Abstract
Microdialysis sampling of the brain is an analytical technique with numerous applications in neuroscience and the neurointensive care of brain-injured human patients. Even so, implanting microdialysis probes into brain tissue causes a penetration injury that triggers gliosis (the activation and proliferation of glial cells) and ischemia (the interruption of blood flow). Thus, the probe samples injured tissue. Mitigating the effects of the penetration injury might refine the technique. The synthetic glucocorticoid, dexamethasone, is a potent anti-inflammatory and immunosuppressant substance. We performed microdialysis in the rat brain for 5 days, with and without dexamethasone in the perfusion fluid (10 µM for the first 24 hrs and 2 µM thereafter). On the 1st and 4th day of the perfusion, we performed dopamine no-net-flux measurements. On the 5th day, we sectioned and stained the brain tissue and examined it by fluorescence microscopy. Although dexamethasone profoundly inhibited gliosis and ischemia around the probe tracks it had only modest effects on dopamine no-net-flux results. These findings show that dexamethasone is highly effective at suppressing gliosis and ischemia but is limited in its neuroprotective activity.
Introduction
The significance of brain microdialysis as an analytical technique in neuroscience is well-established and its application as a tool for the neurointensive care of brain-injured human patients continues to be explored1–10. The power of microdialysis is attributable to its several beneficial attributes: the dialysate samples contain a plethora of interesting and important small molecules,11, 12 (for an extensive review see Westerink and Cremers, 2007) the technique is relatively simple to perform and compatible with the constraints of intensive care, the probes are commercially available or easily built in-house, and the dialysis process prepares the samples for direct, often times on-line, analysis by a broad array of techniques, such as HLPC, CE, and MS. Microdialysis has contributed significantly to the understanding of normal brain function, the pathology of brain disorders and injuries, and the actions of neuroactive drugs, both therapeutic and illicit13–22.
Although microdialysis provides valuable insights into brain chemistry, implanting the probes causes a penetration injury to the brain tissue23–27 that triggers gliosis (the activation and proliferation of glial cells)25–28 and ischemia (interruption of blood flow). Consequently, the tissue sampled by the probe is perturbed from its normal state28. Understanding, and possibly mitigating, the effects of the penetration injury could be a path to enhancing the technique, refining its accuracy and precision, extending the viable microdialysis sampling time window, and enabling deeper insights into the neurochemical aspects of brain function. Given that many neurological disorders, while treatable, are neither preventable nor curable and that the therapies for brain trauma remain limited, enhancing the chemical information output from brain microdialysis procedures stands to be highly significant.
In the present study, we examined the effect of dexamethasone on the probe-induced penetration injury of the rat brain. Dexamethasone is a synthetic, highly potent glucocorticoid with anti-inflammatory and immunosuppressant activity. Several studies have demonstrated that gliosis at brain implants is inhibited by dexamethasone-releasing coatings29–31. Furthermore, Stenken’s group recently reported that dexamethasone inhibits the immune response to subcutaneous microdialysis probes32. We performed microdialysis in the rat brain for 5 days with and without dexamethasone in the perfusion fluid. On the 1st and 4th day of the perfusion we performed dopamine no-net-flux measurements and at the end of the perfusion we sectioned, stained, and examined the brain tissue by fluorescence microscopy to assess gliosis and ischemia.
Materials and Methods
The Supplementary Information contains full details of the Materials and Methods for this study.
Briefly, we performed microdialysis for 5 days in two groups of rats. The rats underwent aseptic stereotaxic surgery under isoflurane anesthesia to position a microdialysis guide cannula (MD-2251, Bioanalytical Systems, Inc. (BASi), West Lafayette, IN) over the striatum. After a four-day post-operative recovery period, the rats were briefly re-anesthetized with isoflurane and returned to the stereotaxic frame. We slowly (~30 min) lowered a microdialysis probe (BASi MD-2204) through the guide cannula into the striatum using the stereotax carrier arm. The probes were perfused continuously for 5 days at 0.610 µL/min by means of a 1.0-mL gas tight syringe (Hamilton, Inc) and a syringe pump (Harvard Apparatus). In one group of rats the probes were perfused with artificial cerebrospinal fluid (aCSF) and in the other group the probes were perfused with aCSF containing dexamethasone-21-phosphate. Upon continuous perfusion with 10-µM dexamethasone, the rats eventually became agitated and damaged the probe or its connecting tubing. So, after 24 hrs of perfusion with 10-µM dexamethasone, the concentration was decreased to 2 µM: this dose did not agitate the animals.
On the first (i.e. starting 24 hrs after probe implantation) and fourth day after implantation of the probe we performed dopamine no-net-flux measurements33. The probes were perfused sequentially with 0, 100, 250 and 1000-nM dopamine and 50-µM ascorbic acid as a preservative for 2 hrs each and 1-hr dialysate samples were collected during the second hour. The dopamine concentrations in the dialysate samples were determined in triplicate by capillary HPLC with radial-flow electrochemical detection34–36 On the fifth day the animals were anesthetized again with isoflurane and were perfused through the heart with saline, paraformaldahyde, and a suspension of fluorescent nanobeads to mark blood vessels 25, 26. The brain tissue was cut horizontally into 30-µm thick sections, labeled with antibody to the glial fibrillary acidic protein (GFAP), a biomarker for astrocytes, and examined by fluorescence microscopy.
Results and Discussion
Microdialysis probes induce gliosis
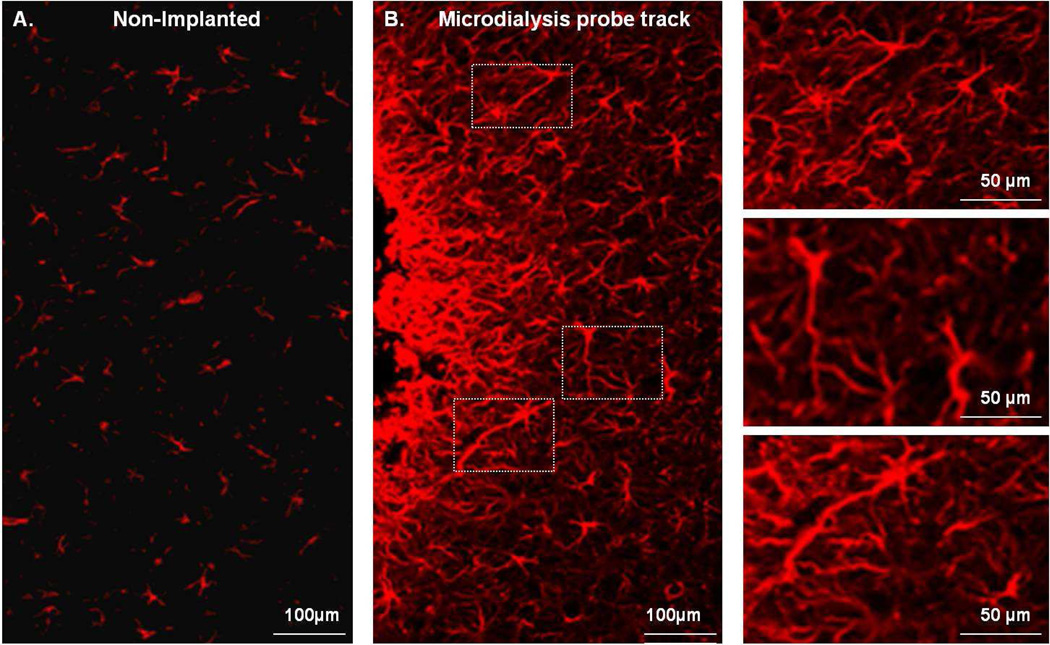
GFAP immunofluorescence reveals profound gliosis at the tracks of microdialysis probes after 5-day perfusions in the rat brain (Fig 1). The GFAP image from the non-implanted brain hemisphere (Fig 1A) resembles that from non-implanted rats (Supplementary Fig 2). Glia surrounding the probe tracks exhibit enlarged cell bodies and thickened and elongated processes (Fig 1B). The images in Fig 1 extend our previous report that gliosis is evident after 24-hr perfusions24. These longer perfusions are relevant to the neurointensive care applications of microdialysis4, 5.
Fig. 1.
The effect of a microdialysis probe on striatal glial cells labeled with GFAP antibody. A) Striatal tissue from a non-implanted brain hemisphere (contralateral to the probe). B) Striatal tissue next to a microdialysis probe track: the edge of the track is on the left side of the image. The right-hand column shows enlargements of the white boxes in panel B.
Dexamethasone inhibits gliosis
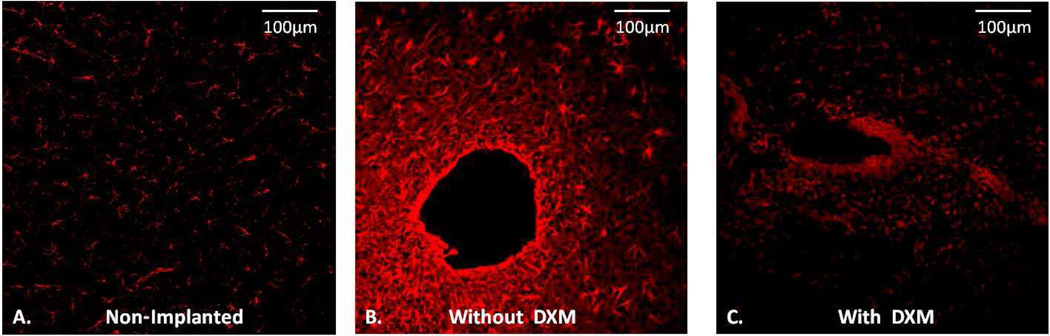
After 5-day perfusions without dexamethasone microdialysis probes are fully engulfed by a glial barrier (Fig 2). The glial barriers were continuous around the entire circumference of the tracks, which maintained their symmetrical shape even when the probes were withdrawn to section the tissue (Fig 2B). The rigidity of the glial barrier is attributable to the proteoglycan secretions of activated glia37. Dexamethasone inhibited the gliosis resulting in a residual, discontinuous, non-rigid glial barrier (Fig 2C). Dexamethasone-perfused tracks showed more GFAP labeling than non-implanted tissues (Fig 2A) but less than tracks perfused without dexamethasone (Fig 2B). After perfusion with dexamethasone, the tracks did not maintain their open circular shape during tissue processing, so they appear distorted and non-symmetric in the images.
Fig. 2.
Retrodialysis of dexamethasone inhibits gliosis. A) GFAP image of striatal tissue from a non-implanted hemisphere contralateral to a microdialysis probe. B) GFAP image of a glial barrier formed after 5 days of microdialysis without dexamethasone. C) GFAP image of a probe track after 5 days of retrodialysis of dexamethasone.
Dexamethasone prevents ischemia
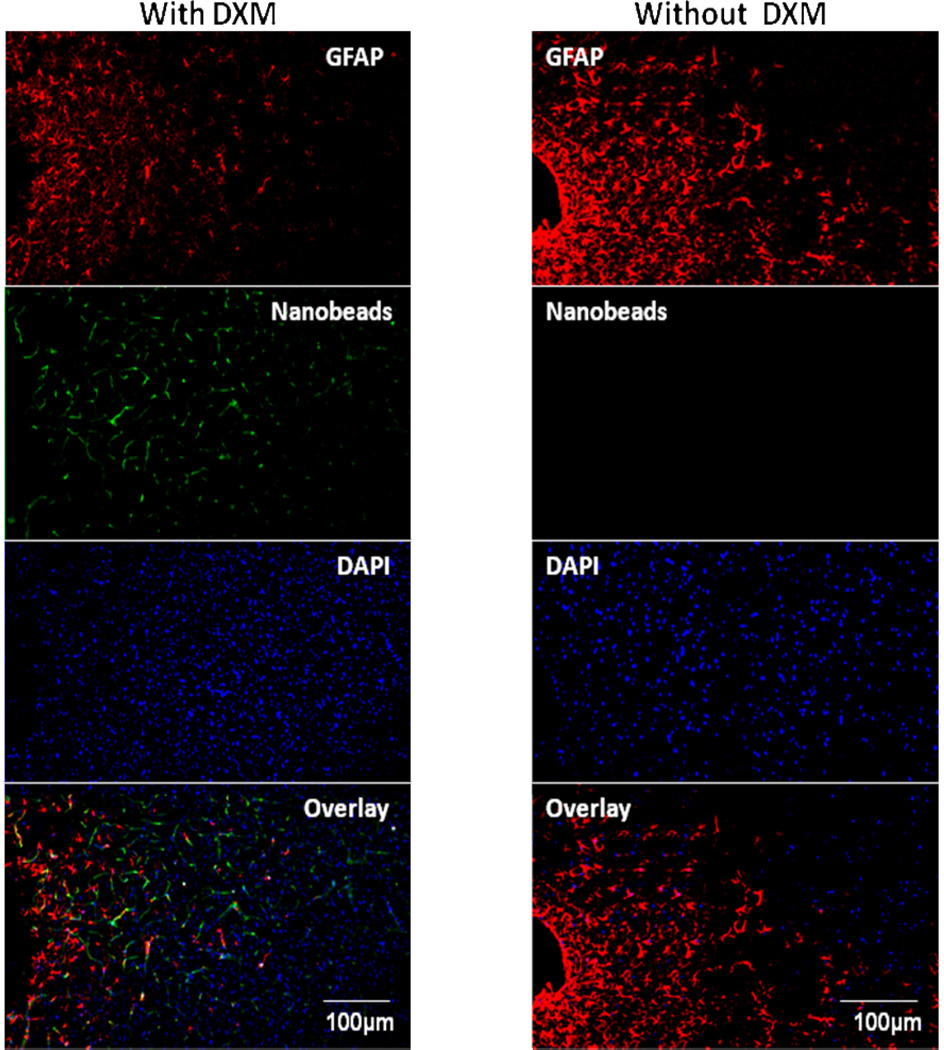
5-day perfusions without dexamethasone caused profound ischemia, as evidenced by a near-total absence of bead-labeled blood vessels near the probe tracks (Fig 3, right panel). However, after 5-day perfusions with dexamethasone bead-laden vessels near the tracks appear essentially normal (Fig 3, left panel). Thus, dexamethasone, in addition to inhibiting gliosis, also prevented ischemia at the probe tracks.
Fig. 3.
A montage of the tissue response after 5 days of microdialysis with (left) and without (right) of dexamethasone. Note the complete absence of nanobeads in the right hand column, indicating profound ischemia in this tissue. The position of the microdialysis probe track is at the far left in both panels.
Objective analysis of GFAP images
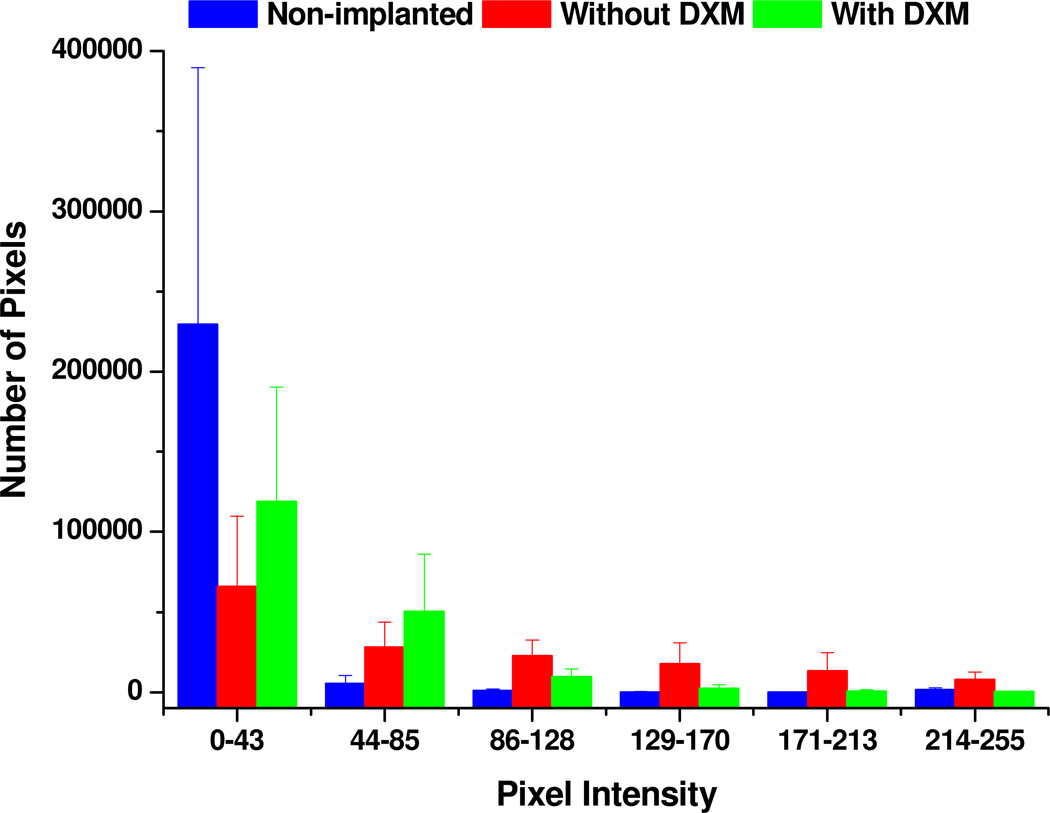
Using Metamorph, we converted 9 images (3 non-implanted, 3 without dexamethasone, and 3 with dexamethasone) to color-coded surface intensity plots (see Supplementary Fig 3) and then to binned intensity histograms (Fig 4). In control images (blue, Fig 4), the majority of pixels are in the lowest intensity bin (0–43) because GFAP labeling is sparse in these tissues. Perfusion without dexamethasone decreases the number pixels in the lowest intensity bin (0–43) and increases the number of pixels in all higher intensity bins (44–255), reflecting the increased GFAP labeling in these tissues (red, Fig 4). Dexamethasone returned the number of pixels in the higher intensity bins (>129) to control levels and increased the number of pixels in the low intensity bins (<128), providing a quantitative measure of the inhibition of gliosis by perfusion with dexamethasone.
Fig. 4.
Comparison of the pixel intensity distribution in images of non-implanted (control) tissue (blue) and tissue dialyzed without (red) and with (green) dexamethasone. The data are reported as the mean and standard deviation of the number of pixels in each intensity bin from three images.
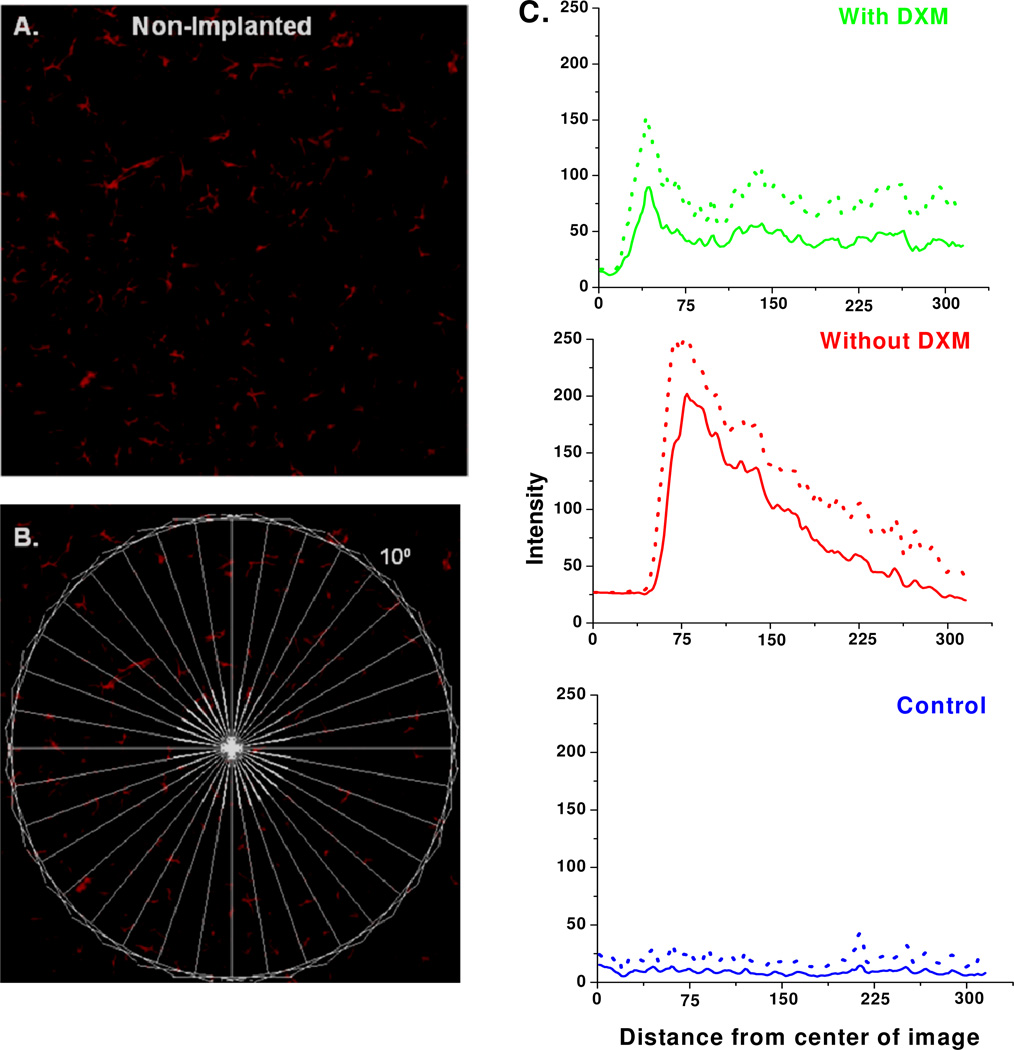
We also used Metamorph to construct line-scan intensity profiles of the GFAP images (Fig 5). The lines were arranged like “spokes” surrounding a “hub” (Fig 5B) centered on the probe track (in non-implanted control tissue, the hub was centered within the striatum). The line-scans from non-implanted tissues (blue, Fig 5C) exhibit only minor variations in intensity with distance from the hub. The line-scans from probe tracks (red and green, Fig 5C) show a region of low intensity near the center of the track where there is no GFAP labeling: the size of this region is affected by the asymmetry of the tracks (see Fig 2). The line-scans from tissues perfused with (green) and without (red) dexamethasone show the profiles of the respective glial barriers. Dexamethasone substantially diminished the height and width of the glial barrier profile.
Fig. 5.
Line scan analysis of GFAP images. A) Non-Implanted control tissue. B) The same image overlaid with the spoke pattern of lines used to construct the line-scans in C. C) Line scan intensity profiles obtained from non-implanted control tissue (blue) and from tissue dialyzed without (red) and with (green) dexamethasone. Data are reported as the mean (solid line) and the standard deviation (dotted line) of 36 lines per image.
Objective analysis of blood vessel images
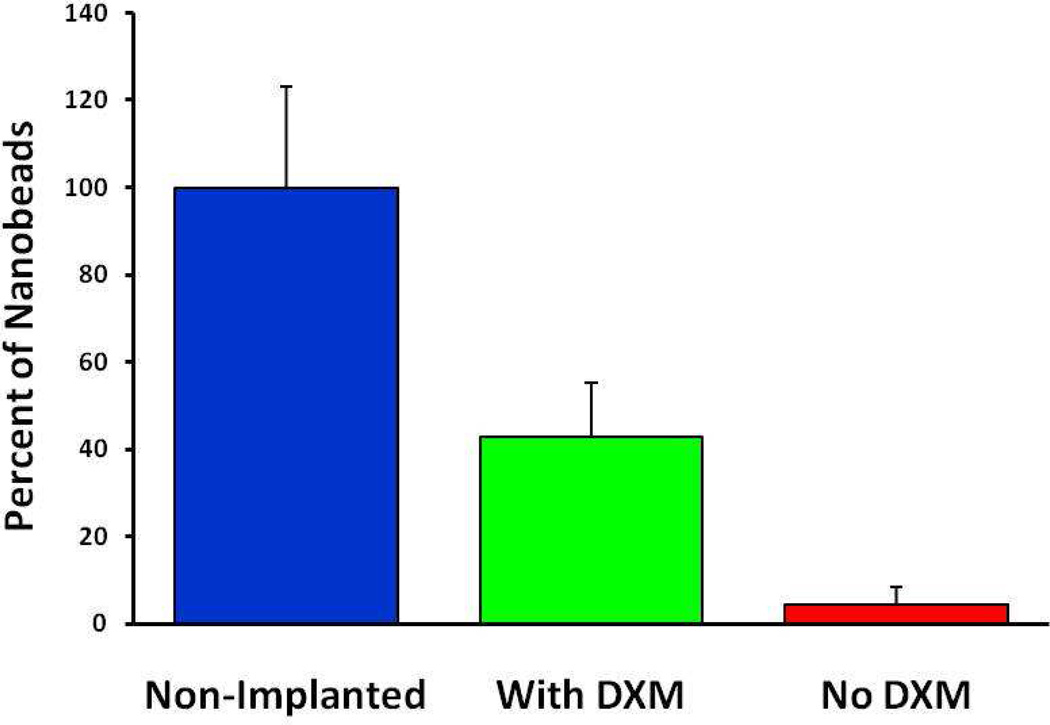
We also used Metamorph software to compare the number of fluorescent pixels in the blood vessel images from implanted and non-implanted tissues. The images were centered on the striatum (non-implanted controls) or the probe tracks (implanted tissues). The results, normalized to the average pixel count from six non-implanted controls, show that in tissues from rats perfused for 5 days without dexamethasone, the number of fluorescent pixels was reduced to 4.4% (red, Fig 6), reflecting profound probe-induced ischemia. In tissues from rats perfused for 5 days with dexamethasone, the number of fluorescent pixels was reduced to 42.8% (green, Fig 6), mainly because the images contained the probe track where blood vessels are completely absent. Thus, Fig 6 confirms quantitatively that dexamethasone inhibited probe-induced ischemia.
Fig. 6.
The number of fluorescent pixels in blood vessel images from non-implanted control tissues (blue) and implanted tissues perfused with (green) and without (red) dexamethasone: the data are normalized with respect to the average pixel count from the non-implanted controls. Tissue with and without dexamethasone were significantly different from one another and from non-Implanted tissue (ANOVA and Tukey posthoc test: F(2,12) = 57.1; p<0.00001).
The effect of dexamethasone on dopamine no-net-flux
Because the impact of dexamethasone on gliosis and ischemia is readily apparent, we became interested in knowing how the drug might affect microdialysis results. To investigate this, we measured no-net-flux curves for dopamine, an important neurotransmitter and the subject of prior microdialysis studies11, 33, 38–41.
Microdialysis extraction curves (see Supplementary Information) are usually linear and aptly described by the following equation42:
| (Eq 1) |
where Cin and Cout are the concentrations at the inlet and outlet of the probe, Cext is the concentration outside the probe, R is the recovery factor, and E is the extraction factor. Since R and Cext are not separable during in vivo experiments, it is practical to replace R·Cext with Cout measured when Cin is zero, which is known as the conventional microdialysis result, Cout,c:
| (Eq 2) |
According to Equation 2, Cout,c is the inverse of the y-intercept of the extraction curve and E is its slope. The x-intercept of the extraction curve corresponds to the condition that Cin and Cout are equal to each other: their common value is called the no-net-flux concentration, Cnnf40, 42–44.
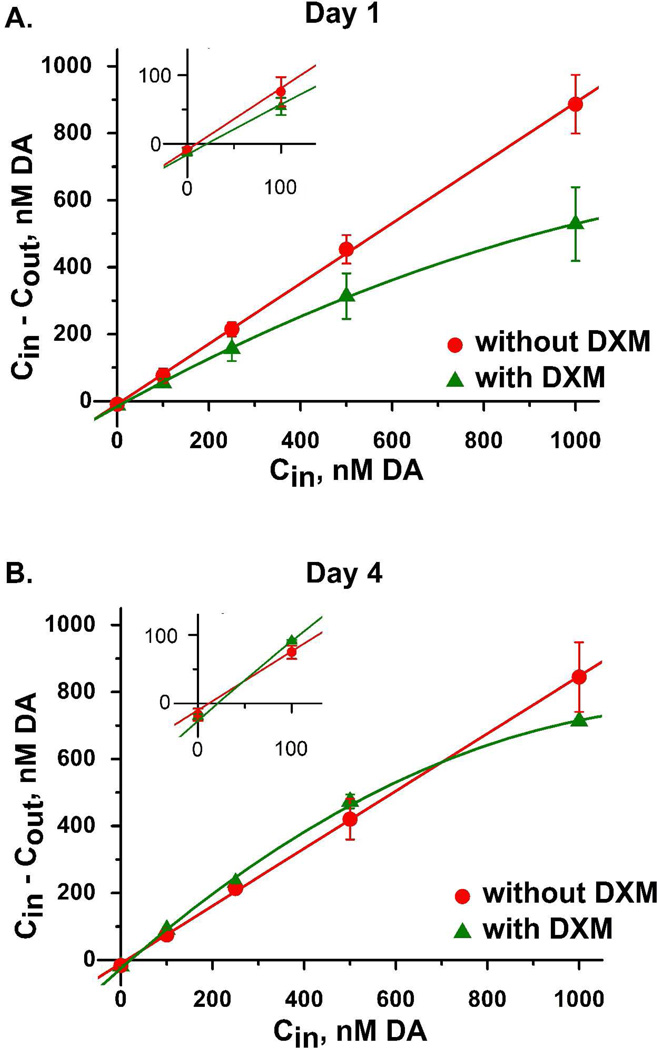
Using probes perfused without dexamethasone, the dopamine extraction curves measured on day 1 and day 4 (red, Fig 7A and 7B) exhibited typical features: the curves were linear with Cout,c and Cnnf values near 10 nM45 (see also Supplementary Table 1). Dexamethasone had no significant effect on the values of Cout,c and Cnnf (Supplementary Table 1). To us, this came as a surprise, as we had anticipated that inhibiting gliosis and ischemia might increase Cout,c and Cnnf towards the higher in vivo dopamine concentrations we have measured with microelectrodes38, 46, 47. This suggests that even though dexamethasone inhibited gliosis and ischemia, it did not protect dopamine terminals from the penetration injury.
Fig. 7.
Dopamine concentration difference plots obtained in the rat striatum on day 1 (A) and day 4 (B) of microdialysis with (green) and without (red) dexamethasone. The data points represent the mean ± the standard error. The solid lines show the linear regression of the data obtained without dexamethasone (red) and the quadratic regression of the data obtained with dexamethasone (green). Insets expand the region near the origin to visualize Cout,c and Cnnf.
Interestingly, however, the extraction curves measured in the presence of dexamethasone appear to be non-linear (green, Fig 7A and 7B, and Supplementary Table 1). The most likely explanation for this involves the Michaelis-Menten kinetics of the dopamine transporter, the protein responsible for the uptake of dopamine by brain tissue. The transporter aids the extraction of dopamine from the microdialysis probe by enabling nearby brain tissue to take up dopamine after it diffuses out of the probe, as originally explained by Smith and Justice48. Since the transporter has a KM value near 200 nM, it is surprising that the extraction curves are linear, as we observed in the absence of dexamethasone, even when the Cin values substantially exceed KM. This indicates that the dopamine is diluted by the time it diffuses to the transporter, such that the transporter does not become saturated with dopamine. The non-linear extraction curve observed with dexamethasone, then, suggests that dopamine more readily diffuses to the transporter, consistent with a reduction of the glial barrier next to the probe, making it easier to saturate the transporter.
Previous microdialysis studies have concluded that steroid use inhibits dopamine uptake, 49–52 prompting speculation that steroids possess a cocaine-like activity that exacerbates their abuse by some users. However, the authors of those prior studies were likely unaware that steroids might have indirect effects on dopamine uptake due to alterations in the tissue reaction to the microdialysis probe. This illustrates that hidden subtleties can impact the interpretation of in vivo analytical measurements.
Conclusion
The results of this study support the conclusion that dexamethasone is highly effective at preventing the gliosis and ischemia triggered by the penetration injury that accompanies the implantation of microdialysis probes into brain tissue, while offering relatively little neuroprotection per se to the dopamine terminals. Nevertheless, we speculate that inhibiting gliosis and ischemia is likely a necessary component of an overall neuroprotective strategy, as activated glia destroy neurons and their processes and prevent their regrowth 28, 53. So, we expect that dexamethasone will contribute to mitigating probe-induced injury, especially if combined with complementary neuroprotective measures (e.g. the use of glutamate antagonists, antioxidants, or radical scavengers, etc.). In closing, we note that there may be restrictions on the uses of dexamethasone, which has failed human trials as a therapeutic agent for traumatic brain injury: human trials were terminated early because dexamethasone increased mortality54.
Supplementary Material
Acknowledgment
This work was supported by NIH grant MH0575989.
References
- 1.Bosche B, Dohmen C, Graf R, Neveling M, Staub F, Kracht L, Sobesky J, Lehnhardt FG, Heiss WD. Stroke. 2003;34:2908–2915. doi: 10.1161/01.STR.0000100158.51986.EB. [DOI] [PubMed] [Google Scholar]
- 2.Dreier JP, Bhatia R, Major S, Drenckhahn C, Lehmann TN, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- 3.Dreier JP, Ebert N, Priller J, Megow D, Lindauer U, Klee R, Reuter U, Imai Y, Einhaupl KM, Victorov I, Dirnagl U. J. Neurosurg. 2000;93:658–666. doi: 10.3171/jns.2000.93.4.0658. [DOI] [PubMed] [Google Scholar]
- 4.Fabricius M, Fuhr S, Willumsen L, Dreier JP, Bhatia R, Boutelle MG, Hartings JA, Bullock R, Strong AJ, M L. Clinical Neurophysiology. 2008;119:1973–1984. doi: 10.1016/j.clinph.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feuerstein D, Manning A, Hashemi P, Bhatia RFM, Ervine M, AJ S, G BM. J. Cereb. Blood Flow and Metab. 2010;30:1343–1355. doi: 10.1038/jcbfm.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meixensberger J, Kunze E, Barcsay E, Vaeth A, Roosen K. Neurol. Res. 2001;23:801–806. doi: 10.1179/016164101101199379. [DOI] [PubMed] [Google Scholar]
- 7.Nordstrom CH. Childs. Nerv. Syst. 2009;26:465–472. doi: 10.1007/s00381-009-1035-z. [DOI] [PubMed] [Google Scholar]
- 8.Sakowitz OW, Sebastian W, Sarrafzadeh AS, Stover JF, Drier JP, Dendorfer A, Benndorf G, Lanksch WR, Unterberg AW. J. Cereb.Blood Flow and Metab. 2001;21:1067–1076. doi: 10.1097/00004647-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Sakowitz OW, Stover JF, Sarrafzadeh AS, Unterberg AW, Kiening KL. J. Trauma. 2007;62:292–298. doi: 10.1097/01.ta.0000203560.03937.2d. [DOI] [PubMed] [Google Scholar]
- 10.Schlenk F, Frieler K, Nagel A, Vajkoczy P, Sarrafzadeh AS. Critical Care. 2008;13:1–9. doi: 10.1186/cc7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry M, Li Q, Kennedy RT. Anal. Chim. Acta. 2009;653:1–22. doi: 10.1016/j.aca.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westerink BH, Cremers TIFH, editors. Handbook of Microdilaysis: Methods, Applications and Perspectivies. London: Academic Press; 2007. [Google Scholar]
- 13.Benveniste H, Diemer NH. Acta. Neuropathol. 1987;74:234–238. doi: 10.1007/BF00688186. [DOI] [PubMed] [Google Scholar]
- 14.Benveniste H, Hansen AJ, Ottosen NS. J. Neurochem. 1989;52:1741–1750. doi: 10.1111/j.1471-4159.1989.tb07252.x. [DOI] [PubMed] [Google Scholar]
- 15.Berger C, Schabitz WR, Georgiadis D, Steiner T, Aschoff A, Schwab S. Stroke. 2002;33:519–524. doi: 10.1161/hs0102.100878. [DOI] [PubMed] [Google Scholar]
- 16.Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. J.Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradberry CW, Nobiletti JB, Elsworth JD, Murphy B, Jatlow P, Roth RH. J. Neurochem. 1993;60:1429–1435. doi: 10.1111/j.1471-4159.1993.tb03305.x. [DOI] [PubMed] [Google Scholar]
- 18.Hashemi P, Bhatia R, Nakamura H, Dreier JP, Graf R, Strong AJ, Boutelle MG. J. Cereb. Blood Flow Metab. 2009;29:166–175. doi: 10.1038/jcbfm.2008.108. [DOI] [PubMed] [Google Scholar]
- 19.Parkin MC, Hopwood SE, Jones DA, Hashemi P, Landolt H, Fabricius MF, Lauritizen M, Boutelle MG, Strong AJ. J. Cerebral Blood Flow and Metab. 2005;25:402–413. doi: 10.1038/sj.jcbfm.9600051. [DOI] [PubMed] [Google Scholar]
- 20.Rittenhouse KD, Pollack GM. Advanced Drug Delivery Reviews. 2000;45:229–241. doi: 10.1016/s0169-409x(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 21.Sakowitz OW, Unterberg AW. Current Opinion in Critical Care. 2006;12:103–111. doi: 10.1097/01.ccx.0000216575.03815.ee. [DOI] [PubMed] [Google Scholar]
- 22.Sarrafzadeh AS, Sakowitz OW, Kiening KL, Benndorf G, Lanksch WR, Unterberg AW. Crit. Care Med. 2002;30:1062–1070. doi: 10.1097/00003246-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. J. Neurosci. Methods. 1999;90:129–142. doi: 10.1016/s0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]
- 24.Hascup ER, Bjerken S, Hascup KN, Pomerleau F, Huettl P, Stromberg I, Gerhardt GA. Brain Res. 2009;1291:12–20. doi: 10.1016/j.brainres.2009.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaquins-Gerstl A, Michael AC. J. Neurosci Methods. 2009;183:127–135. doi: 10.1016/j.jneumeth.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitala CM, Wang Y, Borland LM, Jung M, Shand S, Watkins S, Weber SG, Michael AC. J. Neurosci. Methods. 2008;174:177–185. doi: 10.1016/j.jneumeth.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou F, Braddock JF, Hu Y, Zhu X, Castellani RJ, Smith MA, Drew KL. J. Neurosci. Methods. 2002;119:121–128. doi: 10.1016/s0165-0270(02)00177-2. [DOI] [PubMed] [Google Scholar]
- 28.Stroncek JD, Reichert WM. In: Overview of Wound Healing in Different Tissue Types. Reichert WM, editor. Boca Raton (FL): CRC Press; 2008. [PubMed] [Google Scholar]
- 29.Shain W, Spataro L, Dilgen J, Haverstick K, Retterer S, Isaacson M, Saltsman M, Turner JN. IEEE Transaction on Neural Systems and Rehabilitation Engineering. 2003;11:186–188. doi: 10.1109/TNSRE.2003.814800. [DOI] [PubMed] [Google Scholar]
- 30.Spataro L, Dilgen J, Retterer S, Spence AJ, Isaacson M, Turner JN, Shain W. Exp. Neurol. 2005;194:289–300. doi: 10.1016/j.expneurol.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 31.Zhong Y, Bellamkonda RV. Brain Res. 2007;1148:15–27. doi: 10.1016/j.brainres.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mou X, Lennartz MR, Leoegering DJ, Stenken JA. J. diabetes science and technology. 2011;5:619–631. doi: 10.1177/193229681100500316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Justice JB., Jr J. Neurosci. Methods. 1993;48:263–276. doi: 10.1016/0165-0270(93)90097-b. [DOI] [PubMed] [Google Scholar]
- 34.Jung MC, Weber SG. Anal. Chem. 2005;77:974–982. doi: 10.1021/ac0486241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Zhang J, Xu X, Zhao M, Andrews AM, Weber SG. Anal. Chem. 2010;82:9611–9616. doi: 10.1021/ac102200q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. J. Neurosci. 2001;21:6338–6347. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laabs TL, Wang H, Katagiri Y, MaCann T, Fawcett JW, Geller HM. J. Neurosci. 2007;27:14494–14501. doi: 10.1523/JNEUROSCI.2807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borland LM, Michael AC. J. Neurochem. 2004;91:220–229. doi: 10.1111/j.1471-4159.2004.02708.x. [DOI] [PubMed] [Google Scholar]
- 39.Borland LM, Shi G, Yang H, Michael AC. J. Neurosci. Methods. 2005;146:149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB. J. Neurochem. 2003;86:932–946. doi: 10.1046/j.1471-4159.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H, Peters JL, Michael AC. J. Neurochem. 1998;71:684–692. doi: 10.1046/j.1471-4159.1998.71020684.x. [DOI] [PubMed] [Google Scholar]
- 42.Michael AC, Borland LM, Mitala JJ, Jr, Willoughby BM, Motzko CM. J. Neurochem. 2005;94:1202–1211. doi: 10.1111/j.1471-4159.2005.03265.x. [DOI] [PubMed] [Google Scholar]
- 43.Bungay PM, Morrison PF, Dedrick RL, Chefer VI, Zapata A. In: Handbook of microdialysis: methods, applications, and perspectives. Westerink BHC, Cremers TIFH, editors. Elsevier: Amsterdam; 2007. pp. 131–167. [Google Scholar]
- 44.Chefer VI, Zapata A, Shippenberg TS, Bungay PM. J. Neurosci. Methods. 2006;155:187–193. doi: 10.1016/j.jneumeth.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 45.Kulagina NV, Zigmond MJ, Michael AC. Neuroscience. 2001;102:121–128. doi: 10.1016/s0306-4522(00)00480-2. [DOI] [PubMed] [Google Scholar]
- 46.Peters JL, Michael AC. J. Neurochem. 1998;70:594–603. doi: 10.1046/j.1471-4159.1998.70020594.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Moquin KF, Michael AC. J. Neurochem. 2010;114:150–159. doi: 10.1111/j.1471-4159.2010.06740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith AD, Justice JB., Jr J. Neurosci. Methods. 1994;54:75–82. doi: 10.1016/0165-0270(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 49.Becker JB, Cha JH. Behavioural Brain Research. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- 50.Castner SA, Xiao L, Becker JB. Brain Research. 1993;610:127–134. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- 51.Pacak K, Tjurmina O, Palkovits M, Goldstein DS, Koch CA, Hoff T, Chrousos GP. Neuroendocrinology. 2002;76:148–157. doi: 10.1159/000064522. [DOI] [PubMed] [Google Scholar]
- 52.Sadri-Vakili G, Johnson DW, Janis GC, Gibbs TT, Pierce RC, Farb DH. J. Neurochem. 2003;86:92–101. doi: 10.1046/j.1471-4159.2003.01814.x. [DOI] [PubMed] [Google Scholar]
- 53.Goritz C, Mauch DH, Nagler K, Pfrieger FW. J. Physiology-Paris. 2002;96:257–263. doi: 10.1016/s0928-4257(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 54.Alderson P, Roberts I. BMJ. 1997;314:1855–1859. doi: 10.1136/bmj.314.7098.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marcus HJ, Carpenter K, Price SJ, Hutchinson PJ. J Neurooncol. 2010;97:11–23. doi: 10.1007/s11060-009-9990-5. [DOI] [PubMed] [Google Scholar]
- 56.Cosford RJO, Vinson P, Kukoyi S, Justice JB., Jr J. Neurosci. Meth. 1996;68 doi: 10.1016/0165-0270(96)00057-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.