Abstract
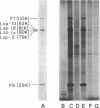
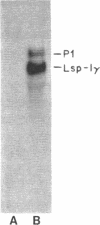
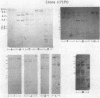
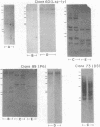
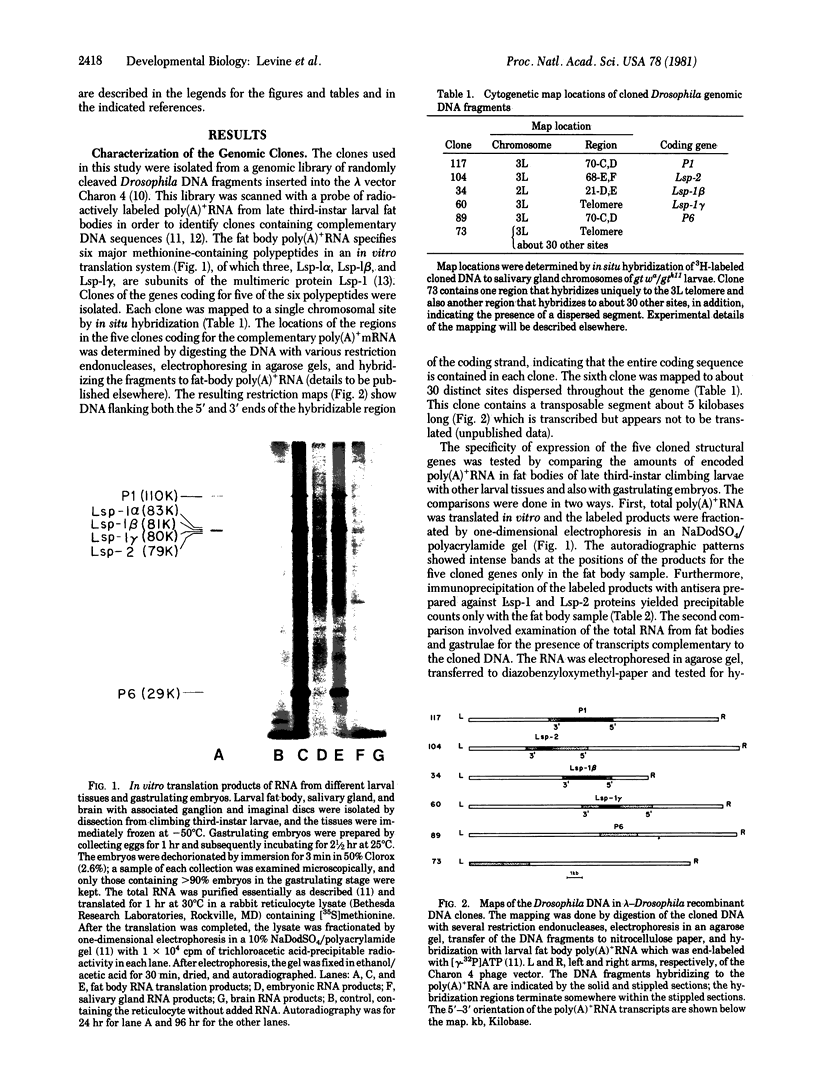
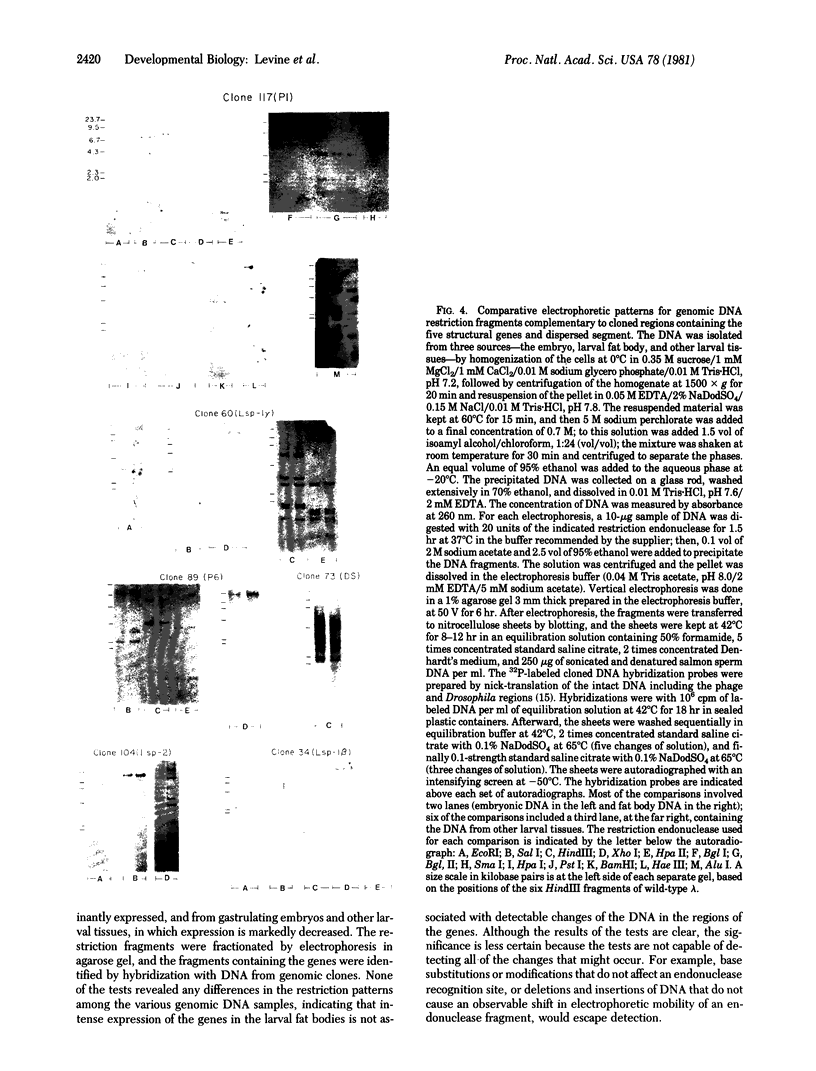
The purpose of this study was to test for the occurrence of changes in the organization, modification, or selective amplification of six developmentally regulated regions of genomic DNA during Drosophila development. Five of the regions contain structural genes, each of which maps at a single chromosomal site; the sixth region contains a dispersed segment that maps at about 30 different sites and appears to be transposable. The RNA transcripts and encoded proteins of the structural genes are major components of the fat body tissue in late third-instar larvae, in contrast to other larval tissues and young embryos in which the transcripts and proteins are hardly detectable. The dispersed segment shows the reverse developmental regulation: the amount of transcript is relatively high in young embryos and low in larval fat bodies. The test for changes in genomic DNA associated with the regulated expression of the six regions was based on comparative restriction mapping of the DNA from these sources. Genomic clones containing the transcribed and also flanking regions of DNA were used as probes to determine the positions of the complementary restriction fragments after electrophoresis in agarose gels. Each test, which involved digesting the genomic DNA samples with one of the endonucleases and probing with one of the clones, produced identical restriction maps for the different samples. The tests are capable of detecting changes in the organization of the cloned regions resulting from addition or removal either of endonuclease cleavage sites or of segments of DNA located between cleavage sites. Because the activities of several of the endonucleases, and probably all, are sensitive to methylation of one of the bases in the DNA recognition sequence, certain modifications of the cloned regions by methylation could also be detected. Although it is not certain that genomic reorganization or modification would have been detected by these tests, the number of endonucleases used was sufficient to provide persuasive evidence that such changes did not occur. Furthermore, the quantitative as well as qualitative similarities of the restriction maps in each test indicated that there was no significant selective amplification of the DNA within the cloned regions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Brock H. W., Roberts D. B. Comparison of the larval serum proteins of Drosophila melanogaster using one and two-dimensional peptide mapping. Eur J Biochem. 1980 May;106(1):129–135. doi: 10.1111/j.1432-1033.1980.tb06003.x. [DOI] [PubMed] [Google Scholar]
- Emmons S. W., Klass M. R., Hirsh D. Analysis of the constancy of DNA sequences during development and evolution of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1333–1337. doi: 10.1073/pnas.76.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W. J., Paro R. Isolation of a hybrid plasmid with homologous sequences to a transposing element of Drosophila melanogaster. Cell. 1980 Apr;19(4):897–904. doi: 10.1016/0092-8674(80)90081-1. [DOI] [PubMed] [Google Scholar]
- Lepesant J. A., Kejzlarova-Lepesant J., Garen A. Ecdysone-inducible functions of larval fat bodies in Drosophila. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5570–5574. doi: 10.1073/pnas.75.11.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Max E. E., Leder P. A kappa-immunoglobulin gene is formed by site-specific recombination without further somatic mutation. Nature. 1979 Aug 2;280(5721):370–375. doi: 10.1038/280370a0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Amplification of genes for chorion proteins during oogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1096–1100. doi: 10.1073/pnas.77.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel E., Dunsmuir P., Rubin G. M. Polymorphisms in the chromosomal locations of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979 Jun;17(2):429–439. doi: 10.1016/0092-8674(79)90169-7. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Tkacheva S. G., Belozersky A. N. Rare bases in animal DNA. Nature. 1970 Mar 7;225(5236):948–949. doi: 10.1038/225948a0. [DOI] [PubMed] [Google Scholar]
- Young M. W., Schwartz H. E. Nomadic gene families in Drosophila. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):629–640. doi: 10.1101/sqb.1981.045.01.081. [DOI] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]