Abstract
While the majority of fractures heal normally, it is estimated that ~10% of fractures exhibit some level of delayed or impaired healing. Although radiography is the primary diagnostic tool to assess the progression of fracture healing, radiographic features only qualitatively correlate with tissue level increases in mineral content and do not quantitatively measure underlying biological processes that are associated with the progression of healing. Specific metaloproteinases have been shown to be essential to processes of both angiogenesis and mineralized cartilage resorption and bone remodeling at different phases of fracture healing. The aim of this study was to determine the potential of using a simple urine based assay of the activity of two MMPs as a means of assessing the biological progression of fracture healing through the endochondral phase of healing. Using a standard mid-diaphyseal murine model of femoral fracture, MMP9 and MMP13 proteins and enzymatic activity levels were quantified in the urine of mice across the time-course of fracture healing and compared to the mRNA and protein expression profiles in the calluses. Both urinary MMP9 and MMP13 protein and enzymatic activity levels, assessed by Western blot, zymogram and specific MMP fluorometric substrate assays, corresponded to mRNA expression and immunohistologic assays of the proteins within callus tissues. These studies suggest that urinary levels of MMP9 and MMP13 may have potential as metabolic markers to monitor the progression of fracture healing.
Keywords: Urine Assay, MMPs, Fracture Healing, Diagnostics
INTRODUCTION
During fracture healing, a repair process is initiated that entails complex interactions among multiple cell types and a recapitulation of many of the developmental processes seen during embryonic endochondral bone formation1–3. Two biological processes of fracture healing that appear to be critical to its progression are vascularization and the resorption of calcified cartilage4–6. Matrix metalloproteinases (MMPs) are one of the major classes of proteolytic enzymes that participate in both the vascular invasion and the normal connective remodeling processes that takes place during calcified cartilage resorption. Previous studies have shown that three specific MMP 9, 13, and 14 contribute to both of these processes, carry out essential roles during both normal skeletal development and during adult fracture repair, and are uniquely activated during endochondral development7–13. A number of other studies have also shown that many of the MMPs are activated during the pathological turnover of cartilage in both rheumatoid and osteoarthritic processes14,15.
Previous transcriptional profiling studies across fracture healing showed that there was an orderly temporal expression of ADAMTS (a disintegrin and metalloproteinase domain with thrombospondin modules) that degrade proteoglycans followed by the specific ordered expression of MMPs that degrade collagen. This suggests that the proteoglycan matrix of cartilage had to be first degraded before the proteolysis of the underlying collagen matrix could proceed. It is interesting to note, in the context of the MMPs, that the expression of TIMP (tissue inhibitor of metalloproteinase) 2/MMP2/ MMP14 and MMP9/MMP13 were temporally expressed as two separate groups in which the former group of genes was expressed earlier than the later16. This has functional relevance since several recent studies have shown that MMP14/MT-1, MMP2 and TIMP2 are associated together in a protein complex in which pro MMP2 is activated in conjunction with MMP14 and TIMP217–19. In regard to the timing of these genes’ expression, it is also consistent with the observation that MMP14 is necessary for post natal growth plate cartilage resorption. Finally, studies of both pathological turnover of articular cartilage, as well as normal developmental remodeling of growth cartilage have shown that MMP9 and MMP13 are also concurrently expressed4,20,21.
The unique temporal expression of the various MMPs and their restricted expression to various tissue types during bone repair suggest that the MMPs may have potential use as markers for assessing the biological progression of endochondral bone formation seen during fracture healing. In one previous study, MMPs were examined in serum samples collected prospectively from patients who had under gone operative treatment for limb fractures. Using MMP-specific ELISA assays these investigators showed observable levels of MMPs 1, 2, 3, 8 and 9 in the serum of these patients, although many of these MMPs did not show significant differences at various times after fracture. However, this study did show that proMMP-1 remained at statistically elevated levels for up to 24 weeks post operation in the group of patients that developed nonunions22. Numerous recent studies have shown that specific MMPs are present in the urine of patients who have a number of different kinds of malignancies23–26. These studies have further shown that MMPs are very sensitive assays for detecting the progression or recurrence of various malignancies after surgical resection and the progression of angiogenesis in the tumors26,27.
In the present study, MMP9 and MMP13 were assessed because of their extensively documented functional roles and temporal expression during endochondral bone formation in both development and in fracture healing4,7,28. Since urine assays are much easier to administer and less costly than serum assays, we assessed if MMPs would be present in urine and could be used to follow the progression of fracture healing in a well developed experimental animal model of fracture healing. In this context, we first assayed the expression of the enzyme at mRNA and protein levels in the calluses tissues. We then assayed the urine to see if urine levels are different and timing between tissue level expression and systemic clearance of any enzyme that spills from the callus tissues. Several different modalities of assay based on either selective detection of specific enzyme activity or immunological based assay were examined to test for sensitivity and specificity of specific types of assays.
MATERIALS AND METHODS
Fracture Model
Research was conducted in conformity with all Federal and USDA guidelines, under an IACUC approved protocol. All studies were performed on male 8–10 weeks old C57 BL/6J (B6) mice. Simple transverse closed unilateral fractures in the left femur of all mice were made using a modification of the method of Bonnarens and Einhorn, 198429 developed for rat femur, as adapted for the femur of mice30. Age, sex, and genetic strain of mice were based on previous data, in which the time course of fracture healing had been established by both histological and selected candidate gene assessment30,31. Fracture callus tissue samples and urine samples were analyzed on the days post fracture as indicated in each figure.
RNA Analysis
After euthanasia, fracture sites were circumscribed by 5 mm on either side of the break and an identical region of tissue was excised from the mid half of the unfractured contralateral femora. All bone tissues were collected into liquid nitrogen and stored at −80°C until used for RNA extraction. RNA samples were collected from calluses at days 5, 7, 10, 14 and 21. RNAs were prepared by powdering a pool of 3 calluses per time point under liquid nitrogen using a mortar and pestle, followed by extraction of the powder using Trizol ® reagent (Invitrogen, Inc). Total RNA prepared from the Trizol® extractions were further purified by RNaeasy ® (Qiagen, Inc) column absorption. RT-PCR: All reagents for the qRT-PCR analysis were from Applied Biosystems, Inc, and plate assays were read in an ABI 7700 Sequence Detector (Applied Biosystems, Foster City, CA). The methods of DNA amplification and qRTPCR analysis were as previously described16. All reagents for the qRT-PCR analysis were from Applied Biosystems, Inc, and plate assays were read on an ABI 7700 Sequence Detector (Applied Biosystems, Foster City, CA). All mRNA levels were normalized to βactin and each analysis was run in triplicate. The fractional cycle number at which the fluorescence passes the fixed threshold (CT values) was used for quantification by using a comparative CT method as previously described by Wang et al., 200616. The fold change in mRNA expression for each time point was plotted in a graph using day 0 as a reference: 2−ΔΔCT (day 0)=1.
Urine Collection and Volume Normalization
Urine was collected at days 3, 5, 7, 14, and 21 after fracture. Mice were individually placed in metabolic cages 24 hours before the time point of assay and urine was collected over a 24 hours period into tubes embedded in pellets of dry ice. For each time point, n=12 mice were used. All urine was stored at −80°C until it was thawed and used for assay. Urinary creatinine levels were used as a means of normalization for the rate of kidney filtration. After collection urine creatinine activity was assessed using a colormetric assay kit from Fisher Scientific (Pittsburg, PA). Urine volumes were then adjusted such that each urine volume had equivalent creatinine activities of 1 mg/dL.
Zymography
Standardized urine samples were mixed with SDS-tris-glycine loading buffer and were subjected to electrophoresis without heat denaturation or thiol-reduction in 10% gelatin pre-cast Ready Gel Zymogram Gels (Bio-Rad Laboratories Inc Hercules, CA 94547). Gels subsequently were soaked in 2.5% Triton X-100 renaturation buffer, and incubated in Novex Zymogram Developing Buffer from Invitrogen (Life Technologies, Carlsbad, CA, 92008, USA) overnight at 37°C. Gels were stained with 0.1% Comassie Blue overnight and then destained in 5% acetic acid, 10% methanol.
Histological Analysis
Fracture calluses were harvested on days 7, 14 and 21. The histological fixation, sectioning, and staining procedures that we had previously established for rodent histological fracture callus assessments were used for these studies31. For this study transverse sections were obtained. Commercially available antibodies were used for immunohistochemical localization of MMP9 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, 95060, USA) and MMP13 (Thermo Scientific Inc. Waltham, MA, 02454, USA). Staining was visualized using the Vectastain ABC kit per the manufacturer’s suggested protocol (Vector Laboratories, Burlingame, CA, 94010, USA).
Western Blot Analysis
Standardized urine samples were resolved on 8% SDS-PAGE gel, then transferred to nitrocellulose paper. Blots were incubated overnight at 4 degrees Celsius with a primary antibody for MMP9 (Santa Cruz Biotech) or MMP13 (Thermo Scientific Inc. Waltham, MA, 02454, USA). After secondary antibody incubation, bands were visualized using the Amersham ECL Western Blotting Detection kit (GE Healthcare Life Sciences Products).
MMP Fluorometric Assays
MMPs 9 and 13 were selectively assayed using enzyme specific fluorescence substrate kits SensoLyte 520 MMP9 and MMP13 Assay Kit (AnaSpec Fremont, CA, 94555). Urine samples were collected as described above and assay volumes were normalized to creatinine output. Enzyme activity was determined using a fluorescently labeled peptide (FRET peptide) as substrate according to the manufacturer’s instructions. Briefly, 50 µl MMP9 or MMP13 substrate solution (100 µM) was mixed with 50 µl or normalized sample in a black 96 well microtiter plate. The change in fluorescence (expressed as relative fluorescence units) was measured at Ex/Em = 490 nm/520 nm every 20 min for up to 1 hr using a luminescence spectrometer (PerkinElmer LS55). Values are expressed as a relative to the levels seen in urine samples that were collected from control mice that did not have fractures. All values were then assessed as a ratio to the control values which are set as 1.
RESULTS
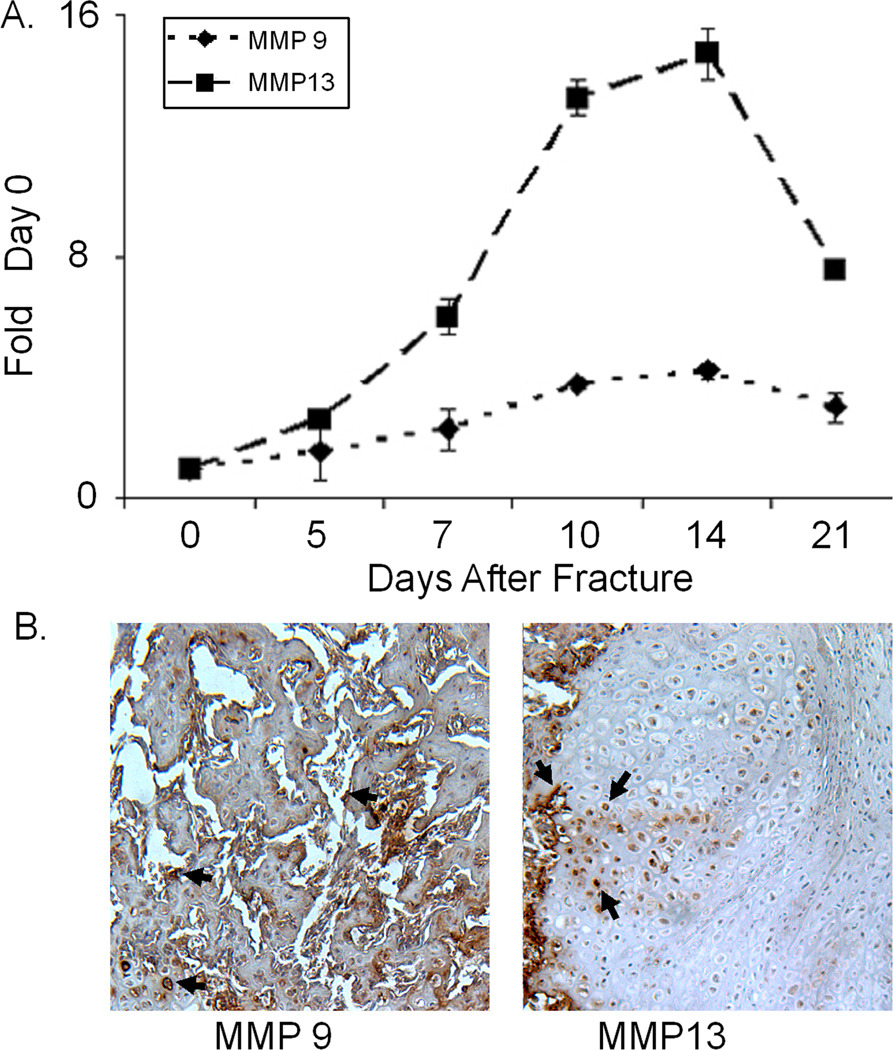
The first set of data that is presented shows the tissue levels of expression of the mRNAs for MMP9 and MPP13 (Figure 1A). Because of its highly restricted expression during chondrocyte hypertrophy, MMP13 showed a much greater fold induction from its baseline expression levels than MMP9 and had a very sharp peak of expression which rapidly declined as the cartilage was resorbed. In contrast, MMP9 is associated both with vascular formation and stromal tissue remodeling showed a much smaller fold of induction and its expression persisted as the callus tissue underwent coupled remodeling. Immunohistological assessment depicted in Figure 1B confirmed the specificity of expression of the two proteases in the callus tissues and showed that MMP13 expression was primarily associated with hypertrophic chondrocytes while MMP9 was associated with small vessels and stromal cells.
Figure 1. Analysis of MMP9 and 13, mRNA, and Protein Expression during Fracture Healing.
A) Messenger RNA expression of MMP9 and MMP13 during endochondral bone formation after fracture. Quantitative RT-PCR analysis of mRNA expression across the time course of fracture healing was carried out on mRNA samples that had been pooled from 3 mice from each time point. All mRNAs were assayed three times and each data point is the mean of the three replicates ±S.D. All data are normalized to β-actin (Ct value / Ct value for β-actin) and presented as the fold change in expression.
B) Immunohistochemical analysis of MMP9 and MMP13 expression in fourteen day post fracture callus tissues. Left side depicts post fracture callus tissue in area of new woven bone. and right hand panel depicts area of callus containing hypertrophic cartilage cells next to the new an area of new bone formation.
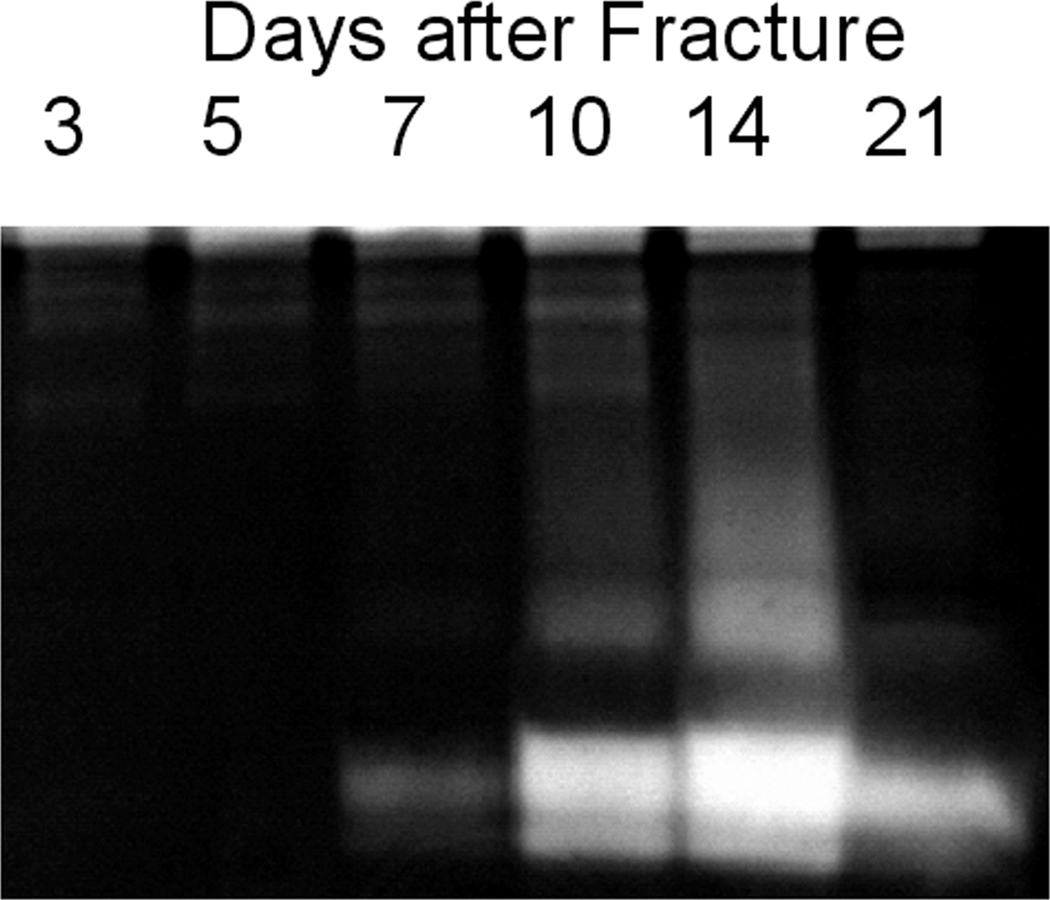
In order to determine if the distinct changes in callus MMP expression might be reflected systemically, we next examined serum and urine samples for non-specific protease enzymatic activity via zymography. The results in Figure 2 showed that gelatinase activity was easily detectable in small aliquots of the urine, while no protease activity was detected in serum following fracture (data not shown). More specifically, urine zymography showed a clear temporal increase in the number and intensity of clearing zones beginning at day 7 and peaking at day 14 during the period when chondrocyte maturation was taking place during endochondral bone formation. The most prominent clearing zones on the gel localized to around ~60 kDa.
Figure 2.
Zymogram of MMP Activity Found in Urine across the Time Course of Fracture Healing. Gels were loaded with urine volumes normalized to 1 micrograms of creatinine. Clearing zones within the gels are indicative of gelatinase activity.
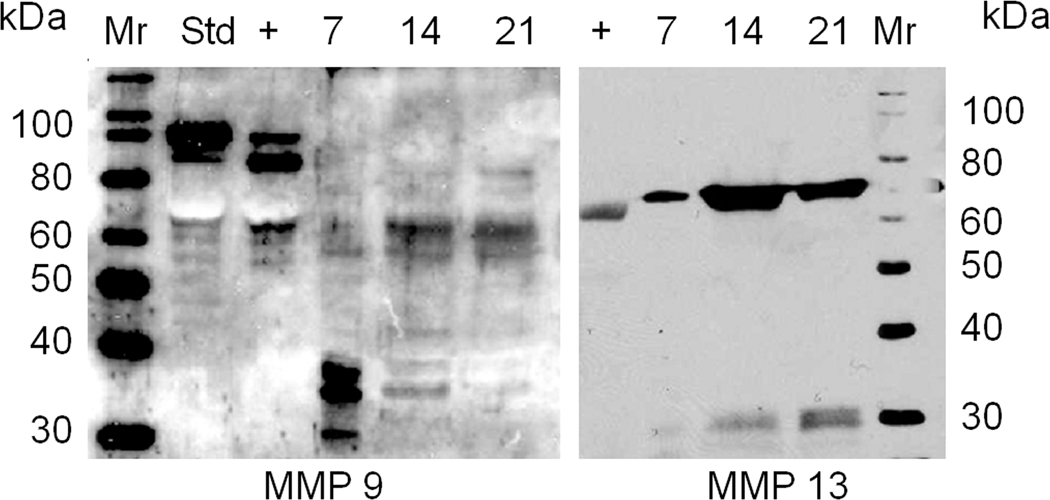
Western blot analysis was next carried out to examine if a portion of the activity that was observed in the zymogram could be attributed to MMP9 and MMP13. For this analysis we assayed the three time points that showed the highest levels of activity in the zymogram and the results of this analysis are shown in Figure 3. As can be seen both enzymes were present in the urine. Both enzymes showed protein levels in the urine that paralleled that of their mRNAs, with MMP13 showing peak protein levels at 14 days post fracture while MMP9 showed levels that appeared to be expressed through 21 days post fracture. It is interesting to note that much of the activity of MMP9 was associated with smaller molecular weight fragments that correspond to fragments seen after the enzyme was allowed to autoactivate and are presumed to be proteolytic fragments of the enzyme. In contrast, MMP13 appeared to be largely in its intact from in the urine.
Figure 3.
Western Blot of Analysis MMP9 and MMP13 Proteins in Urine across the Time Course of Fracture Healing. Urine volumes were standardized to 3 micrograms of creatinine. + indicates recombinant MMP pro-enzyme form that has been incubated in reaction buffer for 60 minutes to self activate the enzyme. Std indicates the enzyme directly added to the gel without self activation.
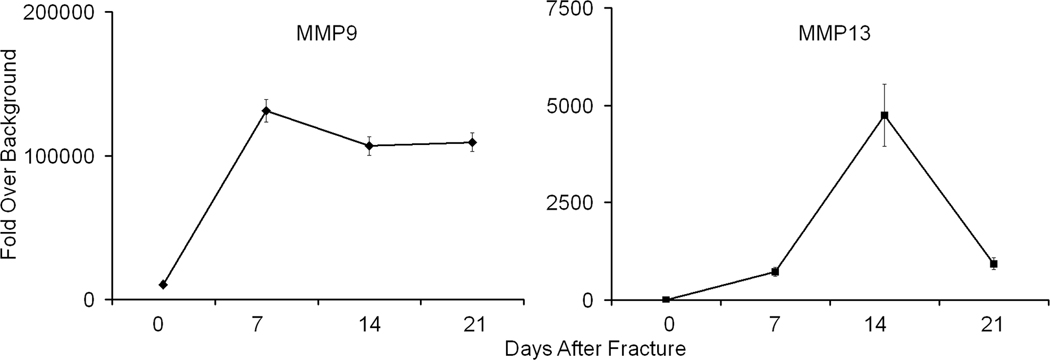
In order to obtain a more quantitative assessment of the amounts of MMP9 and MMP13 that were found in the urine their activity was assayed using specific synthetic substrates for MMP9 and MMP13 that upon enzymatic conversion would show fluorescence. The results from this assay are seen in Figure 4. In this study we compared the three previous times points that showed maximal expression of MMP9 and 13. A comparison of separate assays for MMP9 and MMP13 showed that both enzymes could be easily detected in the urine and once again MMP13 showed very distinct peak of activity at day 14 while MMP9 showed relatively steady level of activity once it had been induced after fracture. Interestingly, this comparison showed that the base line level of MMP9 activity was ~5000 folds that of MMP13 consistent with the broad levels of tissue expression for this protease.
Figure 4.
MMP9 and MMP13 Activities as Determined by MMP Fluorometric Assays of Enzyme Found with Urine Across the Time Course of Fracture Healing. All values are scaled as fold over background which is normalized to 1 which was set to the lowest levels seen for MMP13 in unfractured animals. Note that MMP9 has an inherent level of activity ~ 5,000 fold higher than MMP13 in urine independent of presence of fracture.
DISCUSSION
An ideal biomarker to follow the progression of fracture healing should show a correlation between tissue levels of some type of biological activity within the cells in the callus that can be related to the progression of the regain of biomechanical function. A key biological process that is needed for the regain of mechanical competency is the removal of the calcified cartilage and its replacement and with bony callus that accompanies bridging of the fracture gap. This process is, in part, mediated through the activities of MMP9 and 13, which have been shown to substantially that contribute to the resorption and remodeling of calcified cartilage7,10. The activity of these enzymes would also accompany the revascularization of the bone as cartilage is replaced5,6. In this context, the ability to show a correlation between the levels of MMPs that are seen in the urine and those that are expressed in the callus would suggest that these molecules are reasonable surrogates to aid in the assessment of the progression of fracture healing. The use of MMPs as prognostic tools to assess fracture healing or skeletal repair/fusion in patients is supported by two studies that assessed serum levels of MMPs after osteotomy22,32. In both these studies elevated serum levels of MMP1 and TIMP1 were seen during the repair period for up to ~24 weeks, one in the setting of distraction osteogenesis and the other after rigid internal fixation. In neither of these studies was MMP13 observable by ELISA assay of the serum.
In comparing our findings to those of the above noted clinical studies, the appearance of MMP9 in the urine in our study would be consistent with the findings of elevated levels of this protein in the serum of patients in the study by Weiss et al., 200532. However, we also could detect MMP13 in the urine after fracture. This basic difference among human versus the animal data may be associated with a species difference or could be due to the differences in assaying serum versus urine. It is of interest to note that serum assays contain a much higher background inference than urine assays because of the very high levels of endogenous blood proteins or as noted by the authors of the human studies, due to higher levels of proteolytic breakdown of the MMPs as they undergo auto catalysis in serum than in urine.
A limitation of the present study is that a broad panel of MMPs was not examined nor was the assay of MMPs extended for long periods after fracture but mainly assessed the temporal transit through the endochondral window of bone formation. In this regard, future studies under differing fracture repair conditions and in various models of fracture healing will be quite informative to determine if this assay is useful to identify either atrophic or hypertrophic forms of nonunion. Studies in humans using urine samples will also need to be carried out to determine whether urine based assays will be feasible. Relative to the development of urine based assays, the comparison of the different assay formats suggests that use of specific fluorescent substrates for the various MMPs or the application of an ELISA based assay will provide the greatest sensitivity and specificity. The use of ELISA based assays of human serums are currently in human trials for assessing the progression or presence of various malignancies and may be adaptable for use in assessing the progression of fracture and post orthopedic surgical healing.
CONCLUSION
Using a well defined murine model of closed fracture we show that the appearance of MMPs 9 and 13 in the urine provided a sensitive and quantitative surrogate marker that followed the progression of the expression of the activity of these enzymes in the callus tissues. The assay of the spilled enzyme in the urine assesses in real time the progression of endochondral bone formation and progression of healing of fractures.
ACKNOWLEDGEMENTS
Supported with grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (PO1AR049920) TAE.
Institutional support was provided by the Department of Orthopaedic Surgery Boston University School of Medicine and by Boston University School of Medicine. The authors wish to thank Marcia Moses for her initial discussions concerning the development of our studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87:57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 2.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 3.Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, Tabin CJ. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev. 1998;71:65–76. doi: 10.1016/s0925-4773(97)00203-7. [DOI] [PubMed] [Google Scholar]
- 4.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. [Google Scholar]
- 6.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 7.Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development. 2003;130:4123–4133. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmbeck K, Bianco P, Chrysovergis K, Yamada S, Birkedal-Hansen H. MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth. J. Cell. Biol. 2003;163:661–671. doi: 10.1083/jcb.200307061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega N, Behonick D, Stickens D, Werb Z. How proteases regulate bone morphogenesis. Ann NY Acad Sci. 2003;995:109–116. doi: 10.1111/j.1749-6632.2003.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang K, Vishwanath P, Eichler GS, Al-Sebaei MO, Edgar CM, Einhorn TA, Smith TF, Gerstenfeld LC. Analysis of fracture healing by large-scale transcriptional profile identified temporal relationships between metalloproteinase and ADAMTS mRNA expression. Matrix Biol. 2006;25(5):271–281. doi: 10.1016/j.matbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Behonick DJ, Xing Z, Lieu S, Buckley JM, Lotz JC, Marcucio RS, Werb Z, Miclau T, Colnot C. Role of matrix metalloproteinase 13 in both endochondral and intramembranous ossification during skeletal regeneration. PLoS One. 2007;2(11):e1150. doi: 10.1371/journal.pone.0001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosaki N, Takaishi H, Kamekura S, Kimura T, Okada Y, Minqi L, Amizuka N, Chung UI, Nakamura K, Kawaguchi H, Toyama Y, D'Armiento J. Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice. Biochem Biophys Res Commun. 2007;354(4):846–851. doi: 10.1016/j.bbrc.2006.12.234. [DOI] [PubMed] [Google Scholar]
- 14.Cho TJ, Lehmann W, Edgar C, Sadeghi C, Hou A, Einhorn TA, Gerstenfeld LC. Tumor necrosis factor alpha activation of the apoptotic cascade in murine articular chondrocytes is associated with the induction of metalloproteinases and specific pro-resorptive factors. Arthritis Rheum. 2003;48:2845–2854. doi: 10.1002/art.11390. [DOI] [PubMed] [Google Scholar]
- 15.Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 2004;50:2547–2558. doi: 10.1002/art.20558. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez MJ, Balbin M, Alvarez J, Komori T, Bianco P, Holmbeck K, et al. A regulatory cascade involving retinoic acid, Cbfa1, and matrix metalloproteinases is coupled to the development of a process of perichondrial invasion and osteogenic differentiation during bone formation. J Cell Biol. 2001;155:1333–1344. doi: 10.1083/jcb.200106147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apte SS, Fukai N, Beier DR, Olsen BR. The matrix metalloproteinase-14 (MMP-14) gene is structurally distinct from other MMP genes and is co-expressed with the TIMP-2 gene during mouse embryogenesis. J. Biol. Chem. 1997;272:25511–25517. doi: 10.1074/jbc.272.41.25511. [DOI] [PubMed] [Google Scholar]
- 18.Jo Y, Yeon J, Kim HJ, Lee ST. Analysis of tissue inhibitor of metalloproteinases-2 effect on pro-matrix metalloproteinase-2 activation by membrane-type 1 matrix metalloproteinase using baculovirus/insect-cell expression system. J Biochem. 2000;345:511–519. [PMC free article] [PubMed] [Google Scholar]
- 19.Karagiannis ED, Popel AS. A Theoretical Model of Type I Collagen Proteolysis by Matrix Metalloproteinase (MMP) 2 and Membrane Type 1 MMP in the Presence of Tissue Inhibitor of Metalloproteinase 2. J Biol Chem. 2004;279:39105–39114. doi: 10.1074/jbc.M403627200. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann W, Edgar CM, Wang K, Cho TJ, Barnes GL, Kakar S, et al. Tumor necrosis factor alpha (TNF-alpha) coordinately regulates the expression of specific matrix metalloproteinases (MMPS) and angiogenic factors during fracture healing. Bone. 2005;36:300–310. doi: 10.1016/j.bone.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Kevorkian L, Young DA, Darrah C, Donell ST, Shepstone L, Porter S, et al. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50:131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- 22.Henle P, Zimmermann G, Weiss S. Matrix metalloproteinases and failed fracture healing. Bone. 2005;37:791–798. doi: 10.1016/j.bone.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Monier F, Surla A, Guillot M, Morel F. Gelatinase isoforms in urine from bladder cancer patients. Clin Chim Acta. 2000;299(1–2):11–23. doi: 10.1016/s0009-8981(00)00271-0. [DOI] [PubMed] [Google Scholar]
- 24.Moses MA, Wiederschain D, Loughlin KR, Zurakowski D, Lamb CC, Freeman MR. Increased incidence of matrix metalloproteinases in urine of cancer patients. Cancer Res. 1998;58(7):1395–1399. [PubMed] [Google Scholar]
- 25.Roy R, Louis G, Loughlin KR, Wiederschain D, Kilroy SM, Lamb CC, et al. Tumor-specific urinary matrix metalloproteinase fingerprinting: identification of high molecular weight urinary matrix metalloproteinase species. Clin Cancer Res. 2008;14(20):6610–6617. doi: 10.1158/1078-0432.CCR-08-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27(31):5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan LW, Moses MA, Goley E, Sproull M, Muanza T, Coleman CN, et al. Urinary VEGF and MMP levels as predictive markers of 1-year progression-free survival in cancer patients treated with radiation therapy: a longitudinal study of protein kinetics throughout tumor progression and therapy. J Clin Oncol. 2004;22(3):499–506. doi: 10.1200/JCO.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Yamagiwa H, Tokunaga K, Hayami T, Hatano H, Uchida M, Endo N, Takahashi HE. Expression of metalloproteinase-13 (Collagenase-3) is induced during fracture healing in mice. Bone. 1999;25(2):197–203. doi: 10.1016/s8756-3282(99)00157-x. [DOI] [PubMed] [Google Scholar]
- 29.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- 30.Jepsen KJ, Price C, Silkman LJ, Nicholls FH, Nasser P, Hu B, et al. Genetic variation in the patterns of skeletal progenitor cell differentiation and progression during endochondral bone formation affects the rate of fracture healing. J Bone Miner Res. 2008;23(8):1204–1216. doi: 10.1359/JBMR.080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerstenfeld LC, Alkhiary YM, Krall EA, Nicholls FH, Stapleton SN, Fitch JL, et al. Three-dimensional reconstruction of fracture callus morphogenesis. J Histochem Cytochem. 2006;54:1215–1228. doi: 10.1369/jhc.6A6959.2006. [DOI] [PubMed] [Google Scholar]
- 32.Weiss S, Zimmermann G, Baumgart R, Kasten P, Bidlingmaier M, Henle P. Systemic regulation of angiogenesis and matrix degradation in bone regeneration-distraction osteogenesis compared to rigid fracture healing. Bone. 2005;37(6):781–790. doi: 10.1016/j.bone.2005.06.014. [DOI] [PubMed] [Google Scholar]