Abstract
Large conductance voltage- and Ca2+-activated K+ channels are important in regulating detrusor smooth muscle (DSM) function. Here, we examined systematically how the BK channel pharmacological activation modulates DSM contractility. NS1619, a potent BK channel activator, was utilized as a pharmacological tool to investigate the effect of BK channel activation on rat DSM contractility. Isometric tension recordings of DSM strips isolated from rat urinary bladder were performed systematically under various experimental conditions. NS1619 (30 μM) substantially diminished DSM spontaneous contraction amplitude, muscle force integral, frequency, duration and muscle tone. This effect was blocked by iberiotoxin, a BK channel selective inhibitor. NS1619 inhibited the phasic and tonic contractions in DSM strips pre-contracted with either the cholinergic agonist, carbachol (0.1 μM), or the depolarizing agent, KCl (20 mM and 60 mM KCl). In the presence of elevated KCl, the inhibitory effect of NS1619 was significantly reduced, indicating that BK channel activation is the underlying mechanism of NS1619 action. BK channel activation with NS1619 dramatically decreased the amplitude of electrical field stimulation (EFS)-induced contractions under a range of stimulation frequencies (0.5–50 Hz). In the presence of specific neurotransmitter inhibitors, BK channel activation with NS1619 significantly decreased both cholinergic and purinergic components of EFS-induced contractions. We conclude that BK channel activation with NS1619 significantly inhibited spontaneous, pharmacologically induced and nerve-evoked DSM contractions. Targeting the BK channel with selective openers may offer a unique opportunity to control DSM contractile activity, including pathophysiological conditions such as overactive bladder and detrusor overactivity, regardless of the underlying cause.
Keywords: urinary bladder, K+ channel opener, iberiotoxin, detrusor contractility
1. INTRODUCTION
Several classes of K+ channels have been studied in animal models of abnormal bladder function, and K+ channel openers have gained considerable interest as promising pharmacological targets for detrusor overactivity (Andersson and Arner, 2004; Petkov et al., 2001b). The large conductance voltage- and Ca2+-activated K+ channels also known as BKCa, KCa1.1, Slo, or MaxiK; play an important role in modulating nerve and muscle cell contractility and excitability (Andersson and Arner, 2004; Heppner et al., 1997; Salkoff et al., 2006). The BK channel is composed of 4 pore-forming α subunits with voltage sensors exhibiting a K+ selectivity filter and 4 regulatory, tissue-specific, β1-β4 subunits (Brenner et al., 2000; Meera et al., 2000; Petkov et al., 2001a). Mouse and rat detrusor smooth muscle (DSM) BK channel complex involves pore-forming α and regulatory β1/β4 subunits (Chen and Petkov, 2009; Kita et al., 2010). BK channels are activated by both depolarizing voltages and by an increase in intracellular free Ca2+, thus coupling the membrane potential to intracellular signaling (Petkov, 2009). Opening of the BK channels under physiological conditions leads to hyperpolarization of cell membrane which, in turn, closes voltage-gated Ca2+ channels and relaxes DSM cells (Heppner et al., 1997; Hristov et al., 2008; Petkov et al., 2001a; Petkov and Nelson, 2005). We previously reported that the functional coupling of β-adrenoreceptors, β3 in particular, to BK channel activity is a major inhibitory mechanism of DSM spontaneous contractions (Brown et al., 2008; Hristov et al., 2008; Petkov and Nelson, 2005). BK channel blockers have been shown to increase DSM contractility and excitability (Brown et al., 2008; Herrera et al., 2005; Herrera et al., 2000; Hristov et al., 2011; Hristov et al., 2008; Petkov et al., 2001a). In contrast, BK channel openers lead to hyperpolarization of cell membranes and have been identified as promising candidates to decrease DSM cell excitability and relax smooth muscle (Chang et al., 2010; Layne et al., 2010; Siemer et al., 2000).
NS1619 (1-(2’-hydroxy-5’-trifluoromethylphenyl)-5-trifluoromethyl-2(3H)benzimidazolone) has been shown to be a potent BK channel activator and has the potential to modulate cell excitability in neurons and smooth muscle (Edwards et al., 1994; Holland et al., 1996; Macmillan et al., 1995; Yamamura et al., 2001). NS1619 has been reported to relax smooth muscle with EC50 = 10–30 μM (Chang et al., 2010; Huang et al., 1997; Sheldon et al., 1997).
Here, we hypothesized that pharmacological opening of the BK channel with NS1619 can reduce DSM contractility under various experimental conditions. The aim of the present study was to systematically assess the effects of BK channel activation on spontaneous, pharmacologically induced, and nerve-evoked contractions of isolated rat DSM strips. For the first time, we also systematically evaluated the effects of BK channel activation on all contractile parameters including phasic contraction amplitude, muscle force integral, frequency, duration, and muscle tone. We found that the BK channel opener, NS1619 (30 μM), attenuated all contraction parameters of the spontaneous phasic and tonic contractions as well as that induced by carbachol, potassium, or nerve stimulation. Our study extends early findings (Chang et al., 2010; Sheldon et al., 1997; Siemer et al., 2000), collectively demonstrating the possibility of modulating the contractile status of DSM via targeted BK channel activation.
2. Materials and Methods
2.1 Tissue preparation
Twenty adult male Sprague-Dawley rats (average weight = 350±50 g) were used in this study. All animals were kept under standard laboratory conditions (12-h light/12-h dark). The rats were euthanized with CO2 followed by thoracotomy according to the protocol approved by the Institutional Animal Care and Use Committee of the University of South Carolina (Animal Use Protocol no. 1747). The urinary bladders were removed and placed in ice-cold dissection solution (80 mM monosodium glutamate, 55 mM NaCl, 6 mM KCl, 2 mM MgCl2, 10 mM HEPES and 10 mM glucose, adjusted to pH 7.3 with NaOH). Each bladder was sliced open and rinsed free of urine. The urothelial layer and lamina propria were also removed by careful dissection and only the DSM tissue was used in this study.
2.2 Isometric DSM tension recordings
Isometric contractions of DSM strips were measured as previously described (Chen et al., 2010; Hristov et al., 2011; Hristov et al., 2008). Briefly, small strips (~2 X 5 mm) were cut from the DSM and transferred to a small Petri dish containing Ca2+-free dissection solution. Each strip was secured to an isometric force displacement transducer and was suspended in temperature controlled (37 °C) water jacketed bath containing physiological saline solution (PSS; 119 mM NaCl, 4.7 mM KCl, 24 mM NaHCO3, 1.2 mM KH2PO4, 2.5 mM CaCl2, 1.2 mM MgSO4 and 11 mM glucose, and aerated with 95% O2-5% CO2 (pH 7.4). The DSM strips were initially stretched to 1 g of tension and washed with fresh PSS solution every 15 min during an equilibration period of 45–60 min. Three groups of treatments were set up for strips after the equilibration period. In the first group of DSM strips exhibiting spontaneous contractions, 30 μM NS1619 was added directly to the bath and at least 30–40 min were recorded in the presence of the drug. To assess the BK channel selectivity of NS1619; DSM strips were exposed to the BK channel specific inhibitor, iberiotoxin (IBTX; 200nM). After stabilization of the IBTX-induced phasic and tonic contractions, NS1619 (30 μM) was added to the bath and the effects of these drugs on all contraction parameters were assessed. In the second group, DSM phasic and tonic contractions were induced either with a cholinergic agonist (carbachol) or with a depolarizing agent (KCl), and after stabilization of the phasic and tonic contractions, NS1619 (30 μM) was introduced into the bath.
In the third set of experiments, we elucidated the effect of NS1619 on DSM nerve-evoked contractions. Nerve-evoked contractions were induced by electrical field stimulation (EFS) using a pair of platinum electrodes mounted in the tissue bath in parallel to the DSM strip. The EFS pulses were generated using a PHM-152I stimulator (MED Associates, Inc., Georgia, VT) and the EFS pulse parameters were as follows: 0.75 ms pulse width, 20 V pulse amplitude, 3 s stimulus duration and polarity was reversed for alternating pulses. For EFS studies, after the equilibration period, DSM strips were subjected to either continuous repetitive stimulation with a frequency of 20 Hz at 1-min intervals or to increasing frequencies from 0.5–50 Hz at 3-min intervals. The contractions were recorded using a Myomed myograph system (MED Associates).
2.3 Statistical Analysis
MiniAnalysis software (Synaptosoft, Inc., NJ) was used to analyze the DSM contraction parameters. The tonic and phasic components of the contractions were analyzed separately. A phasic contraction peak was determined as the peak that was superimposed over the tonic component of the entire contraction. In the muscle force integral only the phasic portion was included. Specifically, the DSM contractile activity was quantified by measuring the average phasic contraction amplitude (the difference between the force-time baseline curve and the maximum peak of the contraction), the frequency (contractions per min), muscle force integral (calculated by integrating the area under the force-time baseline curve), the phasic contraction duration (defined as width of contraction at 50% of the amplitude), and the DSM tone (the difference between the zero line and the force-time baseline curve). To compare the phasic contraction parameters for spontaneous, 0.1 μM carbachol or 20 mM KCl-induced contractions, data were normalized to the spontaneous, carbachol or KCl-induced contractions, respectively (taken to be 100%), and expressed as percentages. For the EFS-induced contractions, the maximal contraction amplitude at EFS frequency of 50 Hz under control conditions was taken to be 100% and the data were normalized. To evaluate the effect of NS1619 on phasic contractions, a 10 min period prior to NS1619 application was analyzed for the control and another 10 min period from 35th to 45th min after 30 μM NS1619 application was analyzed. The data are expressed as mean ± S.E.M. for the n, the number of DSM strips isolated from different rats (N= number of rats). All data were analyzed using paired Student’s t test. A P value less than 0.05 was considered significant. Statistical analysis was performed with GraphPad Prism 4.03 software (GraphPad Software, Inc., La Jolla, CA). GraphPad Prism and CorelDraw Graphic Suite X3 software (Corel Co.) was used to illustrate the data.
2.4 Drugs
All drugs were obtained from Sigma-Aldrich (St. Louis, MO). NS1619 was dissolved freshly in DMSO and a final concentration of 30 μM was used for all experiments. The maximum final DMSO concentration was 0.01%. Carbachol, tetrodotoxin, atropine, suramin, and α,β-methylene-ATP were freshly dissolved in distilled water prior to the experiment.
3. RESULTS
3.1 BK channel activation with NS1619 reduces spontaneous phasic and tonic contractions in rat DSM isolated strips
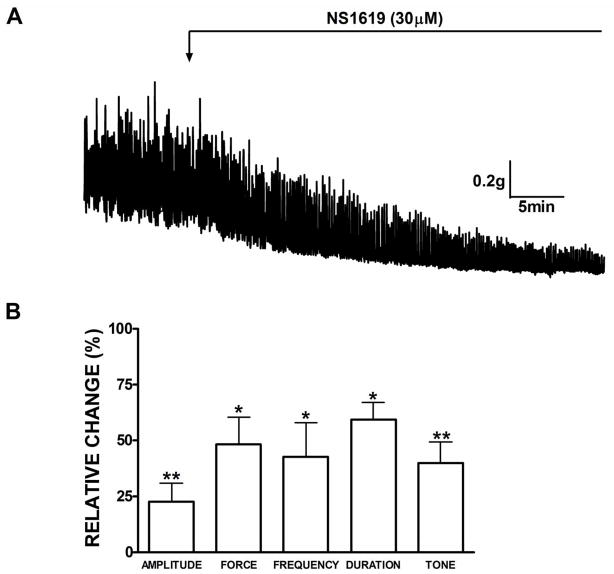
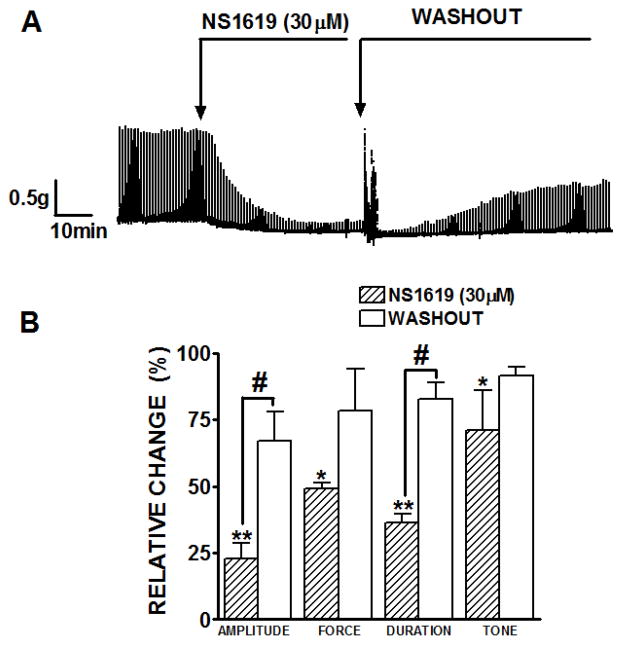
The first experimental series was performed to elucidate how activation of BK channels with NS1619 affects the spontaneous myogenic contractile activity of rat DSM isolated strips. Forty percent of all isolated rat DSM strips (48 strips out of 120 total strips) contracted spontaneously. Usually spontaneous contractions start to appear within the first 20 min after applying the initial tension of 1 g to DSM strips. This experimental series was carried out in the presence of neuronal Na+ channel blocker TTX (1 μM) to avoid any possible neurotransmitter release from the autonomic nerves located in DSM strips. Phasic contractions persisted in the presence of TTX in terms of the amplitude, frequency, and force suggesting a myogenic origin for these contractions. DSM strips undergoing spontaneous contractions were exposed to NS1619 (30 μM). Tension recordings from a representative experiment with NS1619 are shown in Fig. 1A. As illustrated, NS1619 (30 μM) dramatically decreased the DSM spontaneous contractile activity. Summarized data for the effect of NS1619 on all contractile parameters are illustrated in Fig. 1B. There was a statistically significant decrease in phasic contraction amplitude, muscle force integral, frequency of spontaneous contraction, phasic contraction duration and muscle tone. NS1619 reduced statistically significantly the phasic contraction amplitude to 22.5±8.2%, muscle force integral to 48.2±12.1%, frequency to 42.7±15.1%, duration to 59.3±2.5% as well as muscle tone to 39.9±9.3% (n=8, N=7; P<0.01; Fig. 1B). After a washout of NS1619 with fresh PSS, the recovery effect on the spontaneous contractions was significant on the amplitude and muscle force integral of spontaneous contractions (n=4, N=4; P<0.05). BK channel inhibition with IBTX significantly increased the spontaneous phasic contraction amplitude, muscle force integral, and tone compared to the control (n=7, N=3, P<0.05, Fig. 1). The phasic contraction amplitude was increased to 156.3±34.4%, muscle integral force to 163.0±35.8% and tone to 154.4±13.0%. These results are consistent with our previous findings on rat, mouse, and human DSM where we have demonstrated an increase in DSM phasic and tonic contractions in the presence of IBTX (Brown et al., 2008; Hristov et al., 2011; Petkov et al., 2001a). To assess the BK channel selectivity of NS1619 in our experiments, NS1619 was added to the bath in the continued presence of IBTX (Fig. 1C). The BK channel inhibitor, IBTX (200 nM) completely antagonized the inhibitory effects of NS1619 on all contraction parameters (n=7; N=3; Fig. 1D). These results indicate that NS1619 relaxes rat DSM through activation of IBTX-sensitive BK channels.
Fig. 1.
BK channel activation with NS1619 decreases the spontaneous phasic and tonic contractions of rat DSM isolated strips. A) Representative myograph recording obtained from a spontaneously contracting rat DSM strip before and after the addition of 30 μM NS1619. B) Summary data illustrating that NS1619 decreased the amplitude, muscle force integral, frequency and duration of spontaneous phasic contractions, and muscle tone. n=8, N=7, **P<0.005; *P<0.05. C) A representative myograph recording showing lack of NS1619 (30 μM) effect on the 200 nM IBTX-induced phasic and tonic contractions. D) Summary data illustrating the effect of NS1619 on all contraction parameters in the presence of 200 nM IBTX. n=7, N=3, P<0.05; IBTX versus control (ns= not significant; IBTX versus NS1619). Spontaneous contractions were taken to be 100%. Data are means ± S.E.M.
3.2 BK channel activation with NS1619 reduces pharmacologically induced DSM contractions
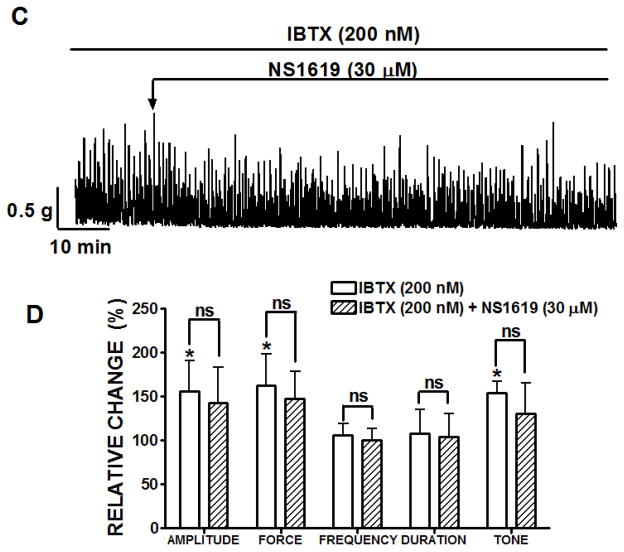
In the next set of experimental series, we sought to test the effect of NS1619 under conditions of either muscarinic receptor stimulation or sustained membrane depolarization. These experiments were also performed in the presence of TTX (1 μM) to inhibit neuronal activity. In the first experimental series, the DSM strips were stimulated with 0.1 μM carbachol, a cholinergic agonist. This low concentration of carbachol rapidly increased the spontaneous contractions of DSM strips (Fig. 2). The DSM strips were allowed to equilibrate for 30 min during the development of carbachol-induced phasic and tonic contractions. Application of NS1619 (30 μM) significantly decreased the amplitude (22.8±4.1%), muscle force integral (15.6±8.4%), frequency (34.1±2.9%), and tone (44.8±6.3%) of carbachol-induced contractions (n=9, N=7; P<0.05), without any significant effect on phasic contraction duration (81.1±12.2%; P>0.05; Fig. 2B).
Fig. 2.
Inhibitory effect of 30 μM NS1619 on 0.1 μM carbachol-induced phasic and tonic contractions of rat DSM isolated strips. A) A representative trace from a DSM isolated strip and the effect of NS1619 on carbachol-induced contractions. Summary data illustrating that NS1619 caused a significant decrease in the amplitude, muscle force integral, frequency and tone. B) NS1619 (30 μM) decreased the amplitude, muscle force integral, frequency and tone of carbachol-induced contractions. 0.1 μM carbachol-induced contractions were taken to be 100%. n=9, N=7, **P<0.005; *P<0.05. Data are means ± S.E.M.
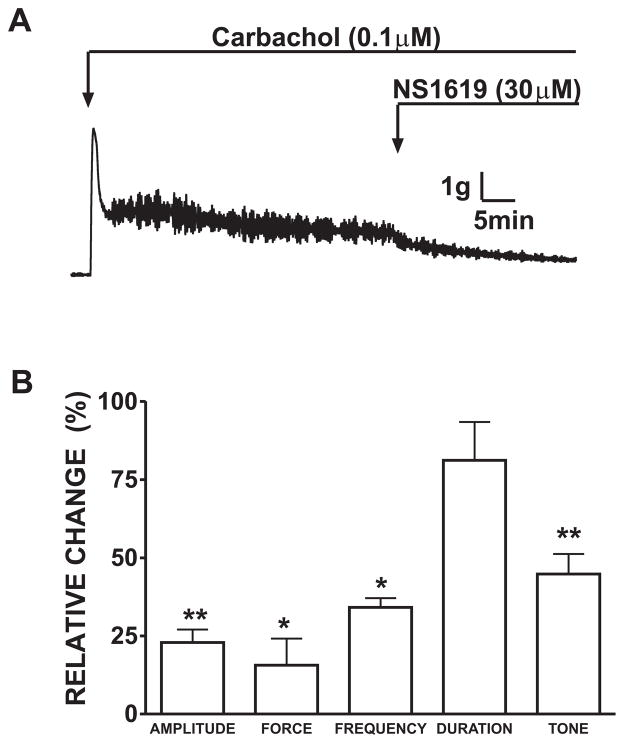
In the next experimental series, we used 20 mM KCl to depolarize DSM strips and induce contractions. In the presence of 20 mM extracellular K+, the rat DSM strips exhibited increased phasic and tonic contractions. As illustrated in Fig. 3, exposure to NS1619 (30 μM) caused a significant inhibition of the 20 mM KCl-induced phasic and tonic contractions. The amplitude of 20 mM KCl-induced phasic contractions was reduced to 39.5±2.6%, muscle force integral to 44.5±8.3%, frequency to 63.0±6.8%, and muscle tone to 47.0±6.6% (n=8, N=6; P<0.05; Fig. 3B). The effect of NS1619 on the duration of the 20 mM K+-induced contractions was insignificant (82.2±3.3%; P>0.05).
Fig. 3.
NS1619 inhibits the KCl-induced phasic and tonic contractions in rat DSM isolated strips. A) A representative recording from a rat DSM isolated strip showing the inhibitory effect of NS1619 (30 μM) on 20 mM K+-induced contractions. B) Summary data illustrating that NS1619 (30 μM) decreased the amplitude, muscle force integral, frequency and tone of 20 mM KCl-induced phasic contractions. 20 mM KCl induced contractions were taken to be 100%. n=8, N=6, **P<0.01; *P<0.05. C) Representative myograph recording of DSM strip contracted by 60 mM KCl and the lack of any significant NS1619 effect. D) Differential effects of NS1619 on the muscle tone in presence of low (20 mM K+) and high (60 mM K+) extracellular KCl concentrations (n=6, N=5; ns=not significant). Data are means ± S.E.M.
To gain a better understanding of BK channel activation; we tested depolarization-mediated DSM contraction elicited by a higher K+ concentration (60 mM K+). This KCl concentration induces a high amplitude tonic contraction in DSM strips (Fig. 3C). Higher concentrations of extracellular K+ in tissue bath raise the equilibrium potential for K+ and inhibit the relaxant effects of K+ channel activators (Trivedi et al., 1995). NS1619 had a relaxant effect in DSM strips pre-contracted by 20 mM KCl; however, this inhibition in DSM strips was much lower at a higher (60 mM) K+ concentration (Fig. 3D). In 20 mM K+ solution; NS1619 significantly inhibited DSM tone to 47.0±6.6% (n=8, N=6; P<0.05). However, NS1619 inhibited DSM tone to only 78.0±5.1% in 60 mM K+ solution and this inhibitory effect was not significant (n=6, N=5; P>0.05). This experimental series provides further support that opening of the BK channel with NS1619 is involved in DSM relaxation.
3.3 BK channel activation with NS1619 reduces the nerve-evoked contractions in DSM isolated strips
Activation of parasympathetic nerves causes DSM contractions during voiding. In pig detrusor, NS1619 at a high concentration of 300 μM has been shown to inhibit nerve-evoked contractility at a lower frequency of 0.05 Hz (Malysz et al., 2004). In this set of experimental series, we sought to explore the ability of NS1619 to reduce DSM neurogenic contractions at both 20 Hz and an increasing frequency range of 0.5–50 Hz. This experimental series was conducted in the absence of TTX. A representative myograph recording in Fig. 4A shows that NS1619 (30 μM) reduced the EFS-induced contractions at a stimulus frequency of 20 Hz. Fig. 4B shows that NS1619 had a significant inhibitory effect on all parameters of nerve-evoked contractions (20 Hz) including amplitude (22.6±5.8%), muscle force integral (49.2±2.0%), duration (36.4±3.2%) and tone (71.2±14.7%) (n=11, N=7; P<0.05). The drug recovery effect was significant on the amplitude (67.1±10.7%) and duration (82.8±5.9%) of the EFS-induced contractions following a washout of NS1619 with fresh PSS from the tissue bath (n=7, N=5; P<0.05; Fig. 4).
Fig. 4.
Inhibitory effect of NS1619 (30 μM) on continuous 20 Hz EFS-induced contractions of DSM isolated strips. A) Representative myograph recordings of EFS-evoked contractions obtained from rat DSM strips before and after the addition of 30 μM NS1619 followed by a washout of the drug. Contractile responses were evoked by repetitive (1/min) EFS (0.75 ms pulse width, 3 s pulse duration, 20 V pulse amplitude). B) Summary data illustrating that NS1619 decreased the peak amplitude, force, duration and tone of 20 Hz EFS-induced contractions n=11, N=7, **P<0.005. The effect of NS1619 on amplitude and duration was significantly reversed after washout of the drug with PSS. #Drug effect vs. washout, n=7, N=5; #P<0.01. Data are means ± S.E.M.
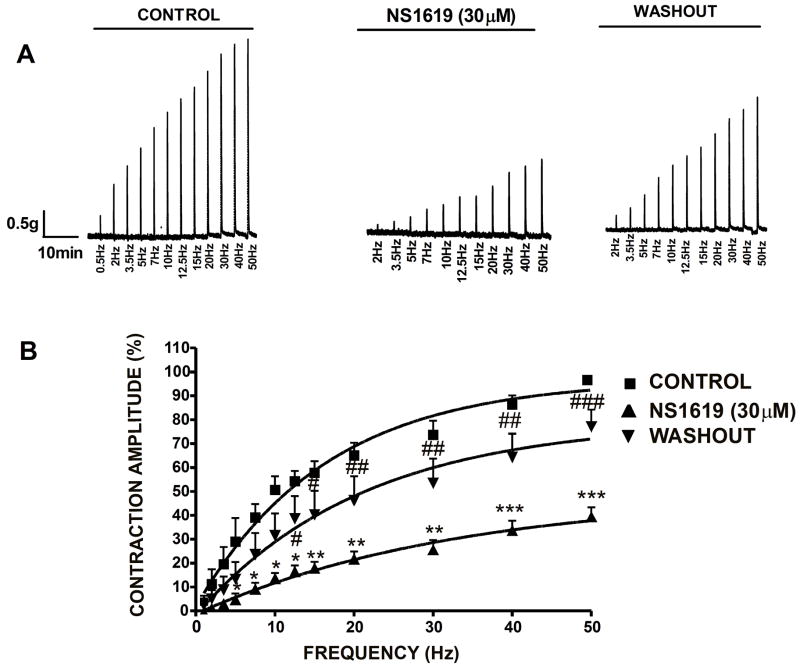
In another group of experiments, the DSM strips were stimulated by increasing EFS frequencies (0.5–50 Hz). The regulation of nerve-evoked contractions by BK channels was assessed by performing frequency-response curve in DSM strips in the presence and absence of NS1619 (30 μM). Following a frequency-response curve under control conditions, DSM strips were pre-incubated for 35 min with NS1619 (30 μM) and a frequency-response curve in the presence of NS1619 was generated again. Fig. 5A shows a representative myograph recording of EFS-induced contractions under control conditions, after exposure to NS1619 (30 μM) and after washout of NS1619. The frequency-response curves in the absence and presence of NS1619 (30 μM), followed by washout of NS1619 are presented in Fig. 5B. NS1619 significantly decreased the maximum amplitude generated in response to all EFS frequencies. Addition of NS1619 (30 μM) to the bath solution inhibited the EFS-induced contraction amplitude to 39.2±4.1% at 50 Hz (n=9, N=7; P<0.005), when compared with control responses (Fig. 5). Washout of NS1619 from the tissue bath with fresh PSS led to significant recovery in EFS-induced contractile responses (n=7, N=7; P<0.05). This experimental series further suggests that BK channel opening can reduce nerve-evoked contractions under a large range of stimulation frequencies.
Fig. 5.
NS1619 (30 μM) decreases the amplitude of the EFS-induced contractions in DSM isolated strips. A) Original trace of EFS-induced (stimulation frequency 0.5–50 Hz) contraction in the absence (control), 35 min after application of 30 μM NS1619 and following washout. B) Frequency-response curve illustrating a decrease in the amplitude of EFS-induced contractions following application of 30 μM NS1619. n=9, N=7, ***P<0.005; **P<0.01; *P<0.05. Washout of NS1619 with PSS showed a partial recovery effect on the amplitude of EFS-induced contractions. n=7, N=7, ###P<0.005; ##P<0.01; #P<0.05. The maximal contraction amplitude at EFS frequency of 50 Hz under control conditions was taken to be 100%. Data are means ± S.E.M.
3.4 BK channel activation with NS1619 reduces both cholinergic- and purinergic-mediated contractions in rat DSM isolated strips
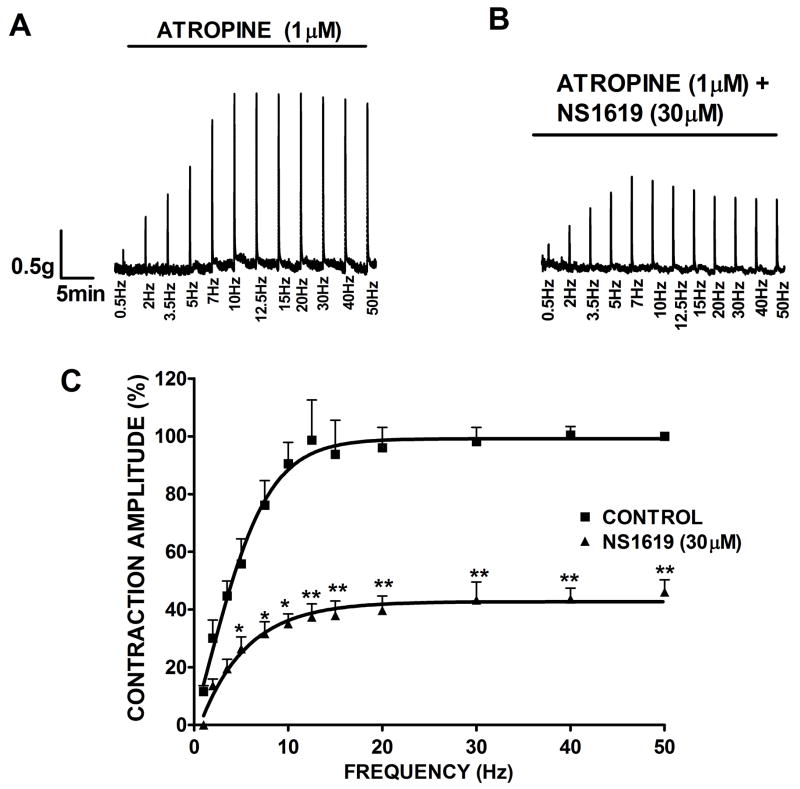
In the final experimental series, we dissected the BK channel contribution to cholinergic and purinergic components by using specific neurotransmitter receptor inhibitors. Atropine (1 μM) was added to the bath before the EFS frequency-response protocol to block the muscarinic acetylcholine receptors. As shown in Fig. 6, in the presence of atropine, the purinergic-mediated DSM contractions increased progressively over lower stimulation frequencies, and above 20 Hz, the frequency-response curve reached a plateau. This observation is consistent with other reports stating that cholinergic signaling has a prominent role at higher frequencies (Brading and Williams, 1990). Activation of the BK channel with NS1619 (30 μM) caused a statistically significant decrease in the purinergic component of EFS-induced contraction amplitude clearly evident above 7 Hz. The contraction amplitude was reduced to 46.0±4.1% at 50 Hz frequency (n=6, N=5; P<0.005).
Fig. 6.
Inhibitory effect of NS1619 on the purinergic EFS-induced contractions. In the presence of 1 μM atropine, activation of BK channel with NS1619 decreases the amplitude of the EFS-evoked contractions in rat DSM isolated strips. Original traces showing EFS-induced contractions (0.5–50 Hz) in the absence (control, A) and after application of 30 μM NS1619 (B). C) EFS frequency-response curves showing a significant decrease in the amplitude of purinergic EFS-induced contractions after application of 30 μM NS1619. The maximal contraction amplitude in the presence of atropine at EFS frequency of 50 Hz under control conditions was taken to be 100%. n=6, N=5, **P<0.005; *P<0.01. Data are means ± S.E.M.
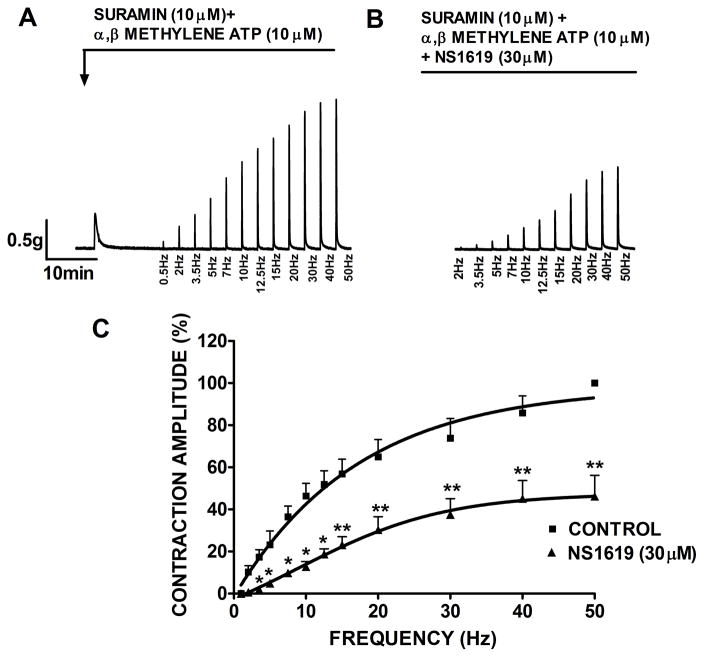
A similar experimental series was performed to probe whether BK channel opening can reduce the cholinergic-component of EFS-induced contractions. Suramin (10 μM) and α,β-methylene ATP (10 μM) were added to the bath solution to block purinergic receptors in DSM strips. α,β-methylene ATP is generally used to block purinergic receptors by initially activating and then rapidly desensitizing the receptors, thus inhibiting its downstream signaling pathway, whereas, suramin is a direct inhibitor of purinergic receptors. BK channel activation with NS1619 (30 μM) significantly decreased the contraction amplitude within the entire frequency range. As shown in Fig. 7, NS1619 reduced the contraction amplitude of the cholinergic component of EFS to 46.2±9.9% at 50 Hz frequency (n=7, N=5; P<0.005). Collectively, these results indicate that BK channel activation inhibits both cholinergic and purinergic nerve-evoked contractions.
Fig. 7.
Inhibitory effect of NS1619 on the cholinergic EFS-induced contractions. In the presence of 10 μM suramin and 10 μM α,β-methylene-ATP, activation of BK channel with NS1619 decreased the amplitude of the EFS-induced contractions in isolated DSM strips. Original recordings of nerve-evoked contractions induced by EFS (frequency of 0.5–50 Hz) in the absence (control, A) and following application of 30 μM NS1619 (B). C) EFS frequency-response curves showing a significant decrease in the amplitude of cholinergic EFS-induced contractions after application of 30 μM NS1619. The maximal contraction amplitude at EFS frequency of 50 Hz under control conditions in the presence of suramin and α,β-methylene-ATP was taken to be 100%. n=7, N=5, **P<0.005; *P<0.01. Data are means ± S.E.M.
4. DISCUSSION
The present study clearly identifies the BK channel as a pharmacological target to control DSM contractility. A major novel aspect of this study is the systematic evaluation of the effects of BK channel activation with NS1619 on all contractile parameters including contraction amplitude, muscle force integral, frequency, duration, and muscle tone. Here, we demonstrate the effect of BK channel activation with NS1619 on all contraction parameters of spontaneous, pharmacologically induced and nerve-evoked contractions in rat DSM isolated strips. Early observations based on the use of BK channel inhibitors and BK channel gene deletion suggest that changes in BK channel activity may contribute to bladder overactivity (Brown et al., 2008; Meredith et al., 2004; Petkov et al., 2001a; Thorneloe et al., 2005). In a recent study, utilizing native human DSM, we have also demonstrated that the BK channel is a key physiological regulator of human DSM excitability and contractility (Hristov et al., 2011). In the current study, a pharmacological and functional approach was used to examine the mechanism of the BK channel opening in the regulation of rat DSM phasic and tonic contractile activity. We investigated the effects of BK channel activation in rat DSM using a well-established BK channel opener, NS1619. At a single-channel level, NS1619 has been shown to increase the open probability of BK channels in Guinea pig DSM (Sheldon et al., 1997). Here, we provide strong evidence that the opening of BK channels with NS1619 (30 μM) in rat DSM isolated strips effectively inhibits contractions under various experimental conditions.
Overactive bladder (OAB) syndrome, often characterized by increased, spontaneous DSM contractions (Brading, 1997), affects ~17% of the adults in the US (Stewart et al., 2003). Recent findings in benign prostatic hyperplasia (BPH) patients with detrusor overactivity and in a partial bladder outlet obstruction (PBOO) rabbit model suggested that downregulation of BK channel contributes to detrusor overactivity (Chang et al., 2010). Furthermore, the authors showed that the BK channel opener, NS1619, reduced the amplitude and frequency of pathologically increased contractions in PBOO bladders (Chang et al., 2010). On the other hand, long-term exposure to PBOO enhances the function of DSM BK channels by increasing the expression of the BK channel β1 subunit as a compensatory mechanism to reduce PBOO-induced detrusor overactivity (Kita et al., 2010). The smooth-muscle specific BK channel β1 subunits plays a key role in the control of DSM contractility (Petkov et al., 2001a). NS1619 has been reported to inhibit contraction amplitude and frequency of spontaneous phasic contractions in Guinea pig detrusor (Imai et al., 2001) and human detrusor (Oger et al., 2010). In line of extending some early findings; our analysis on all spontaneous contraction parameters in isolated rat DSM strips indicates that NS1619 significantly inhibited amplitude, muscle force integral, frequency, duration, and muscle tone of the spontaneous contractions. BK channel pharmacological inhibition with IBTX completely blocked the NS1619-induced relaxation of rat DSM confirming NS1619 selectivity for the BK channel.
A major component of neurogenic stimulus that contributes to DSM contractility is cholinergic input. Neuronally released acetylcholine activates muscarinic receptors in DSM cells and eventually evokes DSM phasic contractions during normal voiding function (Andersson, 2004). To reveal the effect of BK channel opening in decreasing the cholinergic DSM contractions, we stimulated muscarinic receptors directly using carbachol, a chemically stable cholinergic agonist that is resistant to acetylcholinesterase. Unlike the transient DSM contractions induced by physiological release of acetylcholine, carbachol maintains sustained phasic and tonic contractions. It has been reported the NS1619 elicited a decrease in amplitude, area under the curve and freqeuncy of carbachol induced spontaneous contractions in neonatal rat bladder (Ng et al., 2006). Our data demonstrates that NS1619 not only significantly inhibited the phasic contractions but also decreased the tonic contractions evoked by carbachol in rat DSM. Contractile activity was also induced by exposure to KCl (20 mM) which leads to conditions of sustained membrane depolarization inducing high-amplitude phasic contractions. A study by Malysz et al. (Malysz et al., 2004) have earlier reported that NS1619 effectively suppressed 20 mM KCl induced contractility in Guinea pig and rat detrusor as measured by area under the curve. Our results also demonstrate that NS1619 reduced the amplitude, muscle force integral, frequency and tone of the contractions induced by 20 mM K+ indicating that BK channel activation can reduce contractility under conditions of sustained membrane depolarization. These findings are consistent with earlier reports demonstrating that NS1619 inhibited 20 mM KCl-induced contractions in a concentration-dependent manner in isolated Guinea pig DSM strips (Sheldon et al., 1997), isolated rat portal vein and aorta preparations (Edwards et al., 1994).
To further investigate the mechanism of BK channel activation in decreasing DSM contractility, rat DSM strips were pre-contracted under conditions of moderately (20 mM K+) or highly elevated extracellular K+ (60 mM K+). The elevated external K+ decreases the driving force for K+ and changes the K+ reversal potential. NS1619 had reduced ability to relax DSM in the presence of 20 mM extracellular K+ versus physiological concentrations of K+ (compare Fig. 1B and 3B). Increasing K+ concentration to 60 mM further reduces NS1619 inhibitory effect on DSM tone (Fig. 3D). These experiments with increased (20 mM and 60 mM) external K+ provide important mechanistic insights. They reveal that the relaxation produced by NS1619 in DSM is due to a direct BK channel activation and not due to inhibition of voltage-gated Ca2+ channels or any other mechanisms.
To further reveal the mechanism of BK channel opening in decreasing DSM contractility, we also examined the effect of NS1619 on nerve-evoked contractions. Nerve-evoked contractions reflect the activation of DSM purinergic and muscarinic receptors (Andersson and Arner, 2004). Various reports suggest that either pharmacological inhibition or genetic deletion of BK channel significantly increases nerve-evoked contractions (Brading, 1992; Herrera et al., 2005; Meredith et al., 2004; Werner et al., 2007). We showed a significant decrease in the amplitude of the contractile responses to EFS over a wide range of stimulation frequencies. We hypothesized that the effect of NS1619 on nerve-evoked contractions is due to a direct BK channel activation based on a previous finding by Nelson’s group that the BK channel expression appears to be restricted to the smooth muscle with no detectable expression in the nerves present in DSM (Werner et al., 2007). This identifies the BK channel as an important pharmacological target to reduce nerve-evoked DSM contractions. Both cholinergic and purinergic signaling pathways have been reported to play an important role in DSM contractions in rats (Andersson and Arner, 2004; Igawa et al., 1993). Using specific receptor inhibitors, we further dissected the BK channel contribution to cholinergic and purinergic components of EFS-induced neurogenic contractions. BK channel activation caused statistically significant decrease in both cholinergic and purinergic components of EFS-induced neurogenic contractions. By separating the cholinergic and purinergic pathways, we revealed that BK channel activation contributes significantly to decrease neurogenic contractions for both pathways. These results support the observations that genetic deletion of the BK channel in DSM increases the sensitivity to nerve stimulation through both cholinergic and purinergic pathways (Werner et al., 2007).
The present study provides strong evidence that BK channel activation can be a potential approach to control the myogenic and nerve-evoked contractions of DSM. By reducing overall DSM excitability, BK channel activation with NS1619 can significantly diminish spontaneous, pharmacologically induced and nerve-evoked contractions. Our results strongly support the concept that activation of BK channel can be a desirable feature to control bladder function, including pathophysiological conditions of detrusor overactivity regardless of the underlying cause. Selective drug-induced BK channel opening may represent an opportunity for novel pharmacological manipulation of the urinary bladder in humans. Direct evidence from experiments on human DSM is needed to further confirm this concept.
Acknowledgments
We would like to thank Drs. Jennifer G. Schnellmann, Kiril L. Hristov, Shankar Parajuli, Mr. Qiuping Cheng and Mr. Serge Afeli for the critical evaluation of the manuscript.
GRANTS
This study was supported by grants from the National Institutes of Health DK084284 and DK083687 to Georgi V. Petkov.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson KE. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. 2004;3:46–53. doi: 10.1016/s1474-4422(03)00622-7. [DOI] [PubMed] [Google Scholar]
- Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004;84:935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- Brading AF. Ion channels and control of contractile activity in urinary bladder smooth muscle. Jpn J Pharmacol. 1992;58(Suppl 2):120P–127P. [PubMed] [Google Scholar]
- Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50:57–67. doi: 10.1016/s0090-4295(97)00591-8. [DOI] [PubMed] [Google Scholar]
- Brading AF, Williams JH. Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: effects of atropine and alpha,beta-methylene ATP. Br J Pharmacol. 1990;99:493–498. doi: 10.1111/j.1476-5381.1990.tb12956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol. 2008;295:F1149–1157. doi: 10.1152/ajprenal.00440.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Gomes CM, Hypolite JA, Marx J, Alanzi J, Zderic SA, Malkowicz B, Wein AJ, Chacko S. Detrusor overactivity is associated with downregulation of large-conductance calcium- and voltage-activated potassium channel protein. Am J Physiol Renal Physiol. 2010;298:F1416–1423. doi: 10.1152/ajprenal.00595.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Kellett WF, Petkov GV. Voltage-gated K(+) channels sensitive to stromatoxin-1 regulate myogenic and neurogenic contractions of rat urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol. 2010;299:R177–184. doi: 10.1152/ajpregu.00036.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory beta4 subunit in rat and mouse bladder smooth muscle. J Urol. 2009;182:374–381. doi: 10.1016/j.juro.2009.02.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Niederste-Hollenberg A, Schneider J, Noack T, Weston AH. Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br J Pharmacol. 1994;113:1538–1547. doi: 10.1111/j.1476-5381.1994.tb17171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. Ca(2+)-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol. 1997;273:C110–117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Etherton B, Nausch B, Nelson MT. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R402–R409. doi: 10.1152/ajpregu.00488.2004. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol. 2000;279:R60–68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- Holland M, Langton PD, Standen NB, Boyle JP. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol. 1996;117:119–129. doi: 10.1111/j.1476-5381.1996.tb15163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol. 2011 doi: 10.1152/ajpcell.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of beta3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol. 2008;295:C1344–1353. doi: 10.1152/ajpcell.00001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Lau CW, Ho IH. NS 1619 activates Ca2+-activated K+ currents in rat vas deferens. Eur J Pharmacol. 1997;325:21–27. doi: 10.1016/s0014-2999(97)00102-7. [DOI] [PubMed] [Google Scholar]
- Igawa Y, Mattiasson A, Andersson KE. Functional importance of cholinergic and purinergic neurotransmission for micturition contraction in the normal, unanaesthetized rat. Br J Pharmacol. 1993;109:473–479. doi: 10.1111/j.1476-5381.1993.tb13593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Okamoto T, Yamamoto Y, Tanaka H, Koike K, Shigenobu K, Tanaka Y. Effects of different types of K+ channel modulators on the spontaneous myogenic contraction of guinea-pig urinary bladder smooth muscle. Acta Physiol Scand. 2001;173:323–333. doi: 10.1046/j.1365-201X.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- Kita M, Yunoki T, Takimoto K, Miyazato M, Kita K, de Groat WC, Kakizaki H, Yoshimura N. Effects of bladder outlet obstruction on properties of Ca2+-activated K+ channels in rat bladder. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1310–1319. doi: 10.1152/ajpregu.00523.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne JJ, Nausch B, Olesen SP, Nelson MT. BK channel activation by NS11021 decreases excitability and contractility of urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol. 2010;298:R378–384. doi: 10.1152/ajpregu.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan S, Sheridan RD, Chilvers ER, Patmore L. A comparison of the effects of SCA40, NS 004 and NS 1619 on large conductance Ca(2+)-activated K+ channels in bovine tracheal smooth muscle cells in culture. Br J Pharmacol. 1995;116:1656–1660. doi: 10.1111/j.1476-5381.1995.tb16387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malysz J, Buckner SA, Daza AV, Milicic I, Perez-Medrano A, Gopalakrishnan M. Functional characterization of large conductance calcium-activated K+ channel openers in bladder and vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:481–489. doi: 10.1007/s00210-004-0920-y. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci U S A. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- Ng YK, de Groat WC, Wu HY. Muscarinic regulation of neonatal rat bladder spontaneous contractions. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1049–1059. doi: 10.1152/ajpregu.00236.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oger S, Behr-Roussel D, Gorny D, Bernabe J, Comperat E, Chartier-Kastler E, Denys P, Giuliano F. Effects of potassium channel modulators on myogenic spontaneous phasic contractile activity in human detrusor from neurogenic patients. BJU Int. 2010 doi: 10.1111/j.1464-410X.2010.09935.x. [DOI] [PubMed] [Google Scholar]
- Petkov GV. Ion channels. Pharmacology: Principles and Practice. 2009;Chapter 16:385–425. [Google Scholar]
- Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol. 2001a;537:443–452. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of K(ATP) channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol. 2001b;280:R1427–1433. doi: 10.1152/ajpregu.2001.280.5.R1427. [DOI] [PubMed] [Google Scholar]
- Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2005;288:C1255–1263. doi: 10.1152/ajpcell.00381.2004. [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Sheldon JH, Norton NW, Argentieri TM. Inhibition of guinea pig detrusor contraction by NS-1619 is associated with activation of BKCa and inhibition of calcium currents. J Pharmacol Exp Ther. 1997;283:1193–1200. [PubMed] [Google Scholar]
- Siemer C, Bushfield M, Newgreen D, Grissmer S. Effects of NS1608 on MaxiK channels in smooth muscle cells from urinary bladder. J Membr Biol. 2000;173:57–66. doi: 10.1007/s002320001007. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol. 2005;289:F604–610. doi: 10.1152/ajprenal.00060.2005. [DOI] [PubMed] [Google Scholar]
- Trivedi S, Potter-Lee L, Li JH, Yasay GD, Russell K, Ohnmacht CJ, Empfield JR, Trainor DA, Kau ST. Calcium dependent K-channels in guinea pig and human urinary bladder. Biochem Biophys Res Commun. 1995;213:404–409. doi: 10.1006/bbrc.1995.2146. [DOI] [PubMed] [Google Scholar]
- Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol. 2007;292:R616–624. doi: 10.1152/ajpregu.00036.2006. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Ohi Y, Muraki K, Watanabe M, Imaizumi Y. BK channel activation by NS-1619 is partially mediated by intracellular Ca2+ release in smooth muscle cells of porcine coronary artery. Br J Pharmacol. 2001;132:828–834. doi: 10.1038/sj.bjp.0703885. [DOI] [PMC free article] [PubMed] [Google Scholar]