Abstract
Omuralide, a transformation product of the microbial metabolite lactacystin, was the first molecule discovered as a specific inhibitor of the proteasome and is unique in that it specifically inhibits the proteolytic activity of the 20S subunit of the proteasome without inhibiting any other protease activities of the cell. The total syntheses of omuralide and (+)-lactacystin are reported. An important key intermediate is synthesized at an early stage, which allows analogs of these two natural products to be made readily.
Introduction
The proteasome, a high molecular weight, multicatalytic protease complex responsible for most non-lysosomal intracellular protein degradation, is emerging as an important target for cancer chemotherapy because small molecule inhibitors of its catalytic activity induce apoptosis in both in vitro and in vivo models of human malignancies and are proving to have efficacy in early clinical trials.1 A role for the proteasome in programmed cell death was uncovered using small molecular weight, cell-permeable inhibitors, which induce apoptosis in a variety of tumor-derived cell lines.2 Given the importance of the proteasome to normal cellular homeostasis, however, it is likely that inhibitors induce programmed cell death by affecting many apoptosis-associated pathways. Eukaryotic intracellular protein degradation occurs predominantly through the ubiquitin proteasome pathway (UPP) composed of the Ub-conjugating system and the 26S proteasome.3
Lactacystin (1), a microbial natural product that inhibits cell proliferation and induces neurite outgrowth in a murine neuroblastoma cell line, has become a widely used reagent in functional studies of the proteasome.4 The proteasome is composed of a 20S catalytic core particle and additional subunits that are thought to be involved in the recognition and unfolding of ubiquitinated proteins; the composite structure has a sedimentation coefficient of 26S. Lactacystin binds to certain catalytic subunits of the 20S proteasome and inhibits the three best characterized peptidase activities of the proteasome, two irreversibly and all at different rates.5
Omuralide (2; also called clasto-lactacystin-β-lactone), a transformation product of the microbial metabolite lactacystin,6 was originally isolated by Omura and co-workers from cultures of a terrestrial Streptomyces sp.7 Omuralide, remarkable because it was the first molecule discovered to be a truly specific inhibitor of the proteasome, is unique in that it specifically inhibits the proteolytic activity of the 20S subunit of the proteasome without inhibiting any other protease activities of the cell.8 This is an important attribute, which can be utilized in future potential drug design.
Stimulated by the unusual structure of lactacystin, its remarkable biological activity, and the scarcity of natural material, several research groups undertook total syntheses.9,10,11,12,13,14,15 The first total synthesis of lactacystin in 19929 made lactacystin (and radiolabeled lactacystin) available and was instrumental in research, which demonstrated that the biological activity of lactacystin results from its potent, highly selective, and irreversible inhibition of proteasome-mediated peptidase activity.4 Syntheses of omuralide (2) also have been reported.13, 15, 16 Conclusive evidence was obtained that lactacystin is converted in vivo to the equipotent β-lactone omuralide (2), which is the actual agent that acts by acylation of the amino terminal threonine residue of a proteasome subunit. This result was confirmed by X-ray crystallographic studies at 2.4 Å resolution.17,18,19.
Many analogs of 1 were synthesized to discover the most potent agent.20,21 There is an absolute requirement for the β-lactone ring, and the stereochemical fidelity is dictated by that of the natural product. Furthermore, methylation of the y-lactam nitrogen abolishes activity. The one region of the molecule that supported chemical modification was the C4 alkyl group. Replacement of the methyl group at C7 with short aliphatic chains enhanced the potency of the lactone inhibitor. The best activities were recorded for ethyl, n-propyl, isopropyl, and n-butyl, all possessing two to three times the potency of the corresponding (+)-lactacystin-β-lactone.
From the view of medicinal chemistry, an ideal synthesis of a target molecule is one in which a stable key intermediate, which can be used to efficiently assemble a diverse library of analogs of the target on a large scale. Here we report a synthesis of lactacystin (1) and omuralide (2) via an important intermediate (3, Figure 1), leading to the efficient preparation of analogs in which the moieties of 3 can readily be replaced by other groups.
Figure 1.
Retrosynthetic plan for lactacystin and omuralide
Results and Discussion
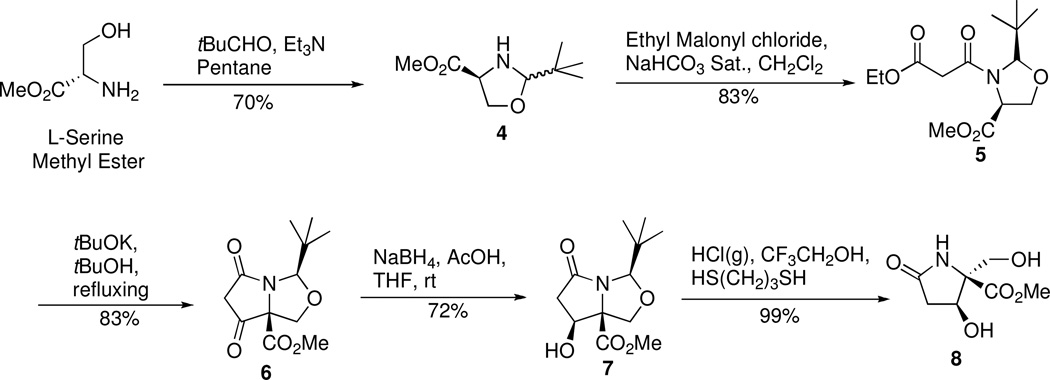
Starting from L-serine methyl ester, β-hydroxylated lactam 8 was prepared in five steps with very high stereoselectivity by an elegant method of Maloney and coworkers for diastereoselectively controlled hydroxylated lactams22 (Scheme 1). Thus, serine-derived oxazolidine 4 was acylated with ethyl malonyl chloride to give N-acyl derivative 5. In our hands, the acylation of 4 in a two-phase reaction medium of saturated aqueous NaHCO3 in methylene chloride gave the best results and made the work up easier than that reported. A Dieckmann condensation followed by reduction generated alcohol 7 and a minor amount of the epimer, which was removed by recrystallization to generate the desired isomer in a 60% overall yield for the two steps. Protection of the β–hydroxyl group of alcohol 7 also was explored. The TBS ether, benzyl ether, and acetate were attempted, all of which, however, resulted in production of an unsaturated lactam side product as a result of in situ elimination of the protected alcohol of 7. This suggests that cis substitution at the beta position of this bicyclic lactam is unstable and favors elimination. Diol 8 was obtained in a quantitative yield by reaction of 7 in a mixture of propane-1,3-dithiol and acidic trifluoroethanol.
Scheme 1.
Synthesis of 8
Key intermediate 12 was prepared as shown in Scheme 2. The two hydroxyl groups of 8 were protected as TBS ethers by treatment with TBS chloride in DMF with imidazole as base. The lactam of resulting bis-TBS ether 9 was protected as its PMB ether (10). Initially, Boc was used as the protecting group for 9; however, in the next step the Boc group migrated from the lactam to the adjacent hydroxyl group after its TBS ether was removed. The TBS ether at the primary alcohol in 10 was then regioselectively deprotected. After screening various acidic and fluoride reagent conditions, TFA/H2O/CH2Cl2 (9/1/1) was found to be the most efficient conditions to prepare alcohol 11, which was Dess-Martin periodinane oxidized to aldehyde 12, the key intermediate.
Scheme 2.
Synthesis of key intermediate 12
Next, the two hydrophobic subunits of the β-hydroxy lactam skeleton were installed (Scheme 3). Aldehyde 12 was treated with 2-propenyl Grignard reagent and trimethylchlorosilane, as was reported earlier for addition to aldehydes with high stereoslectivity,18a and obtained only one stereoisomer of adduct 13. A six-membered ring transition state, with Mg2+ coordinating to the carbonyl groups of the ester and aldehyde, was proposed to account for the stereoselectivity of the addition.18a Hydrogenation of 13 followed by acylation and deprotection of the TBS ether of 15 gave 16; the absolute stereochemistry of 16 was confirmed by X-ray crystallography (Figure 2). Unfortunately, various bases, including LDA and potassium and sodium HMDS, followed by methyl iodide failed to methylate the α-position of the lactam, and only a trace amount of the O-methylated enolate was observed. It was previously reported that Boc-protection of a lactam amide nitrogen assisted in stabilization of the α-anion by formation of a cyclic transition state with the lactam carbonyl group and the lithium cation.10
Scheme 3.
Synthesis of 17
Figure 2.
X-ray crystal structure of 16
Therefore, the PMB group of 15 was exchanged for a Boc protecting group (19) in two steps (Scheme 4). The TBS ether was removed with TBAF in acetic acid; the acetic acid is important, as decomposition was observed when only TBAF was utilized. Methylation of 20 was performed by the method of Donohoe et al.11 with LDA and HMPA, generating 21 in a 60% yield with very high stereoselectivity. After Boc deprotection and hydrolysis of the methyl ester and the acetic ester of 22, the β-lactone was formed by the reported coupling procedure using BOPCl,18a yielding omuralide (2) in a total of 19 steps. Further treatment of 2 with N-acetyl L-cysteine by the reported procedure18a gave (+)-lactacystin (1).
Scheme 4.
Final steps in the synthesis of omularide (2) and lactacystin (1)
Conclusion
We have completed total stereospecific syntheses of omuralide (2) and (+)-lactacystin (1) in 3% (19 steps) and 1.5% (20 steps) overall yields, respectively. Key intermediate 12 was prepared at an early stage of the synthesis; then the two alkyl substituents were introduced at a later stage. The introduction of these substituents permits analog modifications of these natural products. The PMB protecting group was found to be important for the introduction of the isopropyl substituent, and the Boc protecting group was critical for the installation of the methyl group.
Experimental Section
(2R,3S)-Methyl 3-(tert-butyldimethylsilyloxy)-2-((tert-butyldimethylsilyloxy)methyl)-5-oxopyrrolidine-2-carboxylate (9)
To a solution of 8 (5 g, 26.43 mmol) in DMF (200 mL) was added TBSCl (8.76 g, 58.15 mmol) followed by imidazole (7.20 g, 106 mmol) at room temperature. The reaction was stirred overnight. The reaction was quenched by the addition of saturated aqueous NH4Cl solution and then was extracted with EtOAc (3 × 500 mL). The combined organic phases were washed with brine, dried with Na2SO4, and concentrated. The residue was purified by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford 9 (10.4 g, 94%) as a colorless oil. [α]25D +31.19 (c 1.0 CHCl3). 1H NMR (500 MHz, CDCl3) δ 6.30 (s, 1H), 4.30 (dd, J = 6.4, 3.6 Hz, 1H), 4.00 (d, J = 9.8 Hz, 1H), 3.70 (s, 3H), 3.61 (d, J = 9.8 Hz, 1H), 2.60 (dd, J = 16.8, 6.5 Hz, 1H), 2.31 (dd, J = 16.8, 3.6 Hz, 1H), 0.83 (S, 9H), 0.82 (s, 9H), 0.03 (s, 3H), 0.02 (s, 3H), 0.02 (s, 3H), 0.01 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 174.9, 170.0, 73.7, 71.2, 66.4, 52.4, 40.6, 26.3, 26.1, 25.9, 25.8, 25.7, 25.6, 25.4, 25.2, 18.3, 17.9, −4.3, −4.7, −5.1, −5.5, −5.6, −6.0. HRMS (ESI): calcd for [(C19H39NO5Si2) + H]+ 418.2421; found 418.2424.
(2R,3S)-Methyl 3-(tert-butyldimethylsilyloxy)-2-((tert-butyldimethylsilyloxy)methyl)-1-(4-methoxybenzyl)-5-oxopyrrolidine-2-carboxylate (10)
NaH (60% in mineral oil, 1.05 g, 26.33 mmol) was pre-washed with hexanes, and then was suspended in DMF (300 mL). To the mixture was carefully added a solution of 9 (10 g, 23.94 mmol) in DMF (100 mL) at 0 °C. The resulting mixture was allowed to stir for 1 h before PMBCl (4.5 g, 3.90 mL, 28.73 mmol) was added dropwise, followed by TBAI (1.77 g, 4.79 mmol). The reaction mixture was stirred at room temperature overnight then was quenched by adding saturated aqueous NH4Cl solution and was extracted with EtOAc (3 × 500 mL). The combined organic phases were washed with brine (30 mL), dried with Na2SO4, and concentrated. The residue was purified by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford 10 (10.6 g, 82%) as a colorless oil.
[α]25D +55.82 (c 0.8 CHCl3) 1H NMR (500 MHz, CDCl3) δ 7.19 (d, J = 8.5 Hz, 2 H), 6.78 (d, J = 8.5 Hz, 2 H), 4.94 (d, J = 15 Hz, 1 H), 4.56 (t, J = 8.5 Hz, 1 H), 4.98 (dd, J = 12, 50 Hz, 1H), 3.84 (d, J = 12 Hz, 1H), 3.77 (s, 3H), 3.27 (s, 3H), 2.63 (d, J = 8.5 Hz, 1H), 0.89 (s, 9H), 0.81 (s, 9H), 0.10 (s, 6H), 0.09 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 173.9, 170.1, 159.1, 130.6, 128.5, 113.7, 95.0, 73.8, 68.4, 59.0, 55.5, 51.6, 43.5, 39.1, 26.0, 25.9, 25.7, 25.6, 18.3, 17.9, −5.0, −5.4, −5.8. HRMS (ESI): calcd for [(C27H47NO6Si2) + H]+ 538.3013; found 538.3020.
(2R,3S)-Methyl 3-(tert-butyldimethylsilyloxy)-2-(hydroxymethyl)-1-(4-methoxybenzyl)-5-oxopyrrolidine-2-carboxylate (11)
To a stirred solution of TFA/H2O (9/1, 500 mL) was added a solution of 10 (10 g, 18.59 mmol) in CH2Cl2 (50 mL) at room temperature. The reaction was monitored by TLC until the starting material was consumed. Most of the solvent and TFA was evaporated, and the crude product was extracted with EtOAc (3 × 500 mL). The combined organic phase was washed with brine (30 mL), dried with Na2SO4, and concentrated. The residue was purified by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford 11 (5.8 g, 73%) as a colorless oil, which gradually changed to a white solid. [α]25D +10.99 (c 1.6 CHCl3) 1H NMR (500 MHz, CDCl3) δ 7.27 (d, J = 8.5 Hz, 2H), 6.84 (d, J = 8.5 Hz, 2H), 4.73 (d, J = 15.0 Hz, 1H), 4.49 (t, J = 8.2 Hz, 1H), 4.09 (d, J = 15.0 Hz, 1H), 3.95 – 3.71 (m, 4H), 3.55 (s, 3H), 2.65 (ddd, J = 51.3, 16.5, 8.2 Hz, 1H), 0.84 (s, 9H), 0.05 (d, J = 4.8 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 174.3, 170.4, 159.4, 130.0, 129.3, 114.3, 77.5, 77.2, 77.0, 75.1, 68.8, 61.4, 55.4, 52.2, 44.2, 39.0, 25.6, 17.9. HRMS (ESI): calcd for [(C21H33NO6Si) + H]+ 424.2153; found 424.2155.
(2S,3S)-Methyl 3-(tert-butyldimethylsilyloxy)-2-formyl-1-(4-methoxybenzyl)-5-oxopyrrolidine-2-carboxylate (12)
To a solution of 11 (5.0 g, 11.80 mmol) in CH2Cl2 (500 mL) was added Dess-Martin periodinane (3.75 g, 8.85 mmol) in several portion at 0 °C. The resulting mixture was stirred at room temperature for 2 h. Then an aqueous solution of Na2S2SO3 was added very carefully to quench the reaction. To the resulting mixture was added saturated aqueous NaHCO3 solution, which was extracted with CH2Cl2 (3 × 500 mL). The combined organic phase was washed with brine (50 mL), dried with Na2SO4, and concentrated. The residue was purified by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford 12 (4.4 g, 88%), [α]25D +56.99 (c 1.0 CHCl3) 1H NMR (500 MHz, CDCl3) δ 9.76 (s, 1H), 7.02 (d, J = 8.2 Hz, 2H), 6.73 (d, J = 8.1 Hz, 2H), 4.65 (d, J = 14.6 Hz, 1H), 4.46 (t, J = 7.1 Hz, 1H), 4.31 (d, J = 14.6 Hz, 1H), 3.69 (s, 3H), 3.47 (s, 3H), 2.58 (ddd, J = 23.1, 16.4, 7.2 Hz, 2H), 0.76 (s, 9H), −0.04 (d, J = 8.1 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 172.6, 167.6, 159.4, 131.0, 127.2, 113.8, 77.8, 77.5, 77.2, 77.0, 70.7, 55.2, 52.3, 44.4, 39.0, 25.8, 25.4, 25.3, 24.9, 17.7. HRMS (ESI): calcd for [(C21H31NO6Si) + H]+ 422.1999; found 422.1997.
(2S,3S)-Methyl 3-(tert-butyldimethylsilyloxy)-2-((S)-1-hydroxy-2-methylallyl)-1-(4-methoxybenzyl)-5-ox opyrrolidine-2-carboxylate (13)
To a stirred solution of aldehyde 12 (4.0 g, 9.49 mmol) in THF (500 mL) at −40 °C was added TMSCl (5.15 g, 6.0 mL, 47.5 mmol). Isopropenylmagnesium bromide (0.5 M in THF, 57 mL, 28.47 mmol) was then added dropwise at −40 °C. After the mixture was stirred for 2 h at −40 °C, the reaction was quenched with saturated aqueous NH4Cl at that temperature and stirred for an additional 0.5 h. The resulting mixture was extracted with EtOAc (3 × 500 mL). The combined organic phases were washed with brine (30 mL), dried with Na2SO4, and concentrated. The residue was purified by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford 13 (3.8 g, 87%) as a colorless glass, [α]25D +50.00 (c 0.9 CHCl3). 1H NMR (499 MHz, CDCl3) δ 7.17 (d, J = 8.1 Hz, 2H), 6.71 (d, J = 8.1 Hz, 2H), 5.02 (d, J = 51.3 Hz, 2H), 4.78 (d, J = 15.1 Hz, 1H), 4.67 (d, J = 4.7 Hz, 1H), 4.63 – 4.55 (m, 1H), 4.08 (d, J = 5.0 Hz, 1H), 4.01 (d, J = 14.9 Hz, 1H), 3.66 (s, 3H), 3.20 (s, 3H), 2.74 (dd, J = 16.7, 7.5 Hz, 1H), 2.33 (dd, J = 16.8, 4.6 Hz, 1H), 1.70 (s, 3H), 0.74 (s, 9H), −0.03 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 174.1, 171.2, 158.4, 142.7, 129.7, 129.0, 116.6, 113.2, 76.5, 76.3, 69.6, 55.0, 51.4, 45.4, 40.2, 25.3, 20.4, 17.5, −4.7, −5.4. HRMS (ESI): calcd for [(C24H37NO6Si) + H]+ 464.2444; found 464.2446.
(2S,3S)-Methyl 3-(tert-butyldimethylsilyloxy)-2-((S)-1-hydroxy-2-methylpropyl)-1-(4-methoxybenzyl)-5-oxopyrrolidine-2-carboxylate (14)
To a stirred solution of 13 (3.0 g, 6.47 mmol) in EtOH (50 mL) in a single-necked flask was added Pd/C (10%, 300 mg) very carefully at room temperature. The flask was evacuated and connected to a hydrogen balloon through a three-way stopcock adaptor. The solution was evacuated under vacuum for 3 min with stirring, and was filled with H2. This cycle was repeated three times. Then the reaction was stirred overnight under H2. The reaction mixture was filtered through an EtOAc pre-washed Celite layer to give a clear filtrate. The Celite layer was was washed with EtOAc again. The filtrate was evaporated to give 14 (3.0 g, 99%) as a clear colorless oil. [α]25D +21.63 (c 0.8 CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.21 (d, J = 8.5 Hz, 2H), 6.77 (d, J = 8.6 Hz, 2H), 4.73 (dd, J = 8.2, 6.0 Hz, 1H), 4.67 (d, J = 14.9 Hz, 1H), 4.08 (dd, J = 4.7, 3.3 Hz, 1H), 4.04 (d, J = 14.8 Hz, 1H), 3.73 (s, 3H), 3.41 (d, J = 4.8 Hz, 1H), 3.27 (s, 3H), 2.76 (dd, J = 17.1, 8.4 Hz, 1H), 2.43 (dd, J = 17.1, 5.9 Hz, 1H), 1.78 – 1.60 (m, 1H), 0.92 (t, J = 7.1 Hz, 6H), 0.78 (s, 9H), 0.02 (d, J = 4.7 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 174.5, 172.5, 158.9, 130.4, 128.8, 113.6, 76.8, 76.2, 68.2, 55.3, 51.7, 45.4, 40.0, 28.7, 25.5, 25.4, 22.4, 17.7, 17.4, −4.6, −5.2. HRMS (ESI): calcd for [(C24H39NO6Si) + H]+ 466.2632; found 466.2634.
(2S,3S)-Methyl 2-((S)-1-acetoxy-2-methylpropyl)-3-(tert-butyldimethylsilyloxy)-1-(4-methoxybenzyl)-5-oxopyrrolidine-2-carboxylate (15)
To a solution of 14 (2.4 g, 5.15 mmol) in pyridine (20 mL) at 0 °C was added Ac2O (789 mg, 729 µL, 7.73 mmol), followed by DMAP (126 mg, 1.03 mmol). The reaction was allowed to stir overnight at room temperature, and the solvent was evaporated. To the resulting residue was added saturated NH4Cl solution, and the aqueous phase was extracted with EtOAc (3 × 300 mL). The combined organic phase was washed with brine (30 mL), dried with Na2SO4, and concentrated. The residue was purified by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford 15 (2.36 g, 90%) as a colorless oil. [α]25D +2.31 (c 1.0 CHCl3) 1H NMR (500 MHz, CDCl3) δ 7.25 (d, J = 8.5 Hz, 2H), 6.81 (d, J = 8.5 Hz, 2H), 5.49 (d, J = 5.5 Hz, 1H), 4.67 (d, J = 15.5 Hz, 1H), 4.56 (dd, J = 7.0, 3.5 Hz, 1H), 4.35 (d, J = 15.5 Hz, 1H), 3.77 (s, 3H), 3.47 (s, 3H), 2.71 (dd, J = 17.0, 7.0 Hz, 1H), 2.41 (dd, J = 17.0, 3.5 Hz, 1H), 2.09 (s, 3H), 1.89 (m, 1H), 0.94 (d, J = 7.0 Hz, 3H), 0.84 (d, J = 7.0 Hz, 3H),0.85 (s, 9 H), 0.08 (s, 3 H), 0.06)s, 3 H). 13C NMR (126 MHz, CDCl3) δ 174.6, 170.4, 169.0, 158.8, 129.2, 129.1, 113.7, 77.0, 70.0, 55.4, 51.9, 46.6, 40.6, 29.3, 25.6, 21.6, 21.2, 19.0, 17.8, −4.3, −5.1. HRMS (ESI): calcd for [(C26H41NO7Si) + H]+ 508.2762; found 508.2752.
(2R,3S)-Methyl 2-((S)-1-acetoxy-2-methylpropyl)-3-hydroxy-1-(4-methoxybenzyl)-5-oxopyrrolidine-2-carboxylate (16)
15 (2.0 g, 3.94 mmol) was added to a solution of TFA/H2O (10/1, 50 mL) and the resulting reaction mixture was heated to reflux for 3 h. Most of the solvent was evaporated, and the crude product was purified directly by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford 19 (1.4 g, 90%). [α]25D −11.81 (c 0.5 CHCl3). 1H NMR (500 MHz, CDCl3) δ 7.23 (d, J = 8.5 Hz, 2H), 6.84 (d, J = 8.5 Hz, 2H), 5.54 (d, J = 5.8 Hz, 1H), 4.79 (d, J = 15.3 Hz, 1H), 4.68 (dd, J = 7.5, 4.8 Hz, 1H), 4.33 (d, J = 15.3 Hz, 1H), 3.79 (s, 3H), 3.48 (s, 3H), 2.93 (dd, J = 17.7, 7.7 Hz, 1H), 2.62 (dd, J = 17.7, 4.6 Hz, 1H), 2.13 (s, 3H), 1.89 (td, J = 13.1, 6.5 Hz, 1H), 0.97 (d, J = 6.9 Hz, 3H), 0.87 (d, J = 6.6 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 174.5, 170.0, 159.0, 129.7, 128.7, 113.9, 75.8, 69.3, 55.4, 52.3, 46.1, 38.6, 28.9, 21.1. HRMS (ESI): calcd for [(C20H27NO7) + H]+ 394.1842; found 394.1850.
(2R,3S)-Methyl 2-((S)-1-acetoxy-2-methylpropyl)-3-(tert-butyldimethylsilyloxy)-5-oxopyrrolidine-2-carboxylate (18)
To a solution of 15 (1.4 g, 2.76 mmol) in CH3CN/H2O (1/1, 100 mL), was added ceric ammonium nitrate (4 g, 7.30 mmol) at room temperature. The resulting solution was stirred until the TLC showed no more starting material. The solution was extracted with EtOAc (3 × 300 mL), and the combined organic phase was washed with brine (30 mL), dried with Na2SO4, and concentrated. The residue was purified by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford 18 (1.04 g, 97%) as a colorless oil. [α]25D +16.00 (c 0.5 CHCl3). 1H NMR (499 MHz, CDCl3) δ 7.74 (s, 1H), 5.36 (d, J = 5.6 Hz, 1H), 4.23 (s, 1H), 3.68 (s, 3H), 2.61 – 2.35 (m, 1H), 2.10 (d, J = 17.0 Hz, 1H), 2.03 (s, 3H), 1.80 (m, 1H), 0.80 (d, J = 4.6 Hz, 6H), 0.75 (s, 9H), −0.04 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 176.4, 170.3, 169.3, 78.9, 75.5, 73.0, 52.4, 41.2, 29.9, 25.5, 20.9, 19.7, 18.6, 17.7, −5.0, −5.3. HRMS (ESI): calcd for [(C18H33NO6Si) + H]+ 388.2155; found 388.2162.
(2R,3S)-1-tert-butyl 2-methyl 2-((S)-1-acetoxy-2-methylpropyl)-3-(tert-butyldimethylsilyloxy)-5-oxopyrrolidine-1,2-dicarboxylate (19)
To a solution of 18 (900 mg, 2.32 mmol) in THF (20 mL) was added Boc2O (760 mg, 3.48 mmol) at room temperature. Then Et3N (470 mg, 647 µL, 4.64 mmol) was added dropwise followed by DMAP (57 mg, 0.46 mmol). The reaction was then allowed to reflux overnight under N2. The solvent was evaporated, and saturated NH4Cl solution (50 mL) was added. The mixture was extracted with EtOAc (3 × 200 mL). The combined organic phase was washed with brine (30 mL), dried with Na2SO4, and concentrated. The residue was purified by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford 19 (950 mg, 83%) as a colorless oil. [α]25D −41.82 (c 0.6 CHCl3). 1H NMR (499 MHz, CDCl3) δ 5.85 (d, J = 3.6 Hz, 1H), 4.72 (dd, J = 8.3, 6.2 Hz, 1H), 3.60 (s, 3H), 2.83 (dd, J = 18.1, 8.6 Hz, 1H), 2.56 (dd, J = 18.1, 5.9 Hz, 1H), 2.05 (s, 3H), 1.71 (m, 1H), 1.41 (s, 9H), 0.86 (d, J = 6.8 Hz, 6H), 0.77 (s, 9H), 0.04 (s, 3H), −0.00 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 172.1, 169.8, 168.8, 148.4, 74.9, 74.2, 65.3, 52.1, 41.3, 29.2, 27.7, 25.4, 21.6, 21.2, 17.6, 17.3, −4.2, −5.3. HRMS (ESI): calcd for [(C23H41NO8Si) + Na]+ 510.2499; found 510.2502.
(2R,3S)-1-tert-butyl 2-methyl 2-((S)-1-acetoxy-2-methylpropyl)-3-hydroxy-5-oxopyrrolidine-1,2-dicarboxylate (20)
To a solution of TBAF (400 mg, 453 µL, 1.54 mmol) in THF (5 mL) was added AcOH (92 mg, 87 µL, 1.54 mmol) at room temperature. Then a solution of 19 (500 mg, 1.03 mmol) in THF (5 mL) was added dropwise to the reaction. The resulting reaction mixture was stirred overnight. The solution was added to saturated NH4Cl solution (50 mL) and extracted with EtOAc (3 × 100 mL). The combined organic phase was washed with brine (30 mL), dried with Na2SO4, and concentrated. The residue was purified by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford 20 (295 mg, 77%) as a colorless oil. [α]25D +31.19 (c 1.0 CHCl3).1H NMR (500 MHz, CDCl3) δ 5.91 (d, J = 3.6 Hz, 1H), 4.89 (td, J = 8.8, 3.7 Hz, 1H), 3.75 (s, 3H), 2.90 (dd, J = 17.9, 9.0 Hz, 1H), 2.76 (dd, J = 17.9, 8.7 Hz, 1H), 2.22 (t, J = 8.2 Hz, 1H), 2.16 (s, 3H), 1.84 (m, 1H), 1.50 (s, 9H), 0.97 (d, J = 6.9 Hz, 3H), 0.89 (d, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 172.1, 169.7, 169.7, 148.6, 84.8, 75.4, 74.2, 65.5, 52.6, 38.4, 29.2, 27.8, 21.5, 21.1, 17.3. HRMS (ESI): calcd for [(C17H27NO8) + Na]+ 396.1634; found 396.1638.
(2R,3S,4R)-methyl 2-((S)-1-acetoxy-2-methylpropyl)-3-hydroxy-4-methyl-5-oxopyrrolidine-2-carboxylate (21)
To a solution of diisopropylamine (52 mg, 72 µL, 0.52 mmol) in THF (5 mL) was added butyllithium in hexane (200 µL of 2.5 M, 0.52 mmol) at 0 °C. After being stirred for 10 min the solution was cooled to −78 °C and stirred for 10 min. Iodomethane (487 mg, 213 µL, 3.43 mmol)) was added, followed by the dropwise addition of a solution of 20 (160 mg, 0.43 mmol) and HMPA (0.5 mL) in THF (3 mL). After being stirred at −78 °C for 1 h, additional HMPA (0.5 ml) was added. The reaction mixture was stirred at −78 °C for 3 h, and quenched with saturated NH4Cl (20 mL). After being poured into water (30 mL), the products were extracted with EtOAc (3 × 50 mL). The combined organic phase was washed with brine (30 mL), dried with Na2SO4, and concentrated. The residue was purified by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford 21 (100 mg, 60%) as a colorless oil. [α]25D −16.76 (c 0.5 CHCl3). 1H NMR (500 MHz, CDCl3) δ 5.89 (d, J = 3.8 Hz, 1H), 4.89 (dd, J = 9.8, 5.6 Hz, 1H), 3.73 (s, 3H), 2.91 (dq, J = 15.4, 7.7 Hz, 1H), 2.16 (s, 3H), 2.00 (d, J = 5.6 Hz, 1H), 1.92 – 1.73 (m, 1H), 1.51 (s, 9H), 1.32 (d, J = 7.7 Hz, 3H), 0.98 (d, J = 6.9 Hz, 3H), 0.86 (d, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 176.2, 170.3, 169.8, 149.0, 84.9, 75.4, 72.4, 67.1, 52.7, 40.9, 29.4, 28.0, 21.8, 21.3, 17.6, 10.7. HRMS (ESI): calcd for [(C18H29NO8) + Na]+ 410.1791; found 410.1789.
Omuralide (2)
Trifluoroacetic acid (5 mL) was added to a solution of 21 (50 mg, 0.13 mmol) in CH2Cl2 (5 mL) at 4 °C. The reaction mixture was stirred at room temperature for another 2 h and concentrated. The crude product was purified by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford 22 (30 mg, 81%). [α]25D −18.25 (c 0.4 CHCl3). 1H NMR (500 MHz, CDCl3) δ 8.34 (s, 1H), 5.53 (d, J = 6.3 Hz, 1H), 4.32 (d, J = 5.4 Hz, 1H), 3.88 (s, 3H), 2.88 – 2.53 (m, 1H), 2.12 (s, 3H), 1.91 (dt, J = 13.2, 6.6 Hz, 1H), 1.26 (s, 1H), 1.18 (d, J = 7.3 Hz, 3H), 0.92 (t, J = 7.2 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ180.8, 171.4, 170.5, 78.5, 76.0, 75.5, 53.5, 41.3, 30.5, 21.1, 19.6, 18.4, 8.2. HRMS (ESI): calcd for [(C13H21NO6) + Na]+ 310.1267; found 310.1274.
A solution of 0.5 M aqueous NaOH (2 mL of 0.5 M, 1.0 mmol) was cooled to 4 °C and 22 (30 mg) was added, which was was stirred at 4 °C for 64 h. 1 N HCl was added dropwise to the reaction mixture until pH = 1. The resulting solution was extracted with EtOAc (3×50 mL), and the combined organic phase was dried over Na2SO4. The solid was filtered and the filtrate was concentrated in vacuo to provide 23 as a white solid. Compound 23 was suspended in CH2Cl2 at room temperature. Triethylamine (39 mg, 54 µL, 0.39 mmol) and bis(2-oxo-3-oxazolidinyl) phosphinic chloride (19 mg, 0.19 mmol) were added. After being stirred for 2 h, the reaction mixture was poured into water (50 mL). The resulting solution was extracted with EtOAc (3×50 mL), and the combined organic phase was dried over Na2SO4. The solid was filtered, and the filtrate was concentrated. The crude product was purified by flash column chromatography (hexanes/EtOAc 100/0 to 0/100) to afford a solid, which was recrystallized from EtOAc to give β-lactone omuralide 2 (17 mg). [α]23D −93.7° (c 0.25, CH3CN). 1H NMR (499 MHz, Pyridine) δ 10.47 (s, 1H), 7.88 (s, 1H), 5.70 (d, J = 6.1 Hz, 1H), 4.37 (s, 1H), 3.26 – 2.88 (m, 1H), 2.13 (ddd, J = 13.5, 10.5, 6.7 Hz, 1H), 1.48 (d, J = 7.4 Hz, 3H), 1.13 (d, J = 6.9 Hz, 3H), 1.02 (d, J = 6.7 Hz, 3H). 13C NMR (100 MHz, Pyridine) δ 177.8, 172.9, 81.0, 77.5, 71.0, 39.3, 30.3, 20.9, 16.9, 9.3. HRMS (ESI): calcd for [(C10H15NO4) + NH4]+ 231.1344; found 231.1347.
Lactacystin (1)
The conversion of 2 to 1 was conducted following the literature procedure.23 A suspension of 2 (3 mg, 0.014 mmol) in CH2Cl2 was treated with N-acetyl-L-cysteine (2.296 mg, 0.014 mmol) and Et3N (4.27 mg, 6 µL, 0.042 mmol) at 23 °C. The resulting reaction mixture was stirred for 4 h at room temperature and concentrated in vacuo. The solid residue was dissolved in dry pyridine (5 mL) and evaporated under reduced pressure. Regeneration of the free acid by azeotropic distillation with a mixture of THF-HOAc (v/v 5:1) was followed by trituration with a mixture of EtOAc-HOAc (v/v 20:1) to afford lactacystin (2.8 mg) as a colorless glass. 1H NMR (300 MHz, Pyr) δ 9.94 (s, 1H), 8.79 (d, J = 8.0 Hz, 1H), 5.41 (dd, J = 13.0, 6.5 Hz, 2H), 5.34 (d, J = 7.1 Hz, 1H), 4.59 (d, J = 7.0 Hz, 1H), 4.06 (dd, J = 13.5, 4.8 Hz, 1H), 3.85 (dd, J = 13.5, 6.7 Hz, 1H), 3.54 – 3.41 (m, 1H), 2.24 (dd, J = 13.5, 6.8 Hz, 1H), 2.02 (s, 3H), 1.56 (d, J = 7.6 Hz, 3H), 1.25 (d, J = 6.6 Hz, 3H), 1.17 (d, J = 6.8 Hz, 3H).
Supplementary Material
Acknowledgment
The authors are grateful to the National Institutes of Health (Grant R41 CA116971) for financial support of this research. We thank Dr. Charlotte Stern (Northwestern University) for help with the X-ray data.
Footnotes
Supporting Information
Copies of 1H and 13C NMR spectra of all new compounds and copies of 1H NMR spectra of all known compounds. Crystallographic data for 16. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Adams J. Proteasome Inhibitors in Cancer Therapy. Totowa, NJ: Humana Press; 2004. [Google Scholar]
- 2.(a) Orlowski RZ. Cell Death Differ. 1999;6:303. doi: 10.1038/sj.cdd.4400505. [DOI] [PubMed] [Google Scholar]; (b) Grimm LM, Osborne BA. Results Probl. Cell Differ. 1999;23:209. doi: 10.1007/978-3-540-69184-6_10. [DOI] [PubMed] [Google Scholar]; (c) Magnani M, Crinelli R, Bianchi M, Antonelli A. Curr. Drug Targets. 2000;1:387. doi: 10.2174/1389450003349056. [DOI] [PubMed] [Google Scholar]; (d) Hirsch T, Dallaporta B, Zamzami N, Susin SA, Ravagnan L, Marzo I, Brenner C, Kroemer G. J. Immunol. 1998;161:35. [PubMed] [Google Scholar]
- 3.(a) Goldberg AL, Akopian TN, Kisselev AF, Lee DH, Rohrwild M. Biol. Chem. 1997;378:131. [PubMed] [Google Scholar]; (b) DeMartino GN, Slaughter CA. J. Biol. Chem. 1999;274:22123. doi: 10.1074/jbc.274.32.22123. [DOI] [PubMed] [Google Scholar]; (c) Tanahashi N, Kawahara H, Murakami Y, Tanaka K. Mol. Biol. Rep. 1999;26:3. doi: 10.1023/a:1006909522731. [DOI] [PubMed] [Google Scholar]; (d) Zwickl P, Baumeister W, Steven A. Curr. Opin. Struct. Biol. 2000;10:242. doi: 10.1016/s0959-440x(00)00075-0. [DOI] [PubMed] [Google Scholar]; (e) Ciechanover A, Orian A, Schwartz AL. Bioessays. 2000;22:442. doi: 10.1002/(SICI)1521-1878(200005)22:5<442::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Science. 1995;268:726. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 5.Fenteany G, Schreiber SL. J. Biol. Chem. 1998;273:8545. doi: 10.1074/jbc.273.15.8545. [DOI] [PubMed] [Google Scholar]
- 6.Dick LR, Cruikshank AA, Grenier L, Melandri FD, Nunes SL, Stein RL. J. Biol. Chem. 1996;271:7273. doi: 10.1074/jbc.271.13.7273. [DOI] [PubMed] [Google Scholar]
- 7.(a) Õmura S, Fujimoto T, Otoguro K, Matsuzaki K, Moriguchi R, Tanaka H, Sasaki Y. J. Antibiot. 1991;44:113. doi: 10.7164/antibiotics.44.113. [DOI] [PubMed] [Google Scholar]; (b) Õmura S, Matsuzaki K, Fujimoto T, Kosuge K, Furuya T, Fujita S, Nakagawa A. J. Antibiot. 1991;44:117. doi: 10.7164/antibiotics.44.117. [DOI] [PubMed] [Google Scholar]
- 8.(a) Fenteany G, Schreiber SL. J. Biol. Chem. 1998;273:8545. doi: 10.1074/jbc.273.15.8545. [DOI] [PubMed] [Google Scholar]; (b) Tomoda H, Õmura S. Yakugaku Zasshi. 2000;120:935. doi: 10.1248/yakushi1947.120.10_935. [DOI] [PubMed] [Google Scholar]
- 9.Corey EJ, Reichard GA. J. Am. Chem. Soc. 1992;114:10677. [Google Scholar]
- 10.Sunazuka T, Nagamitsu T, Matsuzaki K, Tanaka H, Ōmura S, Smith AB., III J. Am. Chem. Soc. 1993;115:5302. [Google Scholar]
- 11.Donohoe TJ, Sintim HO, Sisangia L, Harling JD. Angew. Chem., Int. Ed. 2004;43:2293. doi: 10.1002/anie.200453843. [DOI] [PubMed] [Google Scholar]
- 12.Hayes CJ, Sherlock AE, Green MP, Wilson C, Blake AJ, Selby MD, Prodger JC. J. Org. Chem. 2008;73:2041. doi: 10.1021/jo7027695. [DOI] [PubMed] [Google Scholar]
- 13.Shibasaki M, Kanai M, Fukuda N. Chemistry--An Asian Journal. 2007;2:20. doi: 10.1002/asia.200600310. [DOI] [PubMed] [Google Scholar]
- 14.Balskus EP, Jacobsen EN. J. Am. Chem. Soc. 2006;128:6810. doi: 10.1021/ja061970a. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda N, Sasaki K, Sastry TVRS, Kanai M, Shibasaki M. J. Org. Chem. 2006;71:1220. doi: 10.1021/jo0524223. [DOI] [PubMed] [Google Scholar]
- 16.Hayes CJ, Sherlock AE, Selby MD. Org. Biol. Chem. 2006;4:193. doi: 10.1039/b516311k. [DOI] [PubMed] [Google Scholar]
- 17. Fenteany G, Standaert RF, Reichard GA, Corey EJ, Schreiber SL. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3358. doi: 10.1073/pnas.91.8.3358. See also: Dick LR, Cruikshank AA, Grenier L, Melandri FD, Nunes SL, Stein RL. J. Biol. Chem. 1996;271:7273. doi: 10.1074/jbc.271.13.7273.
- 18.Groll M, Ditzel L, Löwe J, Stock D, Bochtler M, Bartunik HD, Huber R. Nature. 1997;386:463. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 19.Fenteany G, Standaert RF, Reichard GA, Corey EJ, Schreiber SL. Proc. Natl. Acad. Sci., USA. 1994;91:3358. doi: 10.1073/pnas.91.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Corey EJ, Li W, Nagamitsu T. Angew. Chem. Int. Ed. 1998;37:1676. doi: 10.1002/(SICI)1521-3773(19980703)37:12<1676::AID-ANIE1676>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]; (b) Corey EJ, Li W-DZ. Tetrahedron. 1999;55:3305. [Google Scholar]; (c) Corey EJ, Li W-D. Tetrahedron Lett. 1998;39:7475. [Google Scholar]; (d) Corey EJ, Reichard GA. Tetrahedron Lett. 1993;34:6973. [Google Scholar]; (e) Corey EJ, Li W-DZ. Tetrahedron Lett. 1998;39:8043. [Google Scholar]; (f) Corey EJ, Choi S. Tetrahedron Lett. 1993;34:6969. [Google Scholar]; (g) Corey EJ, Lee D-H, Choi S. Tetrahedron Lett. 1992;33:6735. [Google Scholar]
- 21.Soucy F, Grenier L, Behnke ML, Destree AT, McCormack TA, Adams J, Plamondon L. J. Am. Chem. Soc. 1999;121:9967. [Google Scholar]
- 22.Andrews MD, Brewster AG, Moloney MG. J. Chem. Soc., Perkin Trans. 1. 2002:80. [Google Scholar]
- 23.Corey EJ, Li W. J. Am. Chem. Soc. 1998;120:2330. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.