Abstract
Phosphorylation of L-serine-containing enkephalin analogs has been explored as an alternative to glycosylation in an effort to increase blood-brain barrier (BBB) permeability and CNS bioavailability of peptide pharmacophores. Two enkephalin-based peptides were modified for these studies, a set related to DTLES, a mixed μ/δ-agonist, and one related to DAMGO, a highly selective μ-agonist. Each unglycosylated peptide was compared to its phosphate, its mono-benzylphosphate ester, and its β-D-glucoside. Binding was characterized in membrane preparations from CHO cells expressing human μ, δ and κ-opiate receptors (MOR, DOR and KOR). Antinociception was measured in mice using the 55°C tail flick assay. In order to estimate bioavailability, the antinociceptive effect of each opioid agonist was evaluated after intracerebroventricular (i.c.v.) or intravenous administration (i.v.) of the peptides. Circular dichroism (CD) methods and high field nuclear magnetic resonance (NMR) were used in the presence and absence of sodium dodecylsulfate (SDS) to understand how the presence of a membrane might influence the peptide conformations.
Introduction
Morphine, and its congeners are the most widely prescribed drugs for moderate to severe pain. These “narcotics” produce an array of side effects, including pruritus, miosis, euphoria, sedation, constipation, and respiratory depression. Since their discovery in 1975, much effort has been expended to convert endogenous opioid peptides Met/Leu-enkephalin into analgesics with the hope that they would provide pain relief with reduced side effects. To date, we are aware of only one report of an enkephalin-based peptide being tested as an analgesic in humans. The compound produced potent and fully efficacious analgesia in severe pain states, but required intrathecal administration.1
Many peptide analogs have been synthesized having greatly improved resistance to enzyme degradation and corresponding increases in plasma stability and serum half-life.2,3 The majority of these metabolically stable analogs fail to cross blood-brain barrier (BBB) in appreciable amounts. The BBB is both a physical and metabolic barrier between the CNS and the systemic circulation, which serves to regulate and protect the microenvironment of the brain.4 The BBB includes tight junctions between the endothelial cells of brain capillaries that behave as a continuous lipid bilayer.5
Glycosylation is a post-translational protein modification, which has been shown to increase metabolic stability,6 and has been shown to promote BBB penetration of enkephalins to produce CNS activity after peripheral administration in rodents.7,8 In some cases these glycosides have been shown to produce antinociceptive effects with greatly reduced side effects compared to traditional small molecules.9,10
Circular dichroism (CD) and nuclear Overhauser studies (NOESY) of these glycosylated enkephalins revealed that they adopt restricted and relatively well-defined conformational ensembles in sodium dodecylsulfate (SDS) micelles, whereas they form predominantly random coil conformations in H2O.11 In a related study it was shown that glycosylation of the highly MOR selective peptide Tyr-(D)Ala-NMePhe-Gly(OH) (“DAMGO”) improved bioavailability and in vivo potency.12 These MOR selective analogs showed greater potencies than morphine. Theoretically calculated amphipathicities of these glyco-DAMGO analogs correlated with antinociceptive potency (i.v. administration), presumably reflecting enhanced transport properties.
In this study we report on phosphorylation as an alternative strategy to enhance peptide stability and BBB penetration. Dass and Mahalaksmi showed that phosphorylated enkephalin was stable to proteolysis.13 Further, phosphorylated opioid peptides have been isolated from bovine adrenal medulla, providing further impetus to investigate the effects of phosphorylation on peptide pharmacology.14 The highly selective MOR agonist DAMGO15 and the mixed MOR/DOR agonist YtGFL-amide16,17 were chosen as the opioid “messages” for this study, which have previously been shown to produce centrally mediated effects upon glycosylation. The chemical structures of the lead peptides and their phosphate derivatives studied are depicted in Figure 1.
Figure 1.
Chemical structures of the compounds tested in this study.
Results and Discussion
In vitro binding activity
As shown in Table 1, all four phosphorylated enkephalin derivatives have Ki values of less than 10 nM for binding to the MOR. The phosphate mono-benzyl ester 1b showed an approximate 2-fold increase in affinity for DOR and KOR compared to phosphate 1a, while reducing the affinity of the peptide for MOR. Compounds 2a and 2b had Ki binding values of less than 5 nM for the MOR. The additional benzyl ester on the phosphate 2b increased the affinity for DOR ~25-fold relative to 2a, without affecting the affinity for MOR. These results demonstrate that the addition of a phosphate group to the peptide does not abolish the affinity of either peptide for opioid receptors, and the lipophilic benzyl moiety increased affinity for DOR opioid receptor for both peptide series.
Table 1.
Binding affinities and antinociceptive potencies of the phosphopeptides.
| Peptide | Ki (nM ± S.E.) | Ratio | A50 (95% CI) | |||
|---|---|---|---|---|---|---|
| [3H]DAMGO MOR | [3H]Naltrindole DOR | [3H]U69,593 KOR | MOR:DOR: KOR | i.c.v. (nmol/mouse) | i.v. (μmol/kg) | |
| 1 | 1.4 ± 0.08 | 4.1 ± 0.41 | 34 ± 2.2 | 1:3:15 | 0.068 (0.054–0.086) | 46.4 (35.4–60.7) |
| 1a | 3.9 ± 0.19 | 7.9 ± 0.73 | 310 ± 66 | 1:2:79 | 0.34 (0.22–0.52) | 54.1 (48.5–60.2) |
| 1b | 8.7 ± 0.93 | 3.3 ± 0.33 | 130 ± 6.2 | 3:1:39 | 0.093 (0.053–0.16) | 30% @ 32.0 mg/Kg |
| 1c | 2.4 ± 0.017 | 7.0 ± 1.2 | 49 ± 4.3 | 1:3:20 | 0.02 (0.01–0.04) | 11.4 (8.5–15.2) |
| 2 | 0.68 ± 0.2 | 600 ± 44 | 190 ± 9.3 | 1:880:220 | 0.002 (0.0015–0.003) | 0.135 (0.106–0.172) |
| 2a | 3.8 ± 0.73 | 1400 ± 180 | 31% inhib. @ 10 μM | 1:370:>1000 | 0.042 (0.02–0.07) | 1.5 (1.3–1.8) |
| 2b | 4.2 ± 0.34 | 55 ± 4.6 | 570 ± 9.8 | 1:13:140 | 0.033 (0.02–0.05) | 30% @ 38.1 mg/Kg |
| 2c¶ | 1.3 ± 0.14 | 54% inhib. @ 10 μM | 270 ± 2.5 | 1:>7000:208 | 0.019 (0.012–0.029) | 0.602 (0.470–0.772) |
Values are taken from Lowery et al.12
Peptides were incubated with membranes from CHO cell expressing human opioid receptors. Analgesic potency was evaluated i.c.v. and i.v. in mice. Details are provided in the experimental section.
Analgesic activity in vivo
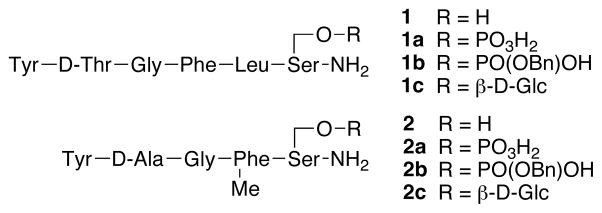
The antinociceptive potency of each compound (i.c.v. or i.v.) is summarized in Table 1. All compounds produced dose-related and time-related antinociceptive effects with full efficacy in the 55°C tail-flick assay following i.c.v. administration in mice (Figure 2 and supplemental info). The data indicate that phosphorylation of the mixed δ/μ agonist (DTLES peptide 1a) and the μ-agonist (DAMGO peptide 2a) did not alter the antinociceptive activity of the parent peptides significantly. The benzyl esters 1b and 2b also showed antinociceptive activity upon i.c.v. administration (data not shown, see supplemental info), but not after i.v. administration. This result suggests that the lipophilic benzyl esters do not cross the BBB in appreciable amounts. The phosphopeptide data were compared to glycopeptide data, which revealed that glycosylation of DTLES peptide (glycopeptide 1c) significantly improved antinociceptive activity upon both i.c.v. and i.v. administration. However, glycosylation of the DAMGO derivative (glycopeptide 2c) did not show improved activity compared to the parent peptide. The glycopeptides also showed superior in vivo activity compared to the phosphates. There was also a trend for increased duration of action from compound 1 to 1c and compound 2 to 2c, respectively.
Figure 2.
Antinociceptive i.c.v. and i.v. dose- and time-response curves for phosphopeptides 1a and 2a in the mouse 55°C warm water tail-flick test. Bars represent SEMs for each data point.
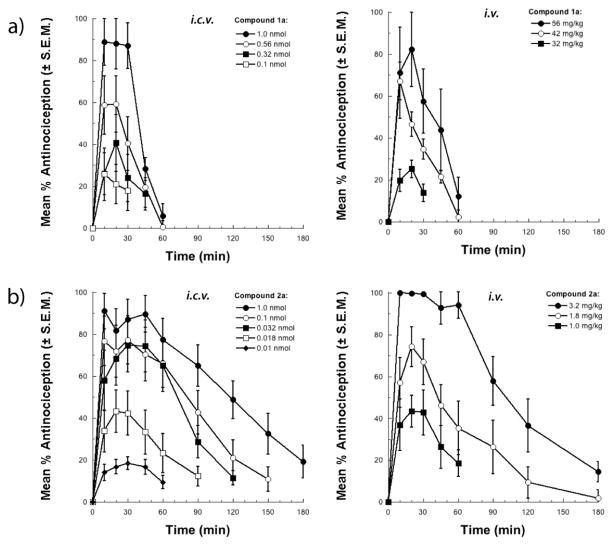
Circular dichroism analysis
The CD spectra for 1a and 2a were recorded in aqueous buffer as well as in the presence of membrane-mimicking SDS micelles (Figure 3). In aqueous buffer the phosphopeptide 1a displayed two maxima at 220 nm and 205 nm. The CD shape is neither random coil nor corresponding to any particular type of fold. Thus, one can conclude that peptide 1a probably exists as a conformational ensemble rather than a single conformer in aqueous buffer. A significant change was observed in the CD shape when the spectrum of 1a was recorded in the presence of SDS micelles; the positive band at 205 nm became a negative band, and the positive maxima at 222 nm shifted to a higher wavelength that reflected an overall conformational change of the molecule. The shape of this CD spectrum is characteristic of a type I/III β-turn conformation as described by Balaram and coworkers.18,19 The type I [φi+1 = −60°, ψi+1= −30°, φi+2 = −90°, ψi+2 ≈ 0°] and type III β-turns [φi+1 = −60°, ψi+1= −30°, φi+2 = −60°, ψi+2 = − 30°] are closely related and cannot easily be distinguished by CD or NMR. Further, the type III β-turn is essentially a single turn of a 310 helix [φi+1 = −60°, ψi+1= −30°, φi+2 = −60°, ψi+2= −30°]. The NOE patterns (vide infra) suggest consecutive turn conformations. Thus, it is probable that the peptide phosphate 1a adopts a 310 helix conformation in a membrane environment in the presence of SDS micelles, unlike in aqueous buffer where the interconversion of multiple turn types occur.
Figure 3.
CD spectra of phosphopeptides 1a and 2a in aqueous buffer and SDS micelles measured at pH 5.5 and 25°C.
The CD behavior of μ-selective 2a was completely different from 1a, and was very similar to the published CD spectrum of DAMGO reported by Doi and colleagues.20 Interestingly, the conformation of the phosphopeptide 2a was independent of the solvent; the peptide displayed more or less similar spectrum in both aqueous buffer as well SDS micelles. There appeared to be a correlation between the CD shape and receptor selectivity as noted by Doi et al. While the MOR-selective peptides related to 1 showed negative bands in the range of 210–230 nm, the mixed MOR-DOR peptide phosphates related to 2 showed positive bands.
NMR Analysis of 1a
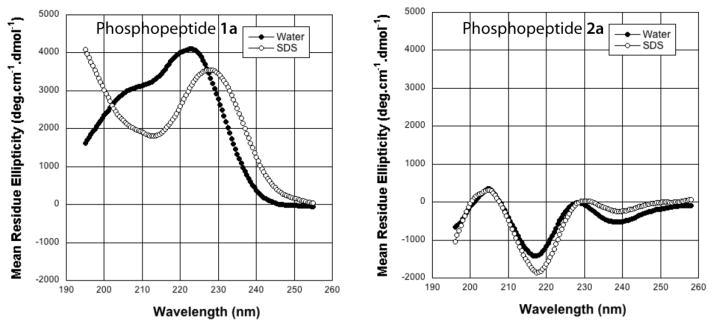
The MOR-DOR-agonist phosphopeptide 1a was subjected to 1H 2D NMR analysis in D2O/H2O buffered to pH 5.5, and in the presence of SDS micelles to obtain additional conformational information. Standard ROESY experiments yielded good quality spectra in D2O/H2O, but failed in the presence of the high M.W. SDS micelles, which required standard NOESY to produce good quality spectra.
The fingerprint regions (CαH Vs. NH) of the NOESY/ROESY spectra for phosphopeptide 1a are shown in Figure 4. In water buffered to pH 5.5, the observation of consecutive dNN(i, i+1) ROEs in the central Gly(3)-Leu(5) segment, along with the lack of any long-range ROEs indicated the presence of localized β-turn conformations.21,22 This was further confirmed by the observation of a single medium-range dαN(i, i+2) ROE between Thr(2) and Phe(4), as well as the diminished temperature coefficient (−4.29 ppb/K°) for the Phe(4) amide protons. Additional medium- and long-range NOEs characteristic of turn and helical conformations were observed in SDS micelles. Many NOEs between side-chain protons of different amino acids were observed (Supplementary data). The NOEs Leu5CδH↔Phe4 aromatic protons and Leu5CδH↔Tyr1 aromatic protons were especially crucial as they indicated proximity (<5Å), and the side-chains might orient on the same side of the backbone to create a hydrophobic core.
Figure 4.
Fingerprint regions (CαH-NH) of NOESY/ROESY spectra for the phosphopeptide 1a in a) SDS micelles and b) water measured at pH 5.5 and 25°C using a mixing time of 300 ms. The medium- and long-range NOE correlations are underlined.
The observation of such a large number of NOEs as shown in Figure 5 for such a short peptide sequence was remarkable. Two consecutive medium-range dαN(i, i+3) NOEs Thr(2)CαH↔Leu(5)NH and Gly(3)CαH↔Ser(6)NH strongly supported a helical type fold. This NOE is also observed in β-turn conformations. Therefore, it is also possible the phosphopeptide adopts two consecutive turns as in a short 310-helix conformation.23 Additional support for the helical type fold comes from the negative deviation of CαH from the random coil values (see the discussion below).
Figure 5.
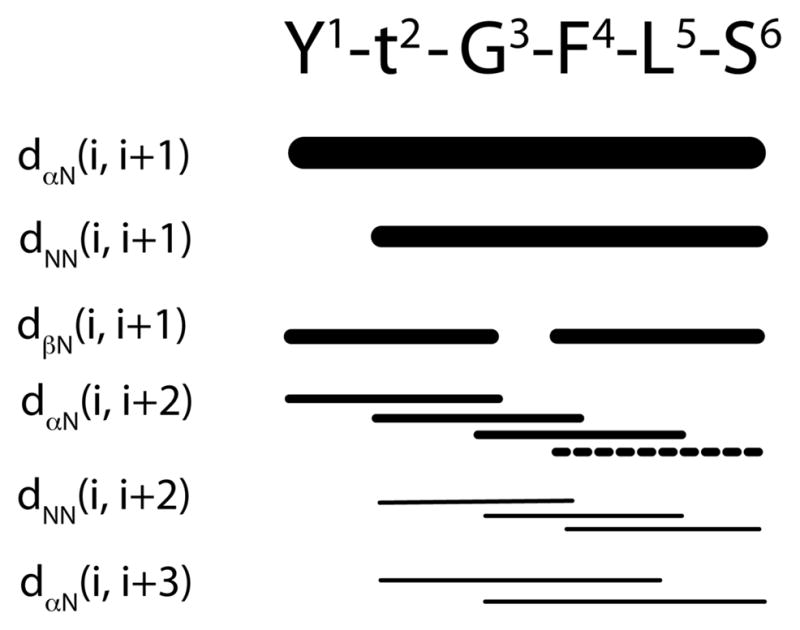
NOE summary for the phosphopeptide 1a in SDS micelles measured at pH 5.5 and 25 °C. The line thickness is approximately proportional to the intensity of the observed NOE cross peaks. The dashed line indicates the signal overlap of the cross-peaks.
Information on hydrogen bonds can be obtained from the amide temperature coefficients (Δδ/ΔT) of the amide bonds. The temperature coefficients for 1a in H2O and in the presence of SDS micelles were obtained in 5°C increments starting at 20°C (Supplementary Data). The Δδ-values for all the amide protons were more negative than −4.6 ppb/K° in water, indicating that the backbone was freely solvated and no intramolecular hydrogen bonds were present.24 In SDS micelles the values for all the amide protons became positive, and the C-terminal amide protons showed temperature coefficients more positive than the hydrogen bond threshold value of −4.6 ppb/K°. The diminished temperature coefficient values of amide protons of Phe(4), Leu(5), Ser(6) and one (but only one) of the terminal NH2 protons in SDS micelles indicated the presence of a hydrogen bond or a high degree of solvent shielding.
The Cα proton chemical shift values are sensitive to the secondary structure of proteins. Sykes and coworkers25 created a chemical shift index based on this principle which allowed us to get the first indication of specific secondary structural elements present in the peptides comparable to CD. Generally, the observation of consecutive negative deviations from random coil (upfield-shifted CαH resonances) indicates a helical conformation. The N-terminal segment residues displayed negative deviations in water, whereas in SDS micelles the C-terminal residues also showed negative deviations. It is not possible to assign a particular secondary structure conformation based solely on these values because the peptide is not long enough, but the differences show that the conformation of phosphopeptide 1a is very sensitive to the solvent milieu.
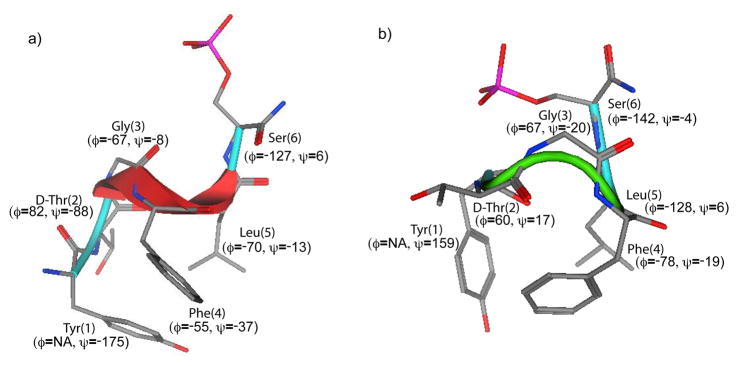
In order to obtain the 3D structures of the peptide phosphates a Stochastic conformational search was applied using distance restraints calculated from NOE data. Examination of low energy structures from the calculation suggests there are two major family of conformers possible for the phosphates. The lowest-energy structures from both the families are shown in Figure 6. The backbone dihedral angle of residues in the middle segment Gly(3), Phe(4) and Leu(5) are helical (Φ= −60° and Ψ= −30°) in one family. At least four residues are necessary to describe a α-helical turn. Therefore, it is possible to describe a 310 type helical fold for this region. Since dihedral values are the same for both type III β-turns and 310 helices, this region can also be described as two type III β-turn – one centered at Gly(3)-Phe(4) dipeptide segment and another centered at Phe(4)-Leu(5) – fused together. Both the family of structures are somewhat closely related to each other. The major difference is dihedral value of Leu(5) residue. The other family has only one type III β-turn centered at Gly(3)-Phe(4) dipeptide segment. The two different yet related family of conformers could be reason for the mixed δ/μ receptor selectivity of this phosphopeptide.
Figure 6.
Lowest energy structure of phosphopeptide 1a from a) Ensemble of helical conformers, and b) Ensemble of turn conformers. The backbone dihedral angle values are indicated in parentheses. Hydrogen atoms have been omitted for clarity.
Effect of Phosphorylation on Peptide Backbone Conformation
The CD and NMR data of the phosphopeptides were compared with data of parent peptides to examine the effect of phosphorylation on peptide conformation. The CD spectrum of 1a (Supplementary data) is significantly different from the parent peptide 1 indicating phosphorylation induced conformational change. Comparison of NOE summary (data not shown- see supplemental info) of phosphopeptide 1a with parent unphosphorylated peptide 1c reveals phosphorylation stabilizes the peptide structure. Many additional NOEs appear in the NOESY spectrum of 1a. The observation of consecutive dαN(i, i+2) and dNN(i, i+2) NOEs for 1a clearly suggest consecutive turns throughout the peptide length which is in contrast to 1 where only few of those NOEs were observed in the middle of the segment. Moreover, the observation of two consecutive medium-range dαN(i, i+3) NOEs Thr(2)CαH↔Leu(5)NH and Gly(3)CαH↔Ser(6)NH, which are not observed for 1, strongly support consecutive turns or short helical type fold for 1a. Additional NMR parameters such as CSI values2525 of Cα protons and temperature coefficient data (data not shown - see supplemental info) also indicate the structure stabilization by the phosphate group attached to the serine side-chain. In the case of phospho-enkephalin analog, Cα protons of all the residues display negative deviation from the random coil value, and most of the C-terminal amide protons are solvent shielded. This indicates they might all be involved in hydrogen bonds. However, the phosphorylation did not alter the conformation of DAMGO analog 2a. The CD and NMR data did not show any significant differences in the conformation by the attachment of phosphate group unlike the phospho-enkephalin analog.
Conclusions
Phosphorylation may provide a useful method to enhance water solubility and effective biodistribution of peptide drugs, but may be less effective than the corresponding serine glucosides, at least for these two short enkephalin sequences. Phosphorylation also stabilized the consecutive type III β-turn, a 310 type helical conformation in the middle segment of the phosphate analog of DTLES sequence, but did not significantly affect the conformation of the DAMGO analog.
The two classes of phosphates differ greatly in their interaction with the SDS micelles, with the more water soluble DAMGO sequence showing a largely electrostatic interaction, while the DTLES sequence17 is aided by additional lipophilic interactions, particularly the Leu(5) side chain residue.26 The phosphate does not interfere with opioid binding. The lipophilic benzyl phosphate ester moiety altered the μ/δ-selectivity, and blocked distribution to the CNS. Further studies with other peptide sequences may be indicated, and will help establish the generality of these findings.
Materials and Methods
Synthesis and Purification of Phosphopeptides
Amino acids, coupling reagents and Rink-amide resin were purchased from Advanced ChemTech (Louisville, USA). The phosphopeptides were synthesized manually by standard solid-phase methods employing fluorenylmethoxycarbonyl (Fmoc) chemistry on rink amide resin. The choice of side chain protecting groups allowed for removal in a single step at the end of the synthesis while the phosphopeptide is still attached to the resin. The side chain protected amino acids used in the synthesis were Fmoc-DThr(But)-OH, Fmoc-Tyr(But)-OH and Fmoc-Ser(OPO(OBz)OH)-OH. The amide couplings were with HBTU/HOBt/DIPEA. Each coupling was performed in a manual peptide synthesis vessel using DMF as solvent by agitating using N2 for 90 minutes. The coupling was monitored by the Kaiser ninhydrin test. Fmoc groups were removed with a solution of 2% piperidine and 2% DBU in DMF. Once the phosphopeptide was assembled and the final Fmoc group was removed, the phosphopeptide was cleaved from the resin with a cocktail F3CCOOH:Et3SiH:H2O:PhOMe:CH2Cl2 (9:0.5:0.5:0.05:1) that also removed the side chain protecting groups. The crude phosphopeptides were precipitated with ice-cold ether, filtered, redissolved in H2O and lyophilized. The phosphopeptides were purified by RP-HPLC with a preparative RP(C-18) column using acetonitrile-water gradient containing 0.1% TFA. Homogeneity of the final glycopeptides was assured by analytical RP-HPLC and mass spectrometry.
Receptor Binding Studies
To determine the affinity and selectivity of the peptides for the μ, δ, and κ opioid receptors, Chinese hamster ovary (CHO) cells that stably expressed one type of human opioid receptor were used as previously described.27 Cell membranes were incubated at 25°C with the radiolabeled ligands in a final volume of 1 ml of 50 mM Tris-HCl, pH 7.5. Incubation times of 60 min were used for the μ-selective peptide [3H]DAMGO and the κ-selective ligand [3H]U69,593, and a 3-hr incubation will be used with the δ-selective antagonist [3H]naltrindole. The final concentrations of [3H]DAMGO, [3H]naltrindole, and [3H]U69,593 were 0.25 nM, 0.2 nM, and 1 nM, respectively. Nonspecific binding was measured by inclusion of 10 μM naloxone for μ and κ binding and 100 mM for δ binding. The binding was terminated by filtering the samples through Schleicher & Scheull No. 32 glass fiber filters using a Brandel 48-well cell harvester. The filters were washed three times with 3 ml of cold 50 mM Tris-HCl, pH 7.5, and were counted in 2 ml of ScintiSafe 30% scintillation fluid (Fisher Scientific, Fair Lawn, NJ). For [3H]U69,593 binding, the filters will be soaked in 0.1% polyethylenimine for at least 30 min before use. IC50 values will be calculated by least squares fit to a logarithm-probit analysis. Ki values of unlabeled compounds will be calculated from the equation Ki = (IC50)/1 + S where S = (concentration of radioligand) (Kd of radioligand).28
Antinociceptive testing
Adult, male ICR mice (25–35 g, Harlan Industries, Cleveland, OH) were used for all in vivo experiments. Mice were housed in groups of 4–5 in standard Plexiglas containers with food and water available ad libitum. Animals were on a 12-h light/dark cycle (lights on at 07:00). Drug administrations were made via the i.c.v. or i.v. routes of administration. Antinociception was assessed using the 55°C warm-water tail flick assay.29 Mice were tested for baseline latencies by immersing the distal third of the tail into the water. Typical baseline latencies were between 1–2 seconds and any mouse with a baseline over 5 seconds was excluded from further procedures. The test compound was administered at t = 0 min and tail flick latencies were measured at various time points after injection. A maximal score of 100% was given to a mouse that did not respond with in 10 seconds to avoid tissue damage. Antinociception was calculated using the following formula: % antinociception = (test latency-baseline latency)/(10-baseline latency) × 100.
Circular Dichroism
All of the circular dichroism experiments were carried out on OLIS DSM-20 spectrophotometer equipped with temperature controller. The glycopeptide stock solutions were prepared by weighing the lyophilized powder to make 05–1.0 mM solution and the pH was adjusted to the desired value. The samples were prepared by diluting the stock solution to 180 μM. The reported data are average of four scans recorded between 200 and 250 nm by using integration time of three seconds, a scan step of 0.5 nm in a cell with a path length of 0.1 cm at 25 °C. All the spectra were baseline corrected and were smoothed using KaleidaGraph Ver. 4 software (Synergy Software, USA).
NMR Spectroscopy
All NMR spectra were recorded on a Bruker DRX600 600MHz spectrometer at 298 °K. Glycopeptide concentration for the NMR experiments varied from 2–3 mM. The micelle samples were prepared by dissolving the peptide and 100 equivalent of perdeuterated SDS in 0.6 ml of phosphate buffer (10 mM) containing 10% D2O. The pH of the each sample was adjusted to 4.5 by using DCl or NaOD as necessary. TSP was added as internal standard for referencing. Two-dimensional nuclear Overhauser enhancement (NOESY)30 and total correlation spectra (TOCSY)31 were acquired using standard pulse sequences and processed using XWINNMR (Bruker Inc) and FELIX2000 (Accelrys Inc, San Diego, CA). Mixing time for TOCSY spectra were either 80 or 100ms and for NOESY spectra was 300 ms. All experiments were 750 increments in t1, 16 or 32 scans each, 1.5 s relaxation delay, size 2 or 4K and the spectral processing was with shifted sine bell window multiplications in both dimensions. The water suppression was achieved by using WATERGATE pulse sequence.32 Coupling constants (3JαH-NH) were measured directly from the 1D spectra wherever possible.
Molecular Modeling
All the structural calculations were done using Molecular Operating Environment (MOE) software 2007.09 (Chemical Computing Inc., Canada). The standard Stochastic Conformational Search protocol was used for generating conformations using AMBER9933 as the force field with 8 Å cutoff for non-bonded interactions. The Stochastic Conformational Search method in MOE generates conformations by randomly sampling local minima of the potential energy surface similar to the Monte Carlo type routine called Random Incremental Pulse Search (RIPS) described by Raber and coworkers.34 RIPS method generates new molecular conformations by randomly perturbing the position of each coordinate of each atom in the molecule by some small amount, typically less than 2 angstroms, followed by energy minimization. The Stochastic Conformational Search method in MOE is similar in spirit except that it is based upon random rotations of bonds (including ring bonds) instead of Cartesian coordinate perturbation. A distance dependent dielectric constant of 4 was used for the calculations. All the inter-residue distance restraints calculated from the NOESY experiments were applied in the form of flat-bottom potential well with a common lower bound of 2.0 Å and the upper bound were obtained from integral volumes of the NOESY peaks. The NOE integral volumes were classified into strong, medium and weak with 3.0, 4.0 and 5.0 Å as upper bound distance. The starting structure for the calculations were having extended conformation. A total of 10000 conformations were generated with energy cut-off of 25 Kcal, which rejected many final conformers that had energy higher than 25 Kcal of the lowest-energy conformer.
Supplementary Material
Acknowledgments
We thank the Office of Naval Research (N00014-05-1-0807 & N00014-02-1-0471), the National Science Foundation (CHE-607917) and the National Institutes of Health (NINDS-NS-052727) for support.
References
- 1.Moulin DE, Max MB, Kaiko RF, Inturrisi CE, Maggard J, Yaksh TL, et al. The analgesic efficacy of intrathecal D-Ala2-D-Leu5-enkephalin in cancer patients with chronic pain. Pain. 1985;23:213–21. doi: 10.1016/0304-3959(85)90099-5. [DOI] [PubMed] [Google Scholar]
- 2.Adessi C, Soto C. Converting a peptide into a drug: strategies to improve stability and bioavailability. Curr Med Chem. 2002;9:963–78. doi: 10.2174/0929867024606731. [DOI] [PubMed] [Google Scholar]
- 3.Hruby VJ. Designing peptide receptor agonists and antagonists. Nat Rev Drug Discov. 2002;1:847–58. doi: 10.1038/nrd939. [DOI] [PubMed] [Google Scholar]
- 4.Abbott NJ, Patabendige A, Dolman D, Yusof S, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Banks WA. The blood-brain barrier as a regulatory interface in the gut-brain axes. Physiol Behav. 2006;89:472–6. doi: 10.1016/j.physbeh.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 7.Polt R, Dhanasekaran M, Keyari CM. Glycosylated neuropeptides: a new vista for neuropsychopharmacology? Med Res Rev. 2005;25:557–85. doi: 10.1002/med.20039. [DOI] [PubMed] [Google Scholar]
- 8.Dhanasekaran M, Polt R. New prospects for glycopeptide based analgesia: glycoside-induced penetration of the blood-brain barrier. Current drug delivery. 2005;2:59–73. doi: 10.2174/1567201052772843. [DOI] [PubMed] [Google Scholar]
- 9.Bilsky EJ, Egleton RD, Mitchell SA, Palian MM, Davis P, Huber JD, et al. Enkephalin glycopeptide analogues produce analgesia with reduced dependence liability. Journal of Medicinal Chemistry. 2000;43:2586–90. doi: 10.1021/jm000077y. [DOI] [PubMed] [Google Scholar]
- 10.Elmagbari NO, Egleton RD, Palian MM, Lowery JJ, Schmid WR, Davis P, et al. Antinociceptive structure-activity studies with enkephalin-based opioid glycopeptides. J Pharmacol Exp Ther. 2004;311:290–7. doi: 10.1124/jpet.104.069393. [DOI] [PubMed] [Google Scholar]
- 11.Palian MM, Boguslavsky VI, O’Brien DF, Polt R. Glycopeptide-membrane interactions: glycosyl enkephalin analogues adopt turn conformations by NMR and CD in amphipathic media. J Am Chem Soc. 2003;125:5823–31. doi: 10.1021/ja0268635. [DOI] [PubMed] [Google Scholar]
- 12.Lowery JJ, Yeomans L, Keyari CM, Davis P, Porreca F, Knapp BI, et al. Glycosylation improves the central effects of DAMGO. Chem Biol Drug Design. 2007;69:41–7. doi: 10.1111/j.1747-0285.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- 13.Dass C, Mahalakshmi P. Phosphorylation of enkephalins enhances their proteolytic stability. Life Sci. 1996;58:1039–45. doi: 10.1016/0024-3205(96)00057-4. [DOI] [PubMed] [Google Scholar]
- 14.Watkinson A, Young J, Varro A, Dockray GJ. The isolation and chemical characterization of phosphorylated enkephalin-containing peptides from bovine adrenal medulla. J Biol Chem. 1989;264:3061–5. [PubMed] [Google Scholar]
- 15.Handa BK, Land AC, Lord JA, Morgan BA, Rance MJ, Smith CF. Analogues of beta-LPH61-64 possessing selective agonist activity at mu-opiate receptors. Eur J Pharmacol. 1981;70:531–40. doi: 10.1016/0014-2999(81)90364-2. [DOI] [PubMed] [Google Scholar]
- 16.Zajac JM, Gacel G, Petit F, Dodey P, Rossignol P, Roques BP. Deltakephalin, Tyr-D-Thr-Gly-Phe-Leu-Thr: a new highly potent and fully specific agonist for opiate delta-receptors. Biochem Biophys Res Commun. 1983;111:390–7. doi: 10.1016/0006-291x(83)90318-2. [DOI] [PubMed] [Google Scholar]
- 17.Gacel G, Daugé V, Breuzé P, Delay-Goyet P, Roques BP. Development of conformationally constrained linear peptides exhibiting a high affinity and pronounced selectivity for delta opioid receptors. J Med Chem. 1988;31:1891–7. doi: 10.1021/jm00118a005. [DOI] [PubMed] [Google Scholar]
- 18.Rao BNN, Kumar A, Balaram H, Ravi A, Balaram P. Nuclear Overhauser Effects and Circular Dichroism as Probes of beta-Turn Conformations in Acyclic and Cyclic Peptides with Pro-X Sequences. J Am Chem Soc. 1983;105:7423–8. [Google Scholar]
- 19.Crisma M, Fasman GD, Balaram H, Balaram P. Peptide models for beta-turns. A circular dichroism study. Int J Pept Protein Res. 1984;23:411–9. [PubMed] [Google Scholar]
- 20.Doi M, Tanaka M, Ikuma K, Nabae M, Kitamura K, Inoue M, et al. Conformational characteristics of receptor-selective opioid peptides. 1H n.m.r. and c.d. spectroscopic studies of delta-kephalin and [Val4]morphiceptin. Biochem J. 1988;251:581–8. doi: 10.1042/bj2510581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penkler LJ, Van Rooyen PH, Wessels PL. Conformational analysis of mu-selective [D-Ala2, MePhe4]enkephalins. Int J Pept Protein Res. 1993;41:261–74. doi: 10.1111/j.1399-3011.1993.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 22.Chou KC. Prediction of tight turns and their types in proteins. Anal Chem. 2000;286:1–16. doi: 10.1006/abio.2000.4757. [DOI] [PubMed] [Google Scholar]
- 23.Pal L, Basu G, Chakrabarti P. Variants of 3(10)-helices in proteins. Proteins. 2002;48:571–9. doi: 10.1002/prot.10184. [DOI] [PubMed] [Google Scholar]
- 24.Cierpicki T, Otlewski J. Amide proton temperature coefficients as hydrogen bond indicators in proteins. J Biomol NMR. 2001;21:249–61. doi: 10.1023/a:1012911329730. [DOI] [PubMed] [Google Scholar]
- 25.Wishart DS, Sykes BD, Richards FM. The chemical shift index: a fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry. 1992;31:1647–51. doi: 10.1021/bi00121a010. [DOI] [PubMed] [Google Scholar]
- 26.Lowery JJ, Raymond TJ, Giuvelis D, Bidlack JM, Polt R, Bilsky EJ. In Vivo Characterization of MMP-2200, a Mixed δ/μ Opioid Agonist, in Mice. J Pharm Exp Ther. 2011;336:767–778. doi: 10.1124/jpet.110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parkhill A, Bidlack J. Several delta-opioid receptor ligands display no subtype selectivity to the human delta-opioid receptor. Eur J Pharmacol. 2002;451:257–64. doi: 10.1016/s0014-2999(02)02241-0. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Prusoff WH. Relationship between inhibition constant (k1) and concentration of inhibitor which causes 50 per cent inhibition (i50) of an enzymatic-reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 29.Jannsen PAJ, Niemegeers CJE, Dorg JGH. The inhibitory effects of fentanyl and other morphine-like analgesics on the warm water-induced tail withdrawl reflex in rats. Arzneim-Forsch. 1963;13:502–5. [PubMed] [Google Scholar]
- 30.Kumar A, Ernst RR, Wüthrich K. A two-dimensional nuclear Overhauser enhancement (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. Biochem Biophys Res Commun. 1980;95:1–6. doi: 10.1016/0006-291x(80)90695-6. [DOI] [PubMed] [Google Scholar]
- 31.Bax A, Davis DG. MLEV-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J Magn Reson. 1985;65:355–60. [Google Scholar]
- 32.Piotto M, Saudek V, Sklenár V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J Biomol NMR. 1992;2:661–5. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Cieplak P, Kollman P. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J Comput Chem. 2000;21:1049–74. [Google Scholar]
- 34.Ferguson DM, Raber DJ. A new approach to probing conformational space with molecular mechanics: random incremental pulse search. J Am Chem Soc. 1989;111:4371–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.