbul/sbp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissling AJ, Makeig S, Braff DL, Light GA. Neurophysiologic markers of abnormal brain activity in schizophrenia. Current Psychiatry Reports. 2010;12:572–8. doi: 10.1007/s11920-010-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Archives of General Psychiatry. 2007;64:521–9. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Archives of General Psychiatry. 2002;59:686–94. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- Schroger E, Giard MH, Wolff C. Auditory distraction: event-related potential and behavioral indices. Clinical Neurophysiology. 2000;111:1450–60. doi: 10.1016/s1388-2457(00)00337-0. [DOI] [PubMed] [Google Scholar]
- Seeber K, Cadenhead KS. How does studying schizotypal personality disorder inform us about the prodrome of schizophrenia? Current Psychiatry Reports. 2005;7:41–50. doi: 10.1007/s11920-005-0024-5. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Bearden CE, Christensen BK, Hawkins K, Heaton R, Keefe RS, Heinssen R, Cornblatt BA. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Archives of General Psychiatry. 2010;67:578–88. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biological Psychiatry. 1991;30:1059–62. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Bilker WB, Siegel SJ, Kohler CG, Gur RE. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Research. 2009;165:27–37. doi: 10.1016/j.psychres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophrenia Research. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biological Psychiatry. 2006;59:762–72. doi: 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Valkonen-Korhonen M, Purhonen M, Tarkka IM, Sipila P, Partanen J, Karhu J, Lehtonen J. Altered auditory processing in acutely psychotic never-medicated first-episode patients. Brain Research. 2003;17:747–58. doi: 10.1016/s0926-6410(03)00199-x. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Hackley SA, Hillyard SA. The effects of channel-selective attention on the mismatch negativity wave elicited by deviant tones. Psychophysiology. 1991;28:30–42. doi: 10.1111/j.1469-8986.1991.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Hillyard SA, Gallen CC, Hampson SR, Bloom FE. Magnetoencephalographic recordings demonstrate attentional modulation of mismatch-related neural activity in human auditory cortex. Psychophysiology. 1998;35:283–92. doi: 10.1017/s0048577298961601. [DOI] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The initial prodrome in psychosis: descriptive and qualitative aspects. The Australian and New Zealand Journal of Psychiatry. 1996;30:587–99. doi: 10.3109/00048679609062654. [DOI] [PubMed] [Google Scholar]
- Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell'Olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, Buckby J. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. The Australian and New Zealand Journal of Psychiatry. 2005;39:964–71. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]