Abstract
Understanding the molecular alterations that confer cancer cells with motile, metastatic properties is needed to improve patient survival. Here we report that p38γ MAPK regulates breast cancer cell motility and metastasis, in part by controlling expression of the metastasis-associated small GTPase RhoC. This p38γ-RhoC regulatory connection was mediated by a novel mechanism of modulating RhoC ubiquitination. This relationship persisted across multiple cell lines and in clinical breast cancer specimens. Using a computational mechanical model based on the finite element method, we demonstrated that p38γ-mediated cytoskeletal changes are sufficient to control cell motility. This model predicted novel dynamics of leading edge actin protrusions, which were experimentally verified and established to be closely related to cell shape and cytoskeletal morphology. Clinical relevance was supported by evidence that elevated expression of p38γ associated with lower overall survival of breast cancer patients. Taken together, our results offer a detailed characterization of how p38γ contributes to breast cancer progression, presents a new mechanics-based analysis of cell motility, and discovers a leading edge behavior in motile cells to accommodate modified cytoskeletal architecture. In summary, these findings not only identify a novel mechanism for regulating RhoC expression but also advance p38γ as a candidate therapeutic target.
Keywords: breast cancer, p38 MAPK, RhoC, metastasis, computational modeling
Introduction
Breast cancer (BC) presents as a dysplastic disease of mammary epithelial cells, which can grow uncontrollably for years yet remain confined within mammary ducts or lobules. If diagnosed at this early stage BC responds well to therapeutic intervention (1). BC becomes much more severe once the cancer cells exit the mammary ducts and metastasize to vital organs. Understanding the molecular alterations that confer metastatic properties to otherwise benign cells is therefore essential to controlling the disease and improving patient survival.
p38 MAPK is a serine/threonine kinase that integrates extracellular stimuli and translates them into cellular responses by phosphorylating a network of downstream effector proteins (2). The p38 arm of the MAPK pathway is effected by four isoforms: p38 α, β, γ and δ. Despite their sequence similarity and shared upstream kinases, the p38 isoforms can have unrelated, and even antagonistic, roles in development and disease (3–7).
p38γ (also known as MAPK12, ERK6, or SAPK3) is expressed predominantly in muscle tissue where it promotes myoblast differentiation into myotubes (8–11). p38γ mRNA is overexpressed in several cancers (12, 13) and helps increase Ras-induced cancer invasion (14, 15). Viewing p38γ function from the developmental perspective led us to hypothesize that p38γ enables mesenchymal-like behavior in BC cells by controlling their motility properties.
Using multiple mechanisms of inhibition we reveal that p38γ is a crucial mediator of BC cell motility and metastasis. Our computational model demonstrates the central role of stress fiber orientation in p38γ-mediated cell motility. Through this in silico experimentation, and subsequent in vitro validation, we discover a novel leading edge behavior in motile BC cells. Mechanistically, p38γ elicits its effects at least in part through RhoC GTPase by affecting RhoC ubiquitination and degradation—a regulatory mechanism never before observed for RhoC. Clinically, high p38γ expression is associated with the basal-like BC subtype and confers a worse prognosis. This work establishes that p38γ is a metastasis-enabling gene responsible for mediating BC cell motility and metastasis, in part by regulating cytoskeletal architecture and modulating RhoC.
Materials and Methods
Cell Lines
Untransfected cell lines were cultured in RPMI 1640 (MDA-MB-231, BT549) or DMEM (Hs578t) supplemented with 10% fetal bovine serum, or DMEM/F12 (MCF-10A) supplemented with 10% horse serum. Selection media for shRNA-transfected MDA-MB-231 cells (“scrambled” or “shp38γ”) was standard cell line media containing 1μg/ml puromycin. MDA-MB-231 stably transfected with both shRNA and RhoC/RhoC G14V were cultured in standard cell line media containing 1μg/ml puromycin and 350μg/ml G418. All cell lines were grown at 37°C in a humidified 5% CO2 incubator.
Phospho-p38γ Immunoprecipitation/Western Blot
Protein was extracted from 70% confluent cells with RIPA buffer. Total protein extracts were incubated with primary antibody (anti-p38γ or anti-phospho-p38) overnight at 4°C. The following morning protein-antibody complexes were captured by incubation with protein A/G beads (Santa Cruz Biotechnology). Immunoprecipitates were run on an SDS-PAGE gel, transferred to a nitrocellulose membrane, and probed with the appropriate reciprocal antibody (anti-p38γ or anti-phospho-p38). All immunoprecipitation and western blot data represent at least 3 independent experiments.
In situ detection/quantification of protein expression
Tumors and Patients
Fresh and formalin-fixed, paraffin-embedded tissue blocks of BC were obtained from the files of the Department of Pathology, University of Michigan Medical Center, Ann Arbor, MI. IRB approval was obtained and the diagnosis was confirmed by morphology. After pathological review, a tissue microarray was constructed from the most representative area using the methodology of Nocito et al. (16). Survival data was obtained from the The University of Michigan's Cancer Registry.
Immunohistochemical Staining and AQUA analysis
Triple immunofluorescence staining was performed as previously described (17) and the AQUA system (HistoRx, New Haven, Connecticut) was used for automated image acquisition and analysis. See Supplemental Methods for the detailed staining and imaging procedure.
Orthotopic Xenografts and Lymphatic Metastasis Analysis
All mouse work was compliant with the University's standards for animal use. 231 scrambled and shp38γ cells were diluted 1:1 with Matrigel (BD Biosciences) to a final concentration of 3 × 106 cells/ml. Athymic nude mice were anesthetized and 1.5 × 105 cells (50 μl) were injected directly into the fourth mammary gland. Tumors were monitored weekly and mice were euthanized once tumor volume approached 2 cm3. Tumors were resected at euthanization, fixed in 10% formalin, and subsequently paraffin embedded. Hematoxylin and eaosin-stained tumor sections were scored for lymphatic metastasis in a single-blind manner by Dr. J. Erby Wilkinson at the ULAM Pathology Core. Lymphatic metastasis was defined as the presence of tumor cells in the inguinal lymph node of the uninjected fourth mammary gland (the contralateral gland, relative to injection site), thus indicating the tumor cells' ability to exit the primary tumor and metastasize to distant sites through the vasculature. The number of mice presenting with lymphatic metastases were compared using Fisher's exact test. Images were captured at room temperature using an Olympus BX-51 upright light microscope with Olympus UPlanApo 10×/0.4 NA and 40×/0.85 NA objectives and an Olympus DP-70 high resolution digital camera with DP controller software.
Statistical analyses
All data is representative of at minimum three independent experiments, and all error bars are ± s.e.m. unless otherwise indicated. All p-values were calculated by Student's two-tailed t-test unless otherwise noted. Expression levels of p38γ and RhoC in TMA samples were compared using Spearman's rank coefficient. Association between p38γ and BC subtype, and the prevalence of lymphatic metastasis between scrambled and shp38γ cells were determined by Fisher's exact test. The relationship between p38γ expression and patient survival was calculated by Kaplan Meier analysis, with surviving patients censored. The log-rank test statistic was used for significance.
Results
p38γ phosphorylation is elevated in an aggressive BC cell line
p38 is activated by dual phosphorylation of the Thr and Tyr residues of the conserved TGY motif (3, 18). To assess p38γ functional relevance in aggressive BC, we assayed phospho-p38γ levels in a representative and widely used aggressive BC cell line, MDA-MB-231 (“231 cells”). Compared to the nontumorigenic mammary epithelial cell line MCF-10A, 231 cells have increased levels of phosphorylated p38γ, despite similar levels of total p38γ (Fig. 1A).
Figure 1. p38γ affects MDA-MB-231 cell motility and may do so by shaping the actin cytoskeleton.
(A) Phospho-p38γ levels are elevated in the MDA-MB-231 BC cell line compared to non-tumorigenic MCF-10A mammary epithelial cells. (B) shRNA knockdown of p38γ in MDA-MB-231 cells. (C) (left) Cell shapes of scrambled and shp38γ cells. (right) Quantification of cell shape difference by aspect ratio measurement (length/width) (*p = 7.82 × 10−7, n = 22 cells for scrambled, n = 27 cells for shp38γ, scale bar = 25 μm). (D) Actin fiber orientation differs between scrambled and shp38γ cells. (**p<0.01, n = 16 for scrambled, n = 22 for shp38γ, scale bar = 20 μm). (E) (left) Representative images from time lapse movies (Movies 1–2) of scrambled and shp38γ cells, demonstrating the characteristic mesenchymal-like motility of scrambled cells (top) and the disjoint crawling motion of shp38γ cells (bottom) (scale bar = 10 μm). (right) shp38γ cells are significantly slower than scrambled cells (*p = 3.34 × 10−5, n = 30 cells per cell line). (F) Preliminary computational models of scrambled and shp38γ cells qualitatively represent the locomotion of each cell type (see text for details). Arrows point to out-of-phase lamellipodial oscillations. The actin fibers are in red and the nucleus is blue.
To investigate the role of p38γ on the metastatic features of 231 cells we used small hairpin RNA (“shRNA”) to stably knock down p38γ expression (Fig. 1B). Importantly, the shRNA did not affect expression of the other three p38 isoforms (Supplementary Fig. S1A). Since there is no p38γ-specific pharmacologic inhibitor, we overexpressed dominant negative p38γ (19) (“DNp38γ”) in MDAMB-231 cells as an additional inhibition method (See Supplementary Methods and Supplementary Fig. S2).
p38γ knockdown alters cell shape and cytoskeletal architecture
Immediately apparent in the p38γ knockdown cells (“shp38γ cells”) was an altered cell morphology compared to scrambled control cells (“scrambled cells”). While scrambled cells exhibited the elongated morphology characteristic of 231 cells and mesenchymal cells in general, shp38γ cells adopted a more rounded shape (Fig. 1C), as did DNp38γ cells (Supplementary Fig. S2C). This change was quantified by the cells' aspect ratio (see Methods). As depicted in Fig. 1C (right), shp38γ cells are significantly less elongated than scrambled cells.
We further asked whether the actin cytoskeleton was modified in shp38γ cells. Immunofluorescent confocal microscopy revealed that shp38γ cells have a strikingly disorganized actin cytoskeleton (Fig. 1D), as do DNp38γ cells (Supplementary Fig. S2E). Scrambled cells exhibit mesenchymal-like polarization with cell-length stress fibers oriented at 3.42° ± 1.89° (mean ± std. dev.) on either side of the normal to the leading edge (Fig. 1D). shp38γ cells retain thick actin bundles resembling stress fibers, but primarily confined to the leading edge and with a bimodal orientation distribution at 61.08° ± 3.94° (mean ± std. dev.) (Fig. 1D). This cluster of actin bundles forms the lamellipodia-like structure present in shp38γ cells (Figs. 1C and D) but shows little similarity to classic lamellipodial cytoskeletal architecture, which normally consists of thin, branched actin filaments forming a protrusive meshwork (20).
p38γ knockdown dramatically alters cell motility and affects other in vitro metastasis-associated properties of MDA-MB-231 cells
Elongated cell shape is one factor that delineates both the mode of motility used by a cell and metastatic from non-metastatic cancer cells in vitro (21, 22). To determine whether the shp38γ cells' rounded shape and modified cytoskeletal architecture indicated a change in metastatic properties we first analyzed cell motility—specifically unstimulated random walk—using time lapse microscopy.
p38γ knockdown profoundly affected the quality of cell motility (Fig. 1E, left) and their speed (Fig. 1E, right). Scrambled control cells had a mesenchymal-like motility, consistent with their appearance and cytoskeletal structure, using long pseudopodial projections to “pull” themselves along in alternating cycles of protrusion and contraction (Fig. 1E, top left, and Movie 1). shp38γ cells, however, remained un-polarized and were unable to form long pseudopodia (Fig. 1E, bottom left, and Movie 2). The cells moved inefficiently using broad lamellipodia-like structures and exhibited detachment anomalies at the rear of the cell. Quantitatively, p38γ knockdown significantly reduced cell speed (Fig. 1E, right).
We also investigated p38γ function in cancer cell invasion and aggressive 3D growth and found that p38γ knockdown significantly impaired both properties (Supplementary Figs. S1B–C). These data demonstrate that p38γ knockdown affects the in vitro metastasis-related properties of aggressive BC cells, and specifically inhibits efficient mesenchymal-like motion in a qualitative and quantitative manner.
Computational modeling reveals that p38γ-induced changes in cytoskeletal architecture influence cell motility
We observed that p38γ knockdown had functional (impaired motility and other metastasis-related properties) and structural (actin cytoskeleton and cell shape) effects on MDA-MB-231 cells. However, there remained the question of whether the change in actin cytoskeletal structure is sufficient to explain the observed impairment of motility. Since it remains inaccessible to experiments, this central question was addressed by computational modeling.
Our computational models use the finite element method to solve the partial differential equations governing the mechanics of cell motility (see Supplementary Materials and Methods for details). We hypothesized that the strikingly different cytoskeletal architectures--polarized stress fibers in scrambled versus bimodal bundles of fibers in shp38γ cells--underlies the observed differences in motility.
To test this hypothesis we first created a computational model of scrambled cell motility. Using the typical dimensions of scrambled cells (Supplementary Table S2/Fig. 1C), their observed cytoskeletal morphology of ±3.42° (Fig. 1D), and the mechanical properties of actin fibers and cell membranes of mammalian cells (Supplementary Table S1) we varied the rates of actin filament protrusion and retraction as model inputs to successfully recreate the observed locomotion of live scrambled cells (Fig. 1F, top, and Movie 3).
To determine whether actin cytoskeletal architecture delineates the motility of the two MDA-MB-231 phenotypes we next created a computational model of shp38γ cells, using the dimensions reported in Supplementary Table S2 and Fig. 1C, the cytoskeletal morphology of actin bundles at ±61.08° (Fig. 1D and Supplementary Fig. S3) and the same mechanical properties of actin fibers and cell membrane as for the scrambled cells (Supplementary Table S1). We further conjectured, and computationally demonstrated, that the observed oscillations in motion of shp38γ cells that are evident in Movie 2 were only possible if the two families of actin bundles at ±61.08° alternate (are out of phase) in their protrusion and retraction (data not shown). When incorporated, these alternating dynamics, albeit not precisely timed, produced a disjoint crawling motion with oscillations of the cell body of the computational shp38γ cell. This motility was remarkably similar to our live shp38γ cell motility (Fig. 1E, bottom, and Movie 3), suggesting that actin cytoskeletal architecture is important for defining shp38γ cell motility. However, further experiments were necessary to separate the influence of actin fiber orientation from actin protrusion/retraction dynamics.
Live scrambled and shp38γ cells have oscillating leading edge protrusions related to modified cytoskeletal architecture
The computational shp38γ cell motility thus revealed the possibility of a previously unobserved aspect of cellular dynamics, namely waves of oscillating left- and right-of-center protrusion at the leading edge of a migrating cell. To test this model prediction we studied in detail the dynamic behavior of the leading edge of scrambled and shp38γ cells stably transfected with RFP-actin.
Using time-lapse microscopy and image edge detection techniques (Supplementary Materials and Methods), we observed remarkable differences in leading edge protrusion between scrambled and shp38γ cells (Figs. 2A and 2C). At first observation, scrambled cells appeared to protrude in one continuous motion while shp38γ cells had alternating left- and right-of-center protrusions, corresponding to the two families of actin bundles at ±61.08° (Fig. 2A). Upon closer analysis, leading edge protrusion in both cell lines actually manifested alternations between left- and right-of-center leading edge regions (Figs. 2B and 2C). (In the case of scrambled cells these regions correspond to the stress fibers at ±3.42°.) However, the cell lines were distinguished by the time half-period between left and right protrusions being much longer (4.83 ± 1.09 minutes) in shp38γ cells than in scrambled cells (1.75 ± 0.14 minutes) (Fig. 2D). This periodicity is evident in the time lapse movies as the disjoint crawling motion of shp38γ cells (Movie 2), contrasting the coordinated protrusion/retraction cycle of scrambled cells (Movie 1). Also note the larger amplitudes of leading edge protrusions in shp38γ cells (Fig. 2C).
Figure 2. The leading edge protrusion dynamics predicted by the computational shp38γ model occur in live cells, and these behaviors differ between scrambled and shp38γ cells.
(A) Kymographs of leading edge protrusion in scrambled and shp38γ cells. (B) Detail showing the “left“ and “right” sides of the leading edges of scrambled and shp38γ cells. (C, D) Dynamics of left and right sides of cell leading edges. Leading edge protrusions from the highlighted regions in (B) are represented graphically (C). The reference is set such that mean forward displacement equals zero; thus, forward displacement greater than the mean of left and right appears positive, and forward displacement less than the mean appears negative. (D) The half-period in (C) (average time between left-right intersections) is significantly greater for shp38γ cells than scrambled cells (*p<0.0072, n = 3 for each cell line). (E) Time lapse images of RFP-actin-transfected scrambled (top) and shp38γ (bottom) cells, with finite element computations for each cell type shown below the corresponding live cell images (from Movie 4) (scale bar = 20 μm). Together with Supplemental Fig. S4 this validates our computational mechanical model of whole cell locomotion using sub-cellular motility data.
These live cell results confirmed the computational model predictions. We repeated our computational cell motility studies with the mean left/right leading edge protrusion amplitudes and time periods, and trailing edge retraction rates (Supplementary Fig. S4A) which we measured via time-lapse microscopy now used as targets to be met by controlling the actin fiber extension/contraction rates in the model (Supplementary Materials and Methods). Incorporating these experimentally-measured dynamics further refined our computational models, showed good agreement between the live and computational cells, and importantly did not fundamentally alter the refined computational cell model results from the initial ones in a qualitative sense (compare Figs. 1F and 2E; Movie 4). Through a series of additional control computational simulations (Supplementary Fig. S4 and Supplementary Results) we determined that, other parameters remaining the same, modifications in cytoskeletal architecture alone are sufficient to influence cell motility in the manner observed experimentally: from creating effective locomotion in live scrambled cells to ineffective locomotion of shp38γ cells.
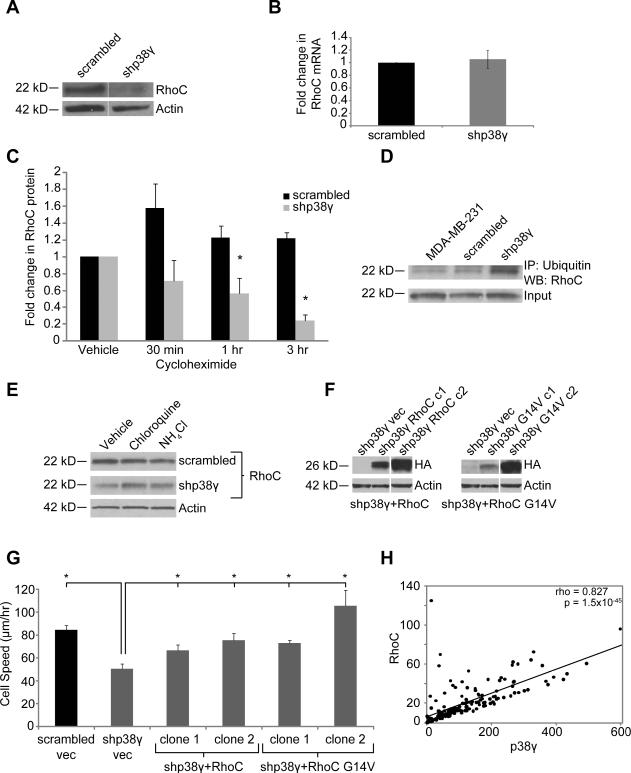
Expression of the cytoskeletal remodeler and metastasis-promoting gene RhoC is decreased in shp38γ cells
Rho-family GTPases (Rac, Rho, and cdc42) are the classic regulators of actin cytoskeletal dynamics driving cell motility (23). Based on the actin cytoskeletal changes and motility defects we observed, we hypothesized that p38γ knockdown affected members of the Rho GTPase family. Interestingly, we found that RhoC GTPase protein levels were downregulated in both shp38γ and DNp38γ cells (Fig. 3A and Supplementary Fig. S2F), with no change in the close homolog RhoA (data not shown). Surprisingly, RhoC mRNA levels were unaffected by p38γ knockdown (Fig. 3B), suggesting p38γ influences RhoC expression at the translational or post-translational level.
Figure 3. p38γ affects RhoC expression by mediating RhoC ubiquitination and lysosomal degradation.
Expression of RhoC GTPase protein (A) but not mRNA (B) is significantly reduced by p38γ knockdown. (C) Cycloheximide treatment reveals that RhoC protein is less stable in shp38γ cells. (D) RhoC ubiquitination is increased in shp38γ cells. (E) RhoC is degraded by the lysosome, as treatment with lysosome inhibitors leads to an increase in RhoC protein in shp38γ cells. (F) shp38γ cells were stably transfected with either HA-tagged RhoC (left) or a constitutively active form of RhoC, RhoC G14V (right). Two clones—one high expressing and one low expressing—were selected for each construct. (G) Re-expressing RhoC in shp38γ cells significantly increases shp38γ cell speed to levels comparable to scrambled cells (*p<0.05). (H) Expression of p38γ and RhoC are strongly positively correlated in clinical BC samples (n=177, rho = 0.827, p=1.5 × 10−45).
p38γ knockdown leads to increased RhoC ubiquitination and degradation
To determine whether p38γ affects RhoC expression at the translational or post-translational level we treated scrambled and shp38γ cells with the translation inhibitor cycloheximide and observed the effect on protein expression. Cycloheximide treatment caused RhoC protein levels to rapidly decrease in shp38γ cells with no corresponding decrease in scrambled cells (Fig. 3C), indicating that RhoC protein is less stable in shp38γ cells.
Upon observing this change in stability we asked whether RhoC was ubiquitinated in shp38γ cells—a common marker of proteins slated for degradation. Upon assaying RhoC ubiquitination we found that levels of ubiquitinated RhoC were increased in shp38γ compared to scrambled cells (Fig. 3D).
To elucidate the RhoC degradation mechanism, we treated cells with either proteasome inhibitors (MG132 or lactacystin) or lysosome inhibitors (ammonium chloride or chloroquine) and observed the effect on RhoC protein levels. Lysosome inhibition caused an increase in RhoC protein (Fig. 3E) whereas proteasome inhibitors had no effect on RhoC expression (Supplementary Fig. S1D), indicating that ubiquitinated RhoC is degraded by the lysosome. Taken together, these data support the conclusion that p38γ affects RhoC expression by mediating RhoC protein stability through regulation of RhoC ubiquitination and lysosomal degradation.
Rescuing RhoC expression restores motility to shp38γ cells
Based on the established roles of RhoC in cell motility and metastasis (24, 25) we hypothesized that decreased RhoC expression in shp38γ cells directly contributes to the impaired motility of shp38γ cells. To test this hypothesis, we stably transfected either RhoC or a constitutively active variant (RhoC G14V) into shp38γ cells and observed the effect on cell motility (Fig. 3F).
Live cell microscopy revealed that re-expressing RhoC significantly increased motility of the shp38γ cells to levels comparable to scrambled control cells (Fig. 3G). Interestingly, the cell speed increase seemed to have a near-linear relationship with RhoC expression/activity. Combined with our previous observations, these data establish a novel mechanistic link whereby p38γ directs cell motility through stabilization of RhoC protein.
p38γ and RhoC expression are strongly positively correlated in human BC tissues
We next asked whether the p38γ-RhoC relationship persists in human BC tissues. To address this question we assayed expression of p38γ and RhoC using AQUA of immunofluorescence signals for each marker in cytokeratin-positive cells from a BC tissue microarray. Analysis of 177 BC specimens revealed a strong positive correlation between p38γ and RhoC expression (Fig. 3H). Taken together with our in vitro results, these data strongly support a p38γ-RhoC relationship in BC, one that likely involves p38γ regulation of RhoC expression.
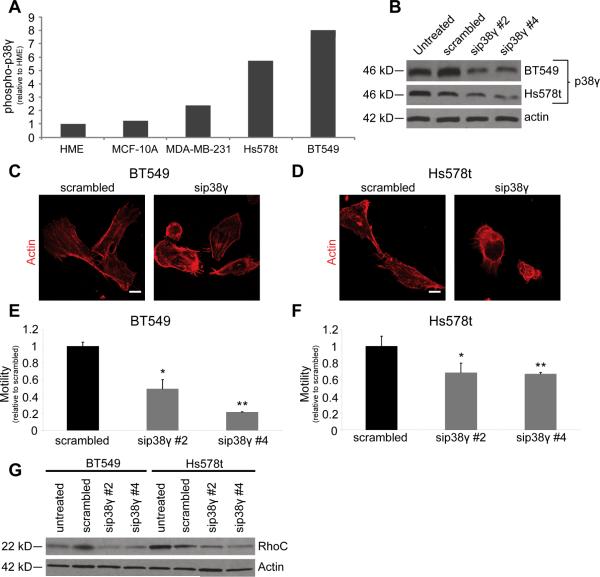
p38γ affects the metastatic properties of other aggressive BC cell lines
Because p38γ specifically alters the actomyosin contractile motility of MDA-MB-231 cells by affecting RhoC expression, we asked whether it plays a similar role in other mesenchymal-like BC cell lines. To address this question we used two additional widely-studied BC cell lines – Hs578t and BT549 – both of which have increased levels of phosphorylated p38γ compared to both MCF-10A and HME non-tumorigenic mammary epithelial cells (Fig. 4A). We transiently knocked down p38γ expression in these two cell lines with siRNA (Fig. 4B) and observed the effects on the actin cytoskeleton and cell motility.
Figure 4. p38γ functions similarly in two additional aggressive BC cell lines.
(A) Levels of phospho-p38γ are elevated in the MDA-MB-231, Hs578t, and BT549 BC cell lines compared to both HME and MCF-10A non-tumorigenic mammary epithelial cells. Graph shows IP/input (phospho-p38γ/total p38γ) quantification of a representative western blot. (B) Transient siRNA knockdown of p38γ in BT549 and Hs578t cells. The siRNA did not affect the other p38 isoforms (data not shown). (C–D) The actin cytoskeleton and cell shape of sip38γ BT549 (C) and Hs578t (D) cells are altered in a manner similar to 231 shp38γ cells (scale bar = 25 μm). (E–F) p38γ knockdown significantly reduces cell motility as measured by a bead motility assay in BT549 (E) and Hs578t (F) cells (For two BT549 siRNAs: *p = 0.0428, **p = 1.64 × 10−5; for two Hs578t siRNAs: *p = 0.0001, **p = 0.0012). (G) RhoC expression is reduced in siRNA-transfected BT549 and Hs578t cells.
siRNA knockdown of p38γ (“sip38γ”) dramatically affected the actin cytoskeletal architecture and cell shape of both cell lines in a manner consistent with 231 shp38γ cells (Figs. 4C–D): sip38γ cells moved significantly less than scrambled cells (Figs. 4E–F). p38γ knockdown in these cell lines also reduced RhoC expression (Fig. 4G). Taken together, these results support our conclusion that p38γ plays a crucial role in promoting motility of aggressive BC cells by modulating RhoC expression.
p38γ is associated with the basal-like BC subtype
Having discovered that p38γ is associated with mesenchymal-like cell motility and is overactivated in three basal-like BC cell lines, we hypothesized that p38γ is associated with the basal-like BC subtype. To test this hypothesis we assayed p38γ expression in 43 BC patient samples that were previously analyzed for molecular subtype by PAM50 (26). We divided the cohort into “high” (top 25%) and “low” (bottom 75%) p38γ-expressing tumors and examined the association between p38γ expression level and molecular subtype. We found that the basal-like subtype is significantly enriched in the high p38γ-expressing tumors (p = 0.018, Fig. 5A), whereas none of the other subtypes examined showed significant association with p38γ expression (data not shown). Taken together with our in vitro data, this clinical data strongly supports an association between high p38γ expression and the basal-like BC subtype.
Figure 5. p38γ is associated with the basal-like BC subtype and impacts metastasis and patient survival.
(A) The basal-like BC subtype is enriched in high (top 25%) compared to low (bottom 75%) p38γ-expressing patients (p = 0.018). (B) Quantification of lymph node metastasis of orthotopically xenografted 231 scrambled and shp38γ cells. Scrambled cells had a significantly higher incidence of metastasis into the contralateral mammary lymph nodes (p = 0.047), as measured by Fisher's exact test. (C) Representative images of 231 scrambled cells invading the ipsilateral lymph node (left) and a metastatic cluster in the contralateral respective mammary gland (right). The intact lymph node capsule is indicated by the arrowhead, while invading scrambled cells are marked by the arrow and magnified in the inset (all scale bars = 100 μm). (D) High p38γ expression is associated with lower overall BC patient survival as determined by Kaplan-Meier analysis (p = 0.013).
p38γ is necessary for BC metastasis in vivo and is clinically associated with lower overall patient survival
Based on our in vitro observations we hypothesized that p38γ knockdown reduces the metastatic potential of MDA-MB-231 BC cells in vivo. To test this hypothesis we orthotopically xenografted athymic nude mice with 231 scrambled and shp38γ cells and allowed tumors to grow for 14 weeks, at which point we resected the tumors and analyzed the contralateral respective lymph node for signs of metastasis (see Materials and Methods). Two-thirds of mice injected with scrambled cells presented with lymphatic invasion and metastasis, compared to one of nine shp38γ-injected mice (Figs. 5B–C). This in vivo data, combined with the extensive body of in vitro data presented here, strongly suggests that p38γ is necessary for metastasis, and that it exerts its effects, at least in part, by promoting cell motility through regulation of RhoC.
As a final determination of the clinical relevance of p38γ expression we assayed p38γ levels in a cohort of 118 BC cases containing patient survival data. Using the same high/low cutoffs as the molecular subtype analysis, we found a significant association between high p38γ expression and lower overall patient survival (p = 0.013, Fig. 5D). This clinical data supports our in vitro and in vivo findings that p38γ is an important mediator of BC cell aggressiveness, establishing that p38γ expression has important implications for BC patient outcome.
Discussion
As the breast is a non-vital organ, primary tumor burden is very rarely the direct cause of cancer-specific mortality. Instead, metastasis of cells from the primary tumor to vital organs results in patient death. Here we demonstrate that p38γ is a novel metastasis-associated gene, which acts in part by affecting cytoskeletal orientation, cell motility, and RhoC.
The importance of the p38 MAPK pathway in cancer has been appreciated (6, 27), but diverse, and sometimes contradictory, roles have been described for p38 in cancer (13). In agreement with other recent findings (15, 28) we show that at least some of the discrepancy in p38 function may be attributed to the distinct contributions of p38 isoforms. The p38 MAPK pathway is ubiquitously utilized in stress-response., It logically follows that metastasizing cancer cells, which encounter an ever-changing milieu of cellular stresses, may gain survival advantages under stressed conditions upon modulation of the appropriate p38 MAPK isoforms; thus, dissecting the contributions of each p38 isoform would allow more precise targeting of specific subsets of cancer. p38γ is an especially promising drug target as, in addition to the data presented here, it is a kinase, has restricted tissue expression (8, 10, 11), and lacks a phenotype when knocked out in mice (29)—possibly indicating that its inhibition may offer a differential detrimental effect on tumor versus normal cells.
Using a computational cell model in combination with live cell microscopy studies we showed that p38γ promotes BC cell motility at least in part by mediating actin cytoskeletal remodeling to create proper stress fiber orientation. Using the computational cell models, we first noted that whereas the observed whole cell locomotion could be produced by simultaneous action of all stress fibers in scrambled cells, the experimentally-observed ineffective locomotion of shp38γ cells was only possible if the action alternated between the two families of actin bundles seen in this cell phenotype—a result that would prove difficult to demonstrate using cell biological techniques alone.
The computational model predicted, and subsequent biological experiments verified, the presence of left-right leading edge protrusion oscillations in both scrambled and shp38γ cells. We note that other groups have identified oscillations in components of the leading edge (30, 31), however these oscillations all involved forward-backward (protrusion-retraction) movement of the leading edge as a whole. To the best of our knowledge the data presented here is thus the first evidence of left-right oscillations in the leading edge. Interestingly, the principle difference between the two cell types studied here (scrambled and shp38γ) lies in the periodicity of these oscillations. Through detailed in silico experimentation (Fig. 2, Supplementary Fig. S4) we determined that leading edge protrusion oscillation periodicity is influenced by and inseparable from cytoskeletal architecture. This inseparability sharpens the question of exactly how leading edge actin dynamics are linked to cell shape and cytoskeletal morphology. While this is a subject for future investigation, we do speculate on the chain of events: p38γ knockdown results in cytoskeletal architecture changes, which lead to alteration of cell shape, compensatory changes in leading edge protrusion periodicity, and ultimately modified whole cell motility.
Oscillation of other components of cell motility, such as cell shape (32) and trailing edge retraction (33), have been shown to be essential for productive cell motion in Dictyostelium (32) and fish keratocytes (33). We expect further investigations to uncover links between these processes and leading edge protrusion oscillations.
p38γ bears analogies with other mediators of normal mesenchymal differentiation such as twist and snail, which have been shown to also be important promoters of cancer progression (34, 35): p38γ functions in muscle cell differentiation, and thus it is consistent that it functions in mesenchymal-like BC cells. Interestingly, p38γ appears to exert its effects independently of classical cell differentiation markers, as p38γ knockdown does not alter expression of epithelial-tomesenchymal transition markers such as vimentin or E-cadherin (data not shown) despite significantly reverting the mesenchymal-like phenotype of aggressive BC cells. Although many other genes have been shown to drive metastatic transition (36), based on the data presented here we propose that p38γ serves as a crucial regulator of the major cytoskeletal changes necessary for the switch to rapid, mesenchymal-like cell motility—independent of differentiation status—at least in part by modulating RhoC.
RhoC is involved in stress fiber formation and actomyosin contraction (37, 38)— both of which are perturbed by p38γ knockdown—and has previously been linked to the p38 MAPK pathway (39). RhoC also drives metastasis in several types of cancer, including breast (37, 40–42), and plays a larger role in stress fiber formation and contraction than RhoA (43); thus we postulated that changes in RhoC expression influence the shp38γ phenotype. Supporting this hypothesis we found that re-expressing RhoC alone was sufficient to restore motility to shp38γ cells—a surprising feat, given the multitude of proteins involved in cell motility (44, 45), which highlights the importance of RhoC in p38γ-mediated cell motility. Further supporting the p38γ-RhoC link is our finding that expression of the two proteins is concurrently altered in clinical BC specimens (Fig. 5A), suggesting that the p38γ-RhoC axis may be functionally significant—and a potentially druggable target—in the clinic.
We discovered that p38γ regulates RhoC expression by preventing RhoC ubiquitination and subsequent lysosomal degradation. Although other p38 isoforms have been linked to protein ubiquitination (46, 47), ours is the first evidence of p38γ contributing to this process. Ubiquitination and protein degradation have recently emerged as important mechanisms for regulating Rho GTPase expression (48), however this is the first demonstration of modulating RhoC ubiquitination as a relatively fast mechanism to regulate RhoC action within a time domain relevant to cell motion. Further research into the specific proteins and mechanisms regulating ubiquitination of RhoC and other Rho GTPases should have significant impact on our understanding of how cell motility and metastasis are regulated.
Supplementary Material
Acknowledgments
We thank the University of Michigan Microscopy and Image Analysis lab for excellent technical support with all microscopy work; Dr. J. Erby Wilkinson and the ULAM Pathology Core for tissue processing and pathologic analysis; and Dr. Dafydd Thomas and the UMCCC Tissue Core for performing the AQUA staining and image acquisition.
Grant Support This work was supported by the Department of Defense Breast Cancer Research Program through a Predoctoral Traineeship award (BC083262) (D.T.R.) and a Multidisciplinary Postdoctoral award (A.C.V.), an NIH Cellular and Molecular Biology Training Grant (T32-GM07315) (D.T.R.), the Burroughs Wellcome Fund (S.D.M.), the Breast Cancer Research Foundation (S.D.M.); and a Department of Energy PECASE award (K.G.). A.C.V. is a member of the Carrera del Investigador Científico (CONICET)
References
- 1.Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430–41. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 2.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 3.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–75. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Huang S, Sah VP, Ross J, Jr., Brown JH, Han J, et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–8. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 5.Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101:4667–79. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 6.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 7.Qi X, Pohl NM, Loesch M, Hou S, Li R, Qin JZ, et al. p38alpha antagonizes p38gamma activity through c-Jun-dependent ubiquitin-proteasome pathways in regulating Ras transformation and stress response. J Biol Chem. 2007;282:31398–408. doi: 10.1074/jbc.M703857200. [DOI] [PubMed] [Google Scholar]
- 8.Tortorella LL, Lin CB, Pilch PF. ERK6 is expressed in a developmentally regulated manner in rodent skeletal muscle. Biochem Biophys Res Commun. 2003;306:163–8. doi: 10.1016/s0006-291x(03)00936-7. [DOI] [PubMed] [Google Scholar]
- 9.Cuenda A, Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem. 1999;274:4341–6. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- 10.Wang XS, Diener K, Manthey CL, Wang S, Rosenzweig B, Bray J, et al. Molecular cloning and characterization of a novel p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:23668–74. doi: 10.1074/jbc.272.38.23668. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Jiang Y, Ulevitch RJ, Han J. The primary structure of p38 gamma: a new member of p38 group of MAP kinases. Biochem Biophys Res Commun. 1996;228:334–40. doi: 10.1006/bbrc.1996.1662. [DOI] [PubMed] [Google Scholar]
- 12.Tang J, Qi X, Mercola D, Han J, Chen G. Essential role of p38gamma in K-Ras transformation independent of phosphorylation. J Biol Chem. 2005;280:23910–7. doi: 10.1074/jbc.M500699200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loesch M, Chen G. The p38 MAPK stress pathway as a tumor suppressor or more? Front Biosci. 2008;13:3581–93. doi: 10.2741/2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi X, Tang J, Loesch M, Pohl N, Alkan S, Chen G. p38gamma mitogen-activated protein kinase integrates signaling crosstalk between Ras and estrogen receptor to increase breast cancer invasion. Cancer Res. 2006;66:7540–7. doi: 10.1158/0008-5472.CAN-05-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loesch M, Zhi HY, Hou SW, Qi XM, Li RS, Basir Z, et al. p38{gamma} MAPK cooperates with c-Jun in trans-activating matrix metalloproteinase 9. J Biol Chem. 2010 doi: 10.1074/jbc.M110.105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nocito A, Kononen J, Kallioniemi OP, Sauter G. Tissue microarrays (TMAs) for high-throughput molecular pathology research. Int J Cancer. 2001;94:1–5. doi: 10.1002/ijc.1385. [DOI] [PubMed] [Google Scholar]
- 17.McCabe A, Dolled-Filhart M, Camp RL, Rimm DL. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. J Natl Cancer Inst. 2005;97:1808–15. doi: 10.1093/jnci/dji427. [DOI] [PubMed] [Google Scholar]
- 18.Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–61. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- 19.Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273:1741–8. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- 20.Ladwein M, Rottner K. On the Rho'd: the regulation of membrane protrusions by Rho-GTPases. FEBS Lett. 2008;582:2066–74. doi: 10.1016/j.febslet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Rajah TT, Abidi SM, Rambo DJ, Dmytryk JJ, Pento JT. The motile behavior of human breast cancer cells characterized by time-lapse videomicroscopy. In Vitro Cell Dev Biol Anim. 1998;34:626–8. doi: 10.1007/s11626-996-0009-7. [DOI] [PubMed] [Google Scholar]
- 22.Partin AW, Schoeniger JS, Mohler JL, Coffey DS. Fourier analysis of cell motility: correlation of motility with metastatic potential. Proc Natl Acad Sci U S A. 1989;86:1254–8. doi: 10.1073/pnas.86.4.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301:43–9. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Hakem A, Sanchez-Sweatman O, You-Ten A, Duncan G, Wakeham A, Khokha R, et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19:1974–9. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:5222–32. doi: 10.1158/1078-0432.CCR-10-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon C, Goepfert H, Boyd D. Inhibition of the p38 mitogen-activated protein kinase by SB 203580 blocks PMA-induced Mr 92,000 type IV collagenase secretion and in vitro invasion. Cancer Res. 1998;58:1135–9. [PubMed] [Google Scholar]
- 28.Chen L, Mayer JA, Krisko TI, Speers CW, Wang T, Hilsenbeck SG, et al. Inhibition of the p38 kinase suppresses the proliferation of human ER-negative breast cancer cells. Cancer Res. 2009;69:8853–61. doi: 10.1158/0008-5472.CAN-09-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabio G, Arthur JS, Kuma Y, Peggie M, Carr J, Murray-Tait V, et al. p38gamma regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 2005;24:1134–45. doi: 10.1038/sj.emboj.7600578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Cho A, Yin H, Schafer DA, Mouneimne G, Simpson KJ, et al. Psidin, a conserved protein that regulates protrusion dynamics and cell migration. Genes Dev. 2011;25:730–41. doi: 10.1101/gad.2028611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiner OD, Marganski WA, Wu LF, Altschuler SJ, Kirschner MW. An actin-based wave generator organizes cell motility. PLoS Biol. 2007;5:e221. doi: 10.1371/journal.pbio.0050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Alamo JC, Meili R, Alonso-Latorre B, Rodriguez-Rodriguez J, Aliseda A, Firtel RA, et al. Spatio-temporal analysis of eukaryotic cell motility by improved force cytometry. Proc Natl Acad Sci U S A. 2007;104:13343–8. doi: 10.1073/pnas.0705815104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnhart EL, Allen GM, Julicher F, Theriot JA. Bipedal locomotion in crawling cells. Biophys J. 2010;98:933–42. doi: 10.1016/j.bpj.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–23. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Golen KL, Wu ZF, Qiao XT, Bao LW, Merajver SD. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000;60:5832–8. [PubMed] [Google Scholar]
- 38.Wu M, Wu ZF, Merajver SD. Rho proteins and cell-matrix interactions in cancer. Cells Tissues Organs. 2007;185:100–3. doi: 10.1159/000101309. [DOI] [PubMed] [Google Scholar]
- 39.van Golen KL, Bao LW, Pan Q, Miller FR, Wu ZF, Merajver SD. Mitogen activated protein kinase pathway is involved in RhoC GTPase induced motility, invasion and angiogenesis in inflammatory breast cancer. Clin Exp Metastasis. 2002;19:301–11. doi: 10.1023/a:1015518114931. [DOI] [PubMed] [Google Scholar]
- 40.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–5. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 41.Ikoma T, Takahashi T, Nagano S, Li YM, Ohno Y, Ando K, et al. A definitive role of RhoC in metastasis of orthotopic lung cancer in mice. Clin Cancer Res. 2004;10:1192–200. doi: 10.1158/1078-0432.ccr-03-0275. [DOI] [PubMed] [Google Scholar]
- 42.Islam M, Lin G, Brenner JC, Pan Q, Merajver SD, Hou Y, et al. RhoC expression and head and neck cancer metastasis. Mol Cancer Res. 2009;7:1771–80. doi: 10.1158/1541-7786.MCR-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu M, Wu ZF, Rosenthal DT, Rhee EM, Merajver SD. Characterization of the roles of RhoC and RhoA GTPases in invasion, motility, and matrix adhesion in inflammatory and aggressive breast cancers. Cancer. 2010;116:2768–82. doi: 10.1002/cncr.25181. [DOI] [PubMed] [Google Scholar]
- 44.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–22. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 45.Rosenthal DT, Brenner JC, Merajver SD. Rho Proteins in Cancer. In: van Golen KL, editor. The Rho GTPases in Cancer. Springer New York; New York: 2010. pp. 29–42. [Google Scholar]
- 46.Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, et al. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–70. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellei B, Maresca V, Flori E, Pitisci A, Larue L, Picardo M. p38 regulates pigmentation via proteasomal degradation of tyrosinase. J Biol Chem. 2010;285:7288–99. doi: 10.1074/jbc.M109.070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nethe M, Hordijk PL. The role of ubiquitylation and degradation in RhoGTPase signalling. J Cell Sci. 2010;123:4011–8. doi: 10.1242/jcs.078360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.