Abstract
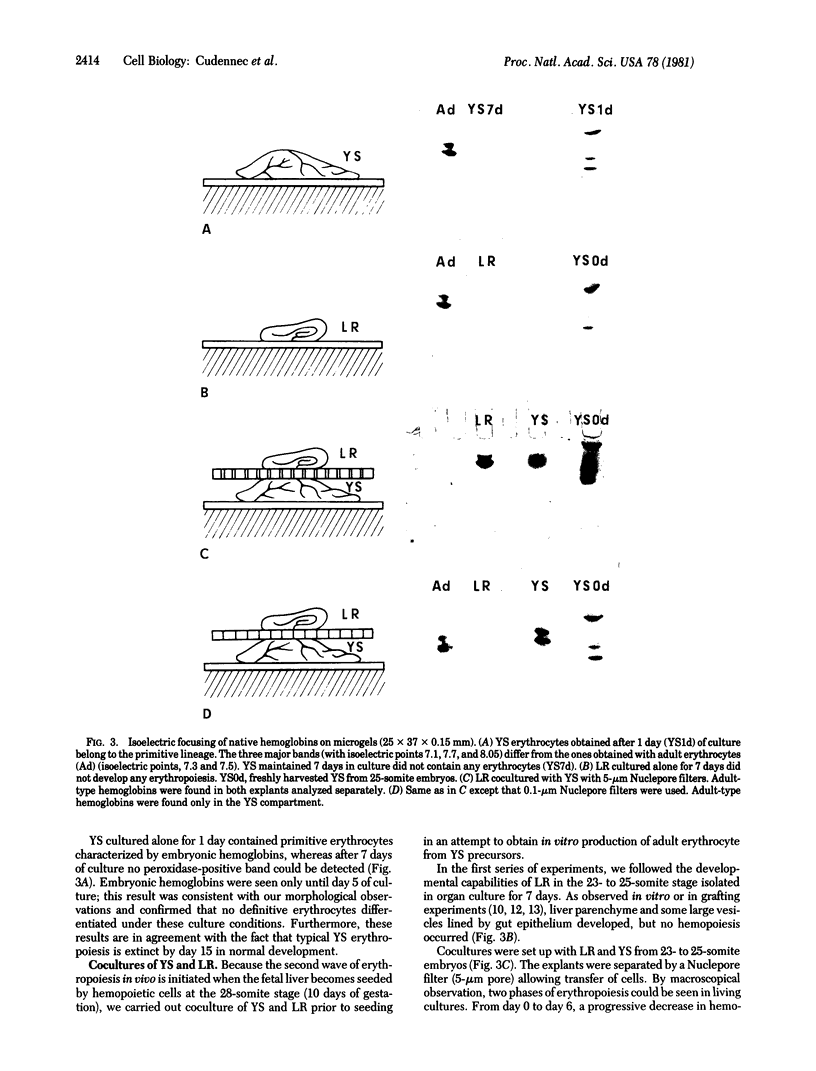
The capacity of yolk sac hemopoietic cells to produce either primitive or definitive erythrocytes was analyzed in vitro under three different experimental conditions. (i) Before the 28-somite stage (prior to colonization of the liver rudiment by hemopoietic cells), yolk sac explanted alone produced solely primitive erythrocytes and only for a short time. (ii) When allowed to colonize a liver rudiment, hemopoietic cells from the yolk sac gave rise to definitive erythrocytes. (iii) These cells could express the same capacity when stimulated by various intraembryonic organs, even if no direct cell--cell contact was established between stimulating tissue and target hemopoietic cells. These results provide evidence that humoral factors present in embryos past the 28-somite stage act on hemopoietic cells, inducing the onset of definitive erythropoiesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. E. Development of the mouse hematopoietic system. I. Types of hemoglobin produced in embryonic yolk sac and liver. Dev Biol. 1968 Jul;18(1):14–29. doi: 10.1016/0012-1606(68)90020-1. [DOI] [PubMed] [Google Scholar]
- Beaupain D., Martin C., Dieterlen-Lièvre F. Are developmental hemoglobin changes related to the origin of stem cells and site of erythropoiesis? Blood. 1979 Feb;53(2):212–225. [PubMed] [Google Scholar]
- Brotherton T. W., Chui D. H., Gauldie J., Patterson M. Hemoglobin ontogeny during normal mouse fetal development. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2853–2857. doi: 10.1073/pnas.76.6.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAIG M. L., RUSSELL E. S. A DEVELOPMENTAL CHANGE IN HEMOGLOBINS CORRELATED WITH AN EMBRYONIC RED CELL POPULATION IN THE MOUSE. Dev Biol. 1964 Oct;10:191–201. doi: 10.1016/0012-1606(64)90040-5. [DOI] [PubMed] [Google Scholar]
- Dieterlen-Lievre F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J Embryol Exp Morphol. 1975 Jun;33(3):607–619. [PubMed] [Google Scholar]
- Fantoni A., Bank A., Marks P. A. Globin composition and synthesis of hemoglobins in developing fetal mice erythroid cells. Science. 1967 Sep 15;157(3794):1327–1329. doi: 10.1126/science.157.3794.1327. [DOI] [PubMed] [Google Scholar]
- Gasic G. J., Boettiger D., Catalfamo J. L., Gasic T. B., Stewart G. J. Aggregation of platelets and cell membrane vesiculation by rat cells transformed in vitro by Rous sarcoma virus. Cancer Res. 1978 Sep;38(9):2950–2955. [PubMed] [Google Scholar]
- Gilman J. G., Smithies O. Fetal hemoglobin variants in mice. Science. 1968 May 24;160(3830):885–886. doi: 10.1126/science.160.3830.885. [DOI] [PubMed] [Google Scholar]
- HUTTON J. J., BISHOP J., SCHWEET R., RUSSELL E. S. Hemoglobin inheritance in inbred mouse strains. I. Structural differences. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1505–1513. doi: 10.1073/pnas.48.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssaint E. Differentiation of the mouse hepatic primordium. I. An analysis of tissue interactions in hepatocyte differentiation. Cell Differ. 1980 Oct;9(5):269–279. doi: 10.1016/0045-6039(80)90026-3. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Jones R. O. Differentiation of the mammalian hepatic primordium in vitro. I. Morphogenesis and the onset of haematopoiesis. J Embryol Exp Morphol. 1973 Aug;30(1):83–96. [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Pure and mixed erythroid colony formation in vitro stimulated by spleen conditioned medium with no detectable erythropoietin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3879–3882. doi: 10.1073/pnas.74.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. R., Moore M. A. Role of stem cell migration in initiation of mouse foetal liver haemopoiesis. Nature. 1975 Dec 25;258(5537):726–728. doi: 10.1038/258726a0. [DOI] [PubMed] [Google Scholar]
- Jotereau F. V., Houssaint E., Le Douarin N. M. Lymphoid stem cell homing to the early thymic primordium of the avian embryo. Eur J Immunol. 1980 Aug;10(8):620–627. doi: 10.1002/eji.1830100809. [DOI] [PubMed] [Google Scholar]
- Kovach J. S., Marks P. A., Russell E. S., Epler H. Erythroid cell development in fetal mice: ultrastructural characteristics and hemoglobin synthesis. J Mol Biol. 1967 Apr 14;25(1):131–142. doi: 10.1016/0022-2836(67)90284-7. [DOI] [PubMed] [Google Scholar]
- Lassila O., Eskola J., Toivanen P., Martin C., Dieterlen-Lievre F. The origin of lymphoid stem cells studied in chick yold sac-embryo chimaeras. Nature. 1978 Mar 23;272(5651):353–354. doi: 10.1038/272353a0. [DOI] [PubMed] [Google Scholar]
- Martin C., Lassila O., Nurmi T., Eskola J., Dieterlen-Liévre F., Toivanen P. Intraembryonic origin of lymphoid stem cells in the chicken: studies with sex chromosome and IgG allotype markers in histocompatible yolk sac-embryo chimaeras. Scand J Immunol. 1979;10(4):333–338. doi: 10.1111/j.1365-3083.1979.tb01359.x. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970 Mar;18(3):279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Burgess A. W., Metcalf D. Similar molecular properties of granulocyte-macrophage colony-stimulating factors produced by different mouse organs in vitro and in vivo. J Biol Chem. 1979 Jun 25;254(12):5290–5299. [PubMed] [Google Scholar]
- Parker J. W., Metcalf D. Production of colony-stimulating factor in mitogen-stimulated lymphocyte cultures. J Immunol. 1974 Feb;112(2):502–510. [PubMed] [Google Scholar]
- Perah G., Feldman M. In vitro activation of the in vivo colony-forming units of the mouse yolk sac. J Cell Physiol. 1977 May;91(2):193–199. doi: 10.1002/jcp.1040910205. [DOI] [PubMed] [Google Scholar]
- Rifkind R. A., Chui D., Epler H. An ultrastructural study of early morphogenetic events during the establishment of fetal hepatic erythropoiesis. J Cell Biol. 1969 Feb;40(2):343–365. doi: 10.1083/jcb.40.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E., Perkins R. G., Zschunke M. A., Hoerl B. J., Maercklein P. B. Plasma membrane vesiculation in 3T3 and SV3T3 cells. I. Morphological and biochemical characterization. J Cell Sci. 1979 Feb;35:229–243. doi: 10.1242/jcs.35.1.229. [DOI] [PubMed] [Google Scholar]
- Wu N. C., Sikkema D. A., Zucker R. M. Mouse fetal hemoglobin. Biochim Biophys Acta. 1978 Sep 26;536(1):306–311. doi: 10.1016/0005-2795(78)90078-8. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk W. J., Emmelot P., Hilkmann H. A., Hilgers J., Feltkamp C. A. Rigid plasma-membrane-derived vesicles, enriched in tumour-associated surface antigens (MLr), occurring in the ascites fluid of a murine leukaemia (GRSL). Int J Cancer. 1979 Jan 15;23(1):62–70. doi: 10.1002/ijc.2910230112. [DOI] [PubMed] [Google Scholar]







