Abstract
HIV-1 release efficiency is directed by late (L) domain motifs in the viral structural precursor polyprotein, Gag, which serve as links to the ESCRT (endocytic sorting complex required for transport) machinery. Linkage is normally through binding of Tsg101, an ESCRT-1 component, to the P7TAP motif in the p6 region of Gag. In its absence, budding is directed by binding of Alix, an ESCRT adaptor protein, to the LY36PXnL motif in Gag. We recently showed that budding requires activation of the inositol 1,4,5-triphosphate receptor (IP3R), a protein that “gates” Ca2+ release from intracellular stores, triggers Ca2+ cell influx and thereby functions as a major regulator of Ca2+ signaling. In the present study, we determined whether the L domain links Gag to Ca2+ signaling machinery. Depletion of IP3R and inactivation of phospholipase C (PLC) inhibited budding whether or not Tsg101 was bound to Gag. PLC hydrolysis of PI(4,5)P2 generates IP3, the ligand that activates IP3R. However, with Tsg101 bound, Gag release was independent of Gq-mediated activation of PLC and budding was readily enhanced by pharmacological stimulation of PLC. Moreover, IP3R was redistributed to the cell periphery and cytosolic Ca2+ was elevated, events indicative of induction of Ca2+ signaling. The results suggest that L domain function, ESCRT machinery, and Ca2+signaling are linked events in Gag release.
Keywords: Human Immunodeficiency Virus type 1 (HIV-1); Tsg101; inositol 1,4,5-triphosphate receptor (IP3R); store operated Ca 2+ entry (SOCE); phosphatidylinositol-(4,5)-bisphosphate (PI(4,5)P2)
INTRODUCTION
The efficiency of HIV-1 release is determined by Late (L) domains located in the C-terminal p6 region of the virus-encoded structural precursor polyprotein Gag 1; 2. These L domain motifs, Pro7-Thr-Ala-Pro (P7TAP) and Leu-Tyr-Pro-Xn-Leu (LYPXnL) recruit the cellular proteins, Tsg101 and Alix, respectively. While the two motifs are each competent to bind their respective cellular partners, the binding of Tsg101 to the P7TAP motif is sufficient to drive release of Gag with high efficiency. Provided the virus has a functional P7TAP motif, Alix can be depleted from the cell 3 and mutations in LYPXnL that impair Alix binding can be introduced without deleterious effects on virus release efficiency 4.
Tsg101 and Alix are components of endocytic trafficking machinery that sort cellular cargo destined for delivery to degradative compartments through late endosome/multivesicular bodies (LE/MVBs) 5; 6. Tsg101 is a component of ESCRT-I (endosomal sorting complex required for transport)-I which normally functions in a sequential manner with ESCRT-0,-II and-III to deliver cargo to intralumenal vesicles of the LE/MVBs through a series of membrane fusion and budding events. When Gag has a functional P7TAP L domain, ESCRT-III is recruited through a mechanism that bypasses the need for ESCRT-II 7; 8. The recruitment of ESCRT-III to viral budding sites on the plasma membrane enables its scission activity to mediate the untethering of the virus particle from the cell 9. In the absence of Tsg101 binding, the adaptor protein Alix directs virus release by linking Gag to ESCRT-III directly 4; 10; 11.
We recently reported that activation of the inositol triphosphate receptor (IP3R) is required for efficient release of HIV-1 Gag as virus-like particles (VLPs) or as fully infectious virions 12. It has been demonstrated that addition of CaCl2 and ionomycin to tissue culture media, which elevates the concentration of free calcium (Ca2+ ) in the cytosol, has an enhancing effect on HIV particle release 13; 14. Our finding suggests that IP3R is the cellular protein that the virus relies on for physiologic provision of Ca2+. IP3R forms a transmembrane channel that “gates” release of intracellular Ca2+ stores with the endoplasmic reticulum (ER) serving as the major store in the cell 15–17. IP3R activation requires binding by its ligand, inositol (1,4,5)-triphosphate (IP3), which is produced along with diacylglycerol (DAG) when phosphatidylinositol-(4, 5)-bisphosphate (PI(4,5)P2) is hydrolyzed by phospholipase C (PLC) 18; 19. It is well documented that receptor activation by IP3 stimulates both Ca2+ release from intracellular stores and Ca2+ entry through channels at the plasma membrane 15–17. Ca2+ entry is responsible for the refilling of the stores. The most supported Ca2+ entry mechanism designated as store operated calcium entry (SOCE) 20; 21 postulates that entry is triggered by the emptying of the IP3R-gated Ca2+ store; hence it is dependent on IP3R activation. A model that has emerged is that refilling requires close apposition of the ER membrane to entry channels on the plasma membrane. Studies also reveal that the elevation in the cytosolic Ca2+ concentration resulting from IP3R activation and entry channel function is essentially confined to the immediate vicinity of the cytoplasmic channel openings 22; 23.
The requirement of efficient viral budding for ESCRT machinery 1; 2, for Ca2+ 13; 14 and for the PI(4,5)P2-PLC-IP3-IP3R-Ca2+ signaling cascade 12 prompted us to determine whether the requirement for IP3R-Ca2+ signaling is linked to L domain function. To this end, the cascade was perturbed by siRNA-targeted depletion of critical components or pharmacological interventions and the effect on release of Gag as virus-like particles (VLPs) was determined. We found that whether or not the release pathway was directed by the binding of Tsg101, the same core components of the cascade, i.e., PLC, IP3R and Ca2+, were required. However, the pathways differed in the mode by which the IP3R-dependent Ca2+ was provided. While Tsg101-independent Gag release relied on SOCE, Tsg101-directed release utilized intracellular Ca2+ stores and, moreover, Tsg101 played an active role in potentiating IP3R-Ca2+ signaling. We conclude that Ca2+ signaling is a critical determinant of budding that is specifically facilitated by Tsg101 binding.
RESULTS
Treatment with CaCl2 and the Ca2+ ionophore ionomycin enhances Gag release in the presence or absence of Tsg101-binding
As noted above, elevation of cytosolic Ca2+ by incubating cells in CaCl2 in the presence of the Ca2+ ionophore ionomycin enhances release of HIV-1 virus particles or the VLPs assembled by Gag expressed alone 13; 14. To determine whether the cytosolic Ca2+ level is also a limiting factor for particle release in the absence of Tsg101 binding to Gag (i.e., when Alix mediates budding), COS-1 cells were transfected with DNA encoding WT Gag or P7L-Gag, a mutant containing a single amino acid substitution in P7TAP that impairs Tsg101 binding 24. The cells were incubated with Ca2+ and ionomycin 48 hrs post-transfection and harvested 4 hrs later. As expected based on previous studies 13; 14, the addition to the media of 2 mM CaCl2 and 10 μM ionomycin increased VLP release (FIGURE 1, panel A, lanes 1 and 2). Release of P7L-Gag VLP was also increased (lanes 3 and 4). A quantitative analysis of VLP release efficiency (Gag signal in [VLP]/[VLP + cell lysate]; panel B) indicated that the CaCl2 plus ionomycin treatment enhanced WT Gag release by ~5-fold. The P7L-Gag release efficiency, which is typically ~3 to 10-fold lower than WT Gag in these cells 24; 25, was enhanced to the level of the untreated WT Gag control (compare lane 4 to lane 1). Thus, WT-and P7L-Gag release were both stimulated by the Ca2+ plus ionomycin treatment, suggesting that cytosolic Ca2+ is a limiting factor in viral particle production.
FIGURE 1. Ionomycin + CaCl2 treatment stimulates both WT Gag and P7L-Gag.
release. Panel A, Cells expressing WT Gag (lanes 1 and 2) or P7L-Gag (lanes 3 and 4) were mock-treated (lanes 1 and 3) or treated with ionomycin (10 μm) and CaCl2 (2 mM) for 4 hr (at 44–48 hr post-transfection). Cell lysates were prepared and VLPs were isolated from the media at 48 hr post-transfection and analyzed by Western blotting. Panel B, Relative release efficiency. Quantitative analysis of VLP release efficiency was based on the Western signal intensity as described in Methods and Materials.
Steady-state levels of IP3R are required for Gag release in the presence or absence of Tsg101-binding
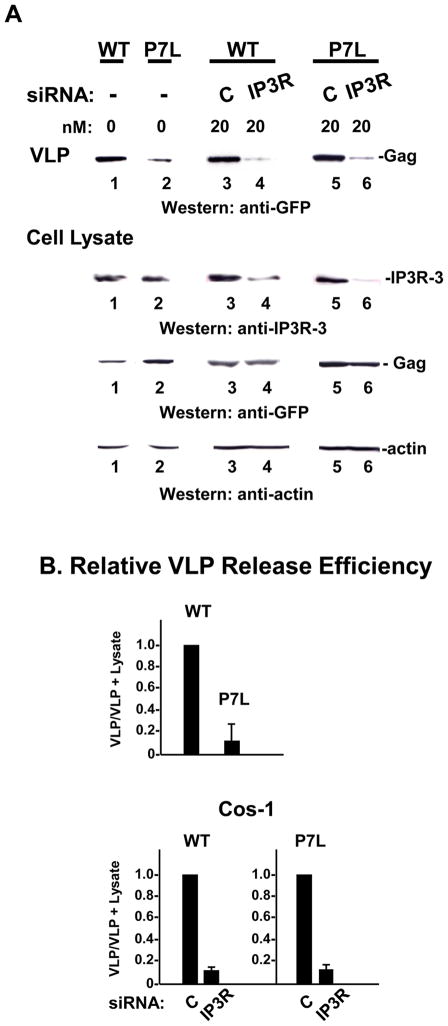
The results above indicated that Ca2+ promotes release whether or not budding is directed by Tsg101 binding to Gag. To determine whether release in the absence of Tsg101 binding also requires IP3R, COS-1 cells were transfected with siRNA targeted against expression of IP3R-3, the major isoform in these cells 26, and the effect on release of P7L-Gag was examined. Since as expected, production of P7L-Gag VLPs was impaired compared to WT Gag (FIGURE 2, panel A, compare lanes 1 and 2 and panel B, top), direct comparison of the samples was facilitated by preparing more of the P7L VLPs and matching the WT-and P7L-VLP control samples (c.f., lanes 3 and 5). In the case of both WT-and P7L-Gag, it was apparent that fewer VLPs were produced in the corresponding samples from IP3R-depleted cells (lanes 4 and 6). Examination of equal amounts of the cell lysates (as reflected by actin) indicated that treatment of the cells with siRNA targeted against IP3R-3 reduced the pool of endogenous protein by 70–90% compared to treatment with control siRNA (compare lanes 3 to 4 and 5 to 6). As all samples contained comparable levels of cell-associated Gag (WT, lanes 3 and 4; P7L-Gag: lanes 5 and 6), the block to VLP production occurred at the level of particle release and resulted in a comparable ~10-fold decrease in release efficiency for both WT and mutant (panel B). The results indicate that the steady-state level of IP3R is required for efficient Gag release in the presence or absence of Tsg101-binding.
FIGURE 2. siRNA-targeted depletion of IP3R-3 inhibits both WT Gag and P7L-Gag release.
Panel A, Cells were mock-transfected (lanes 1 and 2) or transfected with 20 nM of control siRNA (lanes 3 and 5) or siRNA targeted to IP3R type 3 (lanes 4 and 6) 24 hr prior to transfection with DNA encoding WT Gag-GFP (lanes 1, 3 and 5) or P7L-Gag-GFP (lanes 2, 4 and 6). Cell lysates and media were examined by Western analysis. Panel B, Relative release efficiency.
Inhibiting PLC activity impairs Gag release in the presence or absence of Tsg101-binding
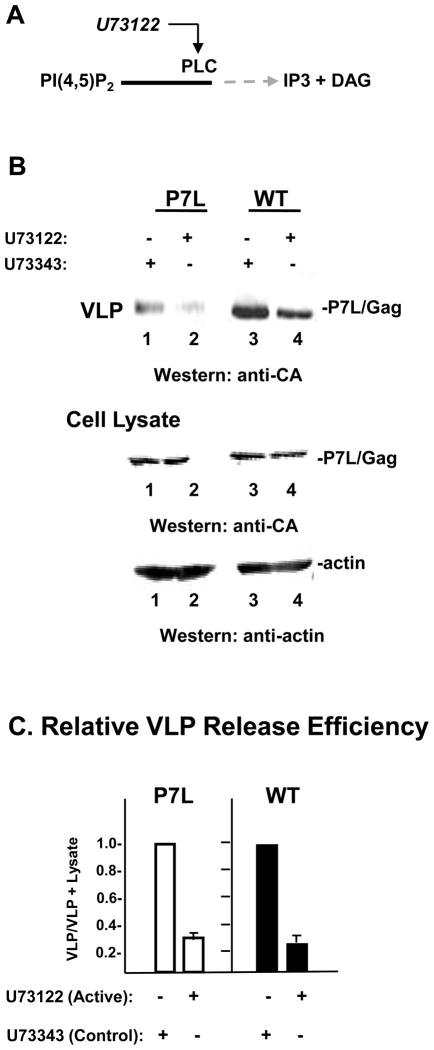
The above findings indicated a role for IP3R in provision of physiologic Ca2+ whether budding is directed through either of the L domains in Gag. Whether functioning directly as a Ca2+ channel gate or indirectly in signaling Ca2+ entry, IP3R requires activation that is initiated by the binding of its ligand, IP3. IP3 is produced mainly from PLC-catalyzed hydrolysis of PI(4,5)P2 on the plasma membrane 18; 19. Although multiple isoforms of PLC exist, they all share the same catalytic site and are uniformly affected by active site activators and inhibitors. We showed previously that WT Gag trafficking and release requires PLC-mediated IP3R activation 12. To determine whether Alix-directed budding also requires IP3R activation, we used the same agent, U73122, which irreversibly binds to the PLC active site 27, to inhibit PLC activity and examine the effect on P7L-Gag release. U73343, an inactive analogue of U73122, was used to control for possible off-target effects. Incubation of cells with U73122 reduced the amount of P7L-Gag VLP detected in the media compared to that produced by cells treated with the inactive control compound (FIGURE 3, panel A, lanes 1 and 2). Consistent with our previous findings 12, production of WT-Gag VLP was also inhibited (lanes 3 and 4). Comparable levels of Gag and actin were detected in lysates whether they were prepared from cells treated with the active inhibitor U73122 or the inactive analog U73343. This observation makes it unlikely that the observed inhibition of VLP release is due to toxic effects. A quantitative assessment of the inhibition indicated that release of WT Gag and P7L-Gag was reduced 3-fold in both cases (panel C). We conclude that the catalytic activity of PLC is important for Gag release in the presence or absence of Tsg101 binding. In a control experiment, we verified that an isoform of PLC (PLCγ) is required for release of both WT and P7L-Gag: We depleted cells of PLCγ using a siRNA whose efficacy was previously described 28 and found that depletion of the endogenous pool of PLCγ inhibited both WT and P7L-Gag release (Supplementary Fig.1).
FIGURE 3. Inhibiting PLC activity impairs both WT Gag and P7L-Gag release.
Panel A, Schematic showing U73122 inhibitory action. Panel B, Cells transfected with DNA encoding P7L-Gag (lanes 1 and 2) or WT Gag (lanes 3 and 4) were treated for 24 hr with the non-active analogue of the U73122 agent, U73343 (0.5 μm, lanes 1 and 3) or the active PLC inhibitor U73122 (0.5 μm, lanes 2 and 4). Proteins in VLP and cell lysates were visualized by Western blotting. Panel C, Relative release efficiency.
Tsg101 binding promotes VLP release following pharmacological stimulation of PLC
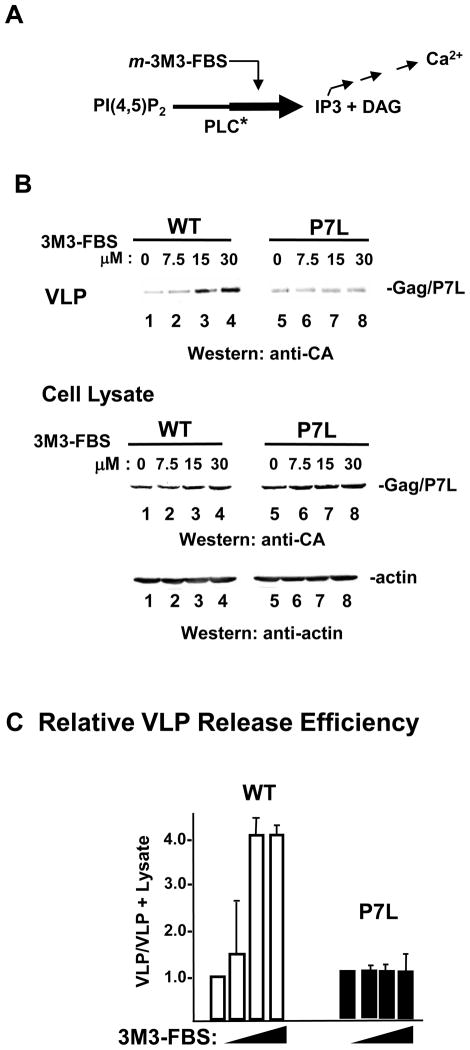
The effect on VLP release of stimulating PLC activity with 2,4,6-trimethyl-N-(meta-3-trifluoromethyl-phenyl)-benzenesulfonamide (m-3M3-FBS) was determined (FIGURE 4). m-3M3-FBS acts on all isoforms of PLC and treatment has been shown to increase cellular IP3 and elevate cytosolic Ca2+ consistent with stimulation of PLC activity 29; 30 as illustrated in panel A. Transfection conditions were used such that comparable levels of WT-and P7L VLP were obtained from untreated cells (panel B top, lanes 1 and 5). The amount of WT Gag VLP detected in the media increased in a dose-dependent manner (panel B top, lanes 2–4) without detectably affecting Gag or actin accumulation inside the cell (panel B bottom, lanes 2–4), indicating that the increase in production was due to enhanced VLP release efficiency. In contrast, no appreciable increase in P7L-Gag release was detected (panel B top, lanes 6–8). Quantitative analysis indicated that the release efficiency of WT Gag was increased 4-to 5-fold while release efficiency of P7L-Gag remained essentially unchanged (panel C). Thus, WT Gag VLP release was more readily enhanced than P7L-Gag VLP release by the PLC activation induced by 3M3-FBS in the concentration range used in the experiment. Considering that both WT-and P7L-Gag VLP release required the Ca2+ signaling cascade (Figs 2 and 3), this finding suggests that the cascade is in a potentiated state in cells expressing the WT Gag protein. Supporting this notion, WT Gag-expressing cells were found to possess a slightly higher cytosolic Ca2+ level than that measured for either mock-transfected or P7L-Gag-expressing cells (Supplementary Fig.2).
FIGURE 4. Tsg101 binding enhances VLP release following pharmacological stimulation of PLC.
Panel A, Schematic representation of the effect of m-3M3-FBS on PLC. Panel B, Cells transfected with DNA encoding WT Gag (lanes 1–4) or P7L-Gag (lanes 5–8) were treated with the indicated dose of the PLC activator, m-3M3-FBS for 3 hr (45–48 hr post-transfection). Proteins were visualized by Western blotting. Panel C, Relative release efficiency.
Tsg101 binding permits VLP release following depletion of PLC activators
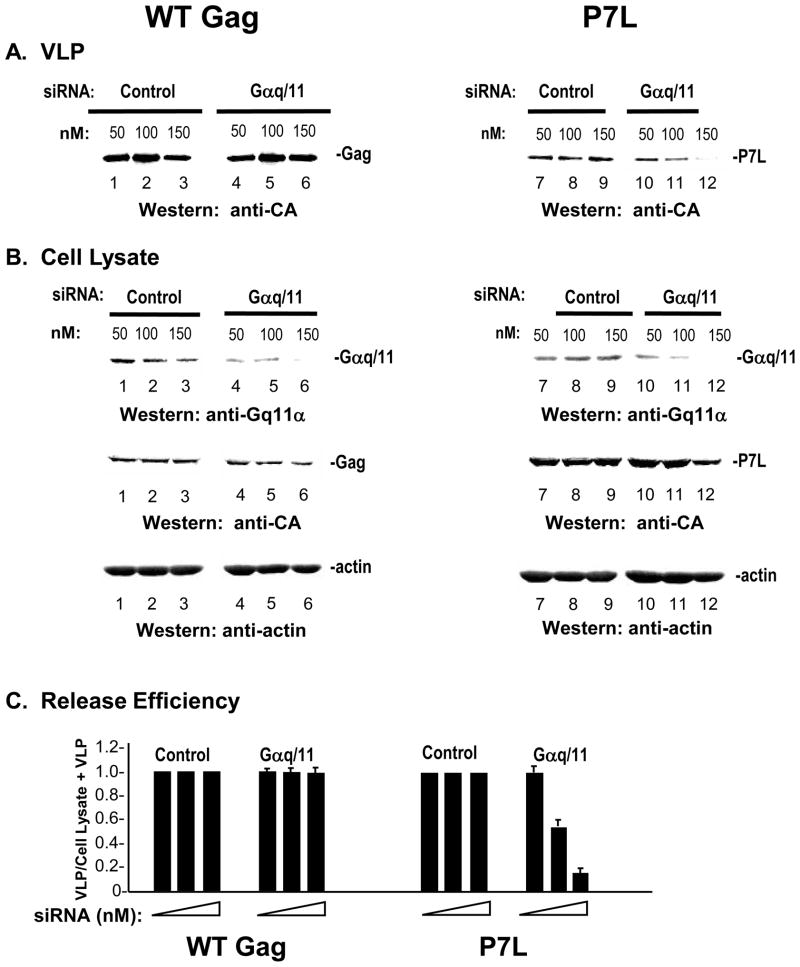
Conformational changes in seven-transmembrane-spanning receptors at the cell surface termed G protein-coupled receptors (GPCRs) leads to activation of heterotrimeric G proteins of which there are four main classes based on their sequence homologies: Gi, Gs, Gq, and G12/13 31. The α subunit of members of the Gq family bind and activate directly the βand indirectly the γ isoforms of PLC 31–33. We determined the effect of depleting the α subunits of Gq family members αq and α11 on release of WT-and P7L-Gag (FIGURE 5). Cells were transfected with either a non-targeting control siRNA or with siRNA targeted to a sequence that is homologous in the α subunit of Gq (Gαq) and the α subunit of G11 (Gα11) as previously described 34 following a depletion regimen used in a previous study of HIV infection 35. After 48 hours, the cells were transfected with DNA encoding WT or P7L-Gag. As shown in panel A, targeted siRNA (referred to here as Gαq/11) had no significant effect on the amount of WT VLP detected in the media (lanes 4–6) compared to the yield from control samples transfected with non-targeting siRNA (lanes 1–3). In contrast, the targeted siRNA reduced P7L-Gag release in a dose-dependent manner (lanes 10–12). As shown in panel B, in cells treated with the targeted siRNA, Gαq/11 protein levels (top), detected with polyclonal antibody directed against a common region in Gαq and Gα11, was reduced to an undetectable level whereas Gag (middle) and actin (bottom) levels were not significantly affected. While VLP release efficiency of the WT Gag was not reduced by depletion of Gαq/11,that of P7L-Gag was reduced by greater than 5-fold at the highest concentration of siRNA tested (panel C). Thus, depletion of Gαq/11 was inhibitory to VLP release when Tsg101 was not bound to Gag. The results indicate that Tsg101 binding makes Gag release independent of Gαq/Gα11-mediated PLC activation.
FIGURE 5. Tsg101 binding permits VLP release following depletion of PLC activators.
COS-1 cells were transfected with the indicated amounts of control siRNA (lanes 1–3 and 7–9) or siRNA targeted to IP3R type 3 (lanes 4–6 and 10–12) 24 hr prior to transfection with DNA encoding WT Gag-GFP (lanes 1–6) or P7L-Gag-GFP (lanes 7–12). Media (panel A) and cell lysates (panel B) were examined by Western analysis. Panel C, Relative release efficiency.
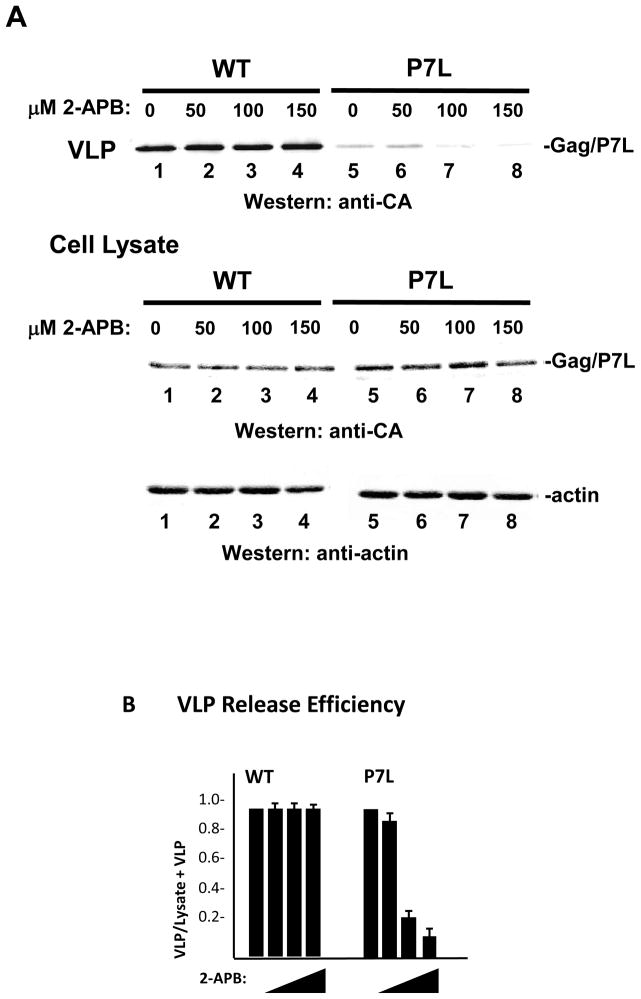
Tsg101 binding permits VLP release following 2-APB-mediated disruption of Ca2+ influx
G proteins of the Gq/11 subfamily are functionally coupled to SOCE 21. We therefore considered the possibility that the indifference of WT Gag release to siRNA-mediated depletion of Gαq/11 reflected a Tsg101-mediated bypass of dependence on Ca2+ influx. To test this, we employed aminoethoxydiphenyl borate (2-APB), an agent that inhibits Ca2+ influx mediated by plasma membrane entry channels such as transient receptor potential (TRP) and Orai1 36; 37. The latter are normally activated by interaction with the ER calcium sensor stromal interaction protein 1 (STIM1) which functions as effector of the coupling between IP3R-gated-store emptying and Ca2+ influx 20; 21. IP3R-mediation of Ca2+ release from stores still occurs during 2-APB treatment. As shown in FIGURE 6, WT Gag VLP production (panel A, lanes 1–4) was not detectably affected by increasing doses of the influx inhibitor. In contrast, production of P7L-Gag VLP was reduced in a dose-dependent manner (lanes 5–8). Intracellular accumulation of the Gag proteins and actin was not significantly affected. Quantitative analysis (panel B) indicated that the release efficiency of P7L-Gag was reduced 10-fold at the highest 2-APB dose tested while that of WT Gag was unchanged. The results support the conclusion that Tsg101 binding relieves Gag from dependence on store-operated Ca2+ entry to achieve efficient VLP release.
FIGURE 6. Tsg101 binding permits VLP release following 2-APB-mediated disruption of Ca2+ influx.
Panel A, Cells transfected with DNA encoding WT Gag (lanes 1–4) or P7L-Gag (lanes 5–8) were treated with the indicated concentration of 2-APB from 24 to 48 hr post transfection in Ca2+-free medium. VLP production and Gag expression levels were compared by Western blotting. Panel B, Relative release efficiency.
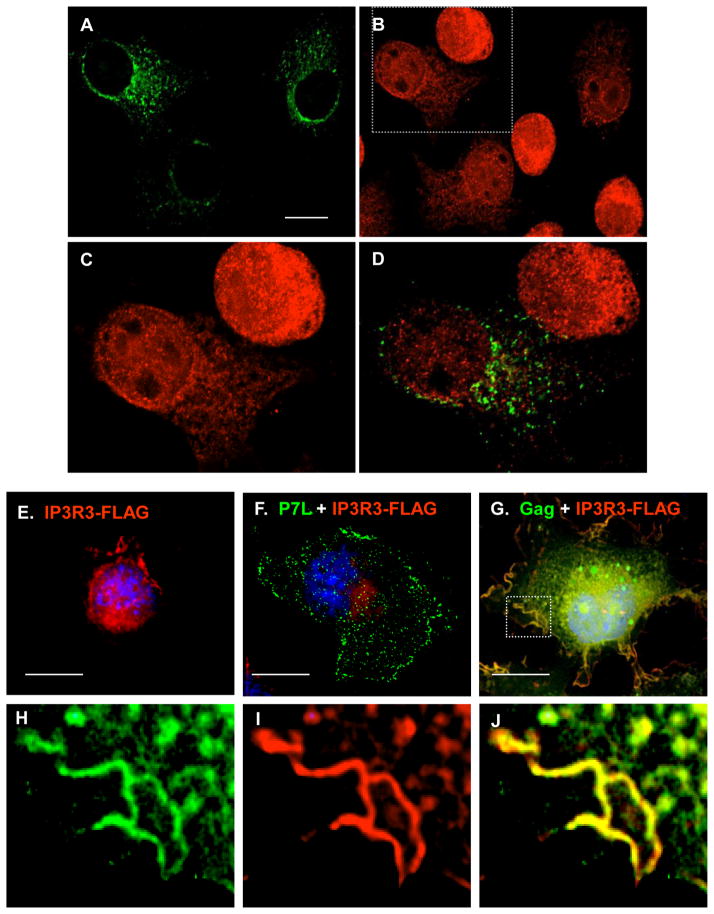
Tsg101-binding correlates with re-distribution of IP3R-FLAG to the plasma membrane
An inference from the results in Figs 5 and 6 is that Tsg101-directed Gag release relies on the internal Ca2+ stores for provision of cytosolic Ca2+. As noted above, emptying of the IP3R-gated Ca2+ stores activates SOCE; this induces a redistribution of ER-associated STIM1 to plasma membrane-proximal regions 20; 21; 38. If Tsg101-directed Gag release promotes utilization of Ca2+ provided from the intracellular stores, the re-distribution of ER and IP3R to the plasma membrane might be an event associated with cells expressing WT Gag. FIGURE 7, panels A–D, shows the effect of Gag expression on the distribution of endogenous IP3R-3 as visualized by confocal microscopy. IP3R-3 exhibited a perinuclear localization in the untransfected cells in the culture, as expected 17, but was re-distributed towards the cell periphery in several of the cells expressing Gag (6 of 40 cells = 15%). Co-localization was not apparent (panel D). To enhance detection of IP3R in the peripheral location and to determine whether the L domain influences IP3R re-distribution, cells transfected with IP3R-3-FLAG and WT-or P7L-Gag were examined. When expressed alone (panel E) or with P7L-Gag (panel F), IP3R-3-FLAG exhibited the perinuclear localization in >95% of the cells, similar to the localization of the endogenous protein in the absence of Gag described above. In contrast, in 78% (39 of 50) of cells co-expressing WT Gag, IP3R-3-FLAG was redistributed. WT Gag co-localized with IP3R-FLAG in two locations: plasma membrane-proximal punctuate structures and plasma membrane. The protein was in plasma membrane-proximal punctuate structures in 33 of 39 cells (85%) and also at the plasma membrane in 6 of 39 cells (15%, panel G). A region at the cell periphery is enlarged in panels H-J. WT Gag co-localized with IP3R-FLAG on the plasma membrane and in the plasma membrane-proximal punctuate region in these cells (panel J; Pearson’s coefficient = 0.6), compared to none of the cells expressing P7L-Gag. The results indicate that IP3R is localized in the plasma membrane-proximal region of cells expressing WT Gag and suggest that this localization is less frequent (or does not occur) in cells expressing P7L-Gag. Thus, facilitating PLC activation and IP3R mobilization to the plasma membrane both require the intact PTAP motif in Gag, inferring that the interaction of Gag with Tsg101 links efficient release to IP3R signaling machinery.
FIGURE 7. Expression of WT-, but not P7L-, Gag induces re-distribution of IP3R.
Panels A–D, WT Gag induces re-distribution of endogenous IP3R. (A) A field of cells including cells that express Gag-GFP (green); (B) Endogenous IP3R-3 (red) in cells shown in panel A. (C) Region in panel B demarcated by the dashed line is enlarged to show the IP3R signal and (D) the merged Gag and IP3R signals. Panels E–J, PTAP motif requirement for Gag-induced re-distribution of FLAG-tagged IP3R-3. (E) Cell expressing FLAG-tagged IP3R-3 alone (red); (F) cell expressing FLAG-tagged IP3R-3 with P7L-Gag-GFP (green); (G) cell expressing FLAG-tagged IP3R-3 with WT Gag-GFP (green). Region at the cell periphery is enlarged in panels H–J to show the Gag signal (panel H), the IP3R-FLAG signal (panel I) and the merged signals (panel J). Scale bar, 10 μm
DISCUSSION
Previously, we reported that IP3R activation is required for plasma membrane localization of Gag and for budding of infectious virus 12. The study provided evidence that the PI(4,5)P2-PLC-IP3-IP3R-Ca2+ signaling cascade is required for HIV-1 production and that PLC-catalyzed hydrolysis of PI(4,5)P2 is a critically important event. However, several studies also have shown that PI(4,5)P2 targets Gag to the plasma membrane 39–44. Thus, viral particle release requires both intact PI(4,5)P2 and PI(4,5)P2 hydrolysis. We recently described evidence that the cellular protein, Sprouty2, functions to maintain PI(4,5)P2 in intact form 45. In the current study, we provide evidence that Tsg101 bound to the PTAP L domain of Gag facilitates utilization of the PI(4,5)P2-PLC-IP3-IP3R-Ca2+ signaling cascade and thus, functions to maintain PI(4,5)P2 in the hydrolyzed form.
In the current study, we onfirm previous findings that VLP release was enhanced by increasing the cytosolic Ca2+ concentration 13; 14, and show that the enhancement of VLP release occurred whether or not Tsg101 was bound to Gag. This suggests that Ca2+ is a limiting cellular factor in viral budding. We present evidence that IP3R-mediated Ca2+ signaling was required whether or not budding was directed by Tsg101. Similarly, in the presence or absence of Tsg101 binding, VLP release was inhibited by depleting the endogenous pool of IP3R or by inhibiting PLC, the enzyme that produces IP3, the IP3R activating ligand. However, Tsg101 binding to Gag differentially facilitated the use of the Ca2+ signaling machinery in a manner that permitted Gag to bypass requirements for G protein-mediated PLC activation (c.f., Fig. 5) or for SOCE (c.f., Fig. 6). The results suggest that, in addition to linking Gag to ESCRT machinery, Tsg101 binding also promotes Gag utilization of the IP3R-gated Ca2+ stores.
If WT Gag budding utilizes Ca2+ released from stores and is independent of SOCE, how are the stores refilled during the course of Gag assembly? Possibly, store refilling does not require overt Ca2+ influx 46 or may be mediated by a non-SOCE channel 47; 48 as exemplified by the arachidonic acid-regulated channel (ARC). ARC requires PLC-mediated hydrolysis of PI(4,5)P2 to IP3 and DAG: Arachidonic acid is a product of DAG metabolism and is insensitive to 2-APB 49. It is interesting to note that the process of cytokinesis which, like viral budding, requires Tsg101 and other ESCRTs 50–52, and exhibits a strict reliance on the IP3R-gated stores for Ca2+ provision 53–56. It has been suggested that exclusive utilization of the stores for Ca2+ provision is important for the sequential occurrence of events during the process 57. Possibly, this same necessity pertains to the virus budding process.
The fact that enhancement was more readily detected for WT Gag release in contrast to P7L-Gag release following treatment with m-3M3-FBS, an agent known to activate all isoforms of PLC activation 29, and that WT Gag release was not inhibited by depletion of Gαq and Gα11, i.e., G proteins required for PLC activation, suggests that Tsg101 binding to Gag facilitates the PLC activation process. Recent studies suggest that PLC is autoinhibited in the basal state 58; 59. Possibly, Tsg101, alone or in conjunction with binding partners (including Gag), binds PLC and releases the enzyme from its autoinhibited state. Interestingly, Everett et. al. 59 found that events leading to release from autoinhibition occur at or near membranes. Based on this model, both the PI(4,5)P2-binding determinants in the N-terminal MA domain of Gag that target it to the plasma membrane and the Tsg101-binding motif in the C-terminal p6 region might be required for Tsg101-mediated PLC activation. We recently reported that mutations in the PI(4,5)P2-binding pocket of the MA domain in EIAV Gag engendered the prototype “lollipop” phenotype characteristic of disruptions in the L domain 60. Together with our observations here, these results suggest that virus release involves “cross-talk” between the MA and p6 domains in Gag. It is also noteworthy that Gq/11 proteins, whose PLC activation function we speculate to be assumed by Tsg101 during VLP release, links with Rho 61, as does Tsg101 (via Rho kinase) 51. Thus, an important consequence of Tsg101 binding beyond recruitment of ESCRT machinery is, possibly, release of PLC autoinhibition, resulting in activation and hydrolysis of PI(4,5)P2 with production of IP3, and the ensuing activation of IP3R. One implication of this model is that all 3 proteins (Gag, Tsg101 and IP3R or PLC) be together on the plasma membrane. This has been technically difficult to assess because when we over-express Tsg101, most of the signal is in the cell interior 62, making it difficult to discern the population at the cell periphery. Alternatively, the mechanism may involve spatially distinct interactions.
In summary, our studies show that HIV-1 Gag release, whether directed by Tsg101 or not, requires IP3R activation. However, with Tsg101 bound, budding preferentially utilized the internal Ca2+ stores rather Ca2+ influx. Additionally, Tsg101 potentiated Gag utilization of the IP3R-Ca2+ signaling machinery. These findings could provide a novel basis for development of anti-viral strategies targeted at calcium signaling dynamics.
MATERIALS AND METHODS
Plasmids and reagents
Plasmids encoding Gag-GFP and P7L-Gag-GFP were described previously 25. Non-targeting siRNA controls and targeting siRNAs with previously described sequences (IP3R 63; PLCγ1 28; Gαq/11 34) were obtained from Dharmacon. Polyclonal rabbit antibodies used were: anti-HIV-1 CA 64, anti-Gαq/11 (Upstate), and anti-PLCγ1 (Cell Applications, Inc). Mouse monoclonal antibodies used were: anti-GFP (Clonetech), anti-IP3R type 3 (BD Biosciences), anti-FLAG, and anti-actin (Sigma). Dye-conjugated secondary antibodies used were: IRDye 800 anti-rabbit IgG (Rockland); TRITC-tagged and Alexa Fluor 680-tagged anti-mouse IgG (Molecular Probes). Ionomycin, Fura Red and Pluronic F-127 were from Invitrogen. U73122 and U73343 were from Sigma. m-3M3-FBS (2,4,6-Trimethyl-N-(m-3-trifluoromethylphenyl)benzenesulfonamide) and 2-APB (2-aminoethoxydiphenyl borate) were from Calbiochem.
Cell culture, transfection, and harvest
COS-1 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% fetal bovine serum and 1% streptomycin + penicillin solution. For transfection of siRNA: Nontargeting or targeting siRNA was transfected using Lipofectamine 2000 (Invitrogen) or DharmaFECT (Dharmacon) and incubated as previously described prior to transfection with DNA: 24 hrs for depletion of IP3R-3 63; 48 hrs for depletion of Gαq/11 35 and 72 hrs for depletion of PLCγ1 28. Transfection of DNA was done using Fugene 6 (Roche). Tissue culture media and cells were harvested 48 hours following DNA transfection except when otherwise stated in the text. VLPs were isolated from collected tissue culture media and lysates were prepared from collected cells as previously described 12.
Treatment with small molecule activators and inhibitors
Treatment with CaCl2 plus ionomycin: At 48 hours post-transfection, the tissue culture media was replaced with treatment media according to Perlman and Resh14 and incubated for 4 hours according to Grigorov et. al. 13. Treatment with activators and inhibitors: Stock solutions were prepared using dimethyl sulfoxide (DMSO) as solvent. Unless otherwise stated, at 24 hrs post-transfection tissue culture media was removed, replaced with DMEM containing DMSO alone or drug at concentrations indicated in the text. DMEM containing a volume of DMSO equivalent to the highest volume of stock solution in the treatment set was used as negative control. Treatment periods were 24 hrs for 2-APB, U73122 and U73343 and 3 hours for m-3M3-FBS.
Confocal microscopy
COS-1 cells were grown to 40% confluency on large square coverslips (22 × 22mm) in six well plates in DMEM supplemented with fetal bovine serum and antibiotics as previously described 25. Briefly, 48 hours following transfection, cells on coverslips were fixed in 3.7% formaldehyde, and permeabilized with 0.1% Triton X-100. For detection of endogenous IP3R-3 and FLAG-tagged IP3R-3, coverslips were incubated with either anti-IP3R-3 or anti-FLAG mouse monoclonal antibody followed by incubation with TRITC (red)-tagged secondary antibody. Nucleus was stained with Hoeschst (Molecular Probes). Confocal images were captured with an inverted fluorescent/dic Zeiss Axiovert 200M microscope operated using Axiovision version 4.5 software. Figures show images captured at the center z plane. Protein co-localization was assessed in 10–15 cells by determination of Pearson’s coefficient of correlation 65 using Image J software and regarded as significant when a value of 0.6 or higher (equivalent to a 95% level of confidence for that number of cells) was observed.
Western analysis
Proteins were separated by electrophoresis through 4.5% (for detection of IP3R) or 10% (for detection of other proteins) SDS-polyacrylamide gels and electroblotted onto nitrocellulose membrane. Following incubation with appropriate primary and secondary antibodies, proteins were visualized using an infrared-based imaging system (Odyssey, LI-COR Biosciences). For analysis of release efficiency, densitometric measurements of bands on the immunoblot corresponding to Gag in VLP and Gag in cell lysates were made using NIH Image software. Release efficiency was defined as the ratio of the signal intensity value for the VLP-associated Gag to the sum of the values for VLP-associated Gag plus cell lysate-associated Gag (VLP/VLP + Cell Lysate).
Supplementary Material
Acknowledgments
We thank Dr. Guo-Wei Tian in the Central Microscopy Imaging Center facility at Stony Brook for assistance with cytosolic calcium measurements. G.M. was supported by W. Burghardt Turner Postdoctoral Fellowship (NSF-HRD-funded SUNY AGEP, Grant #35583). This study was supported by NIH R01 award AI068463 (to C.A.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bieniasz PD. Late budding domains and host proteins in enveloped virus release. Virology. 2006;344(1):55–63. doi: 10.1016/j.virol.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 2.Freed EO. Viral Late Domains. J Virol. 2002;76(10):4679–4687. doi: 10.1128/JVI.76.10.4679-4687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin-Serrano J, Yaravoy A, Perez-Caballero D, Bieniasz PD. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc Natl Acad Sci USA. 2003;100(21):12414–12419. doi: 10.1073/pnas.2133846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujii K, Hurley JH, Freed EO. Beyond Tsg101: the role of Alix in ‘ESCRTing’ HIV-1. Nat Rev Microbiol. 2007;5(12):912–916. doi: 10.1038/nrmicro1790. [DOI] [PubMed] [Google Scholar]
- 5.Hurley J. The ESCRTcomplexes. Crit Rev Biochem Mol Biol. 2010;45(6):463–487. doi: 10.3109/10409238.2010.502516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roxrud I, Stenmark H, Malerød L. ESCRT & Co. Biol Cell. 2010;102(5):293–318. doi: 10.1042/BC20090161. [DOI] [PubMed] [Google Scholar]
- 7.Langelier C, von Schwedler UK, Fisher RD, De Domenico I, White PL, Hill CP, Kaplan J, Ward D, Sundquist WI. Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. J Virol. 2006;80(19):9465–9480. doi: 10.1128/JVI.01049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pincetic A, Medina G, Carter C, Leis J. Avian sarcoma virus and human immunodeficiency virus, type 1 use different subsets of ESCRT proteins to facilitate the budding process. J Biol Chem. 2008;283:29822–30. doi: 10.1074/jbc.M804157200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurley J, Hanson PI. Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat Rev Mol Cell Biol. 2010;11(8):556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dussupt V, Javid MP, Abou-Jaoudé G, Jadwin JA, de La Cruz J, Nagashima K, Bouamr F. The nucleocapsid region of HIV-1 Gag cooperates with the PTAP and LYPXnL late domains to recruit the cellular machinery necessary for viral budding. PLoS Pathog. 2009;5(3):e1000339. doi: 10.1371/journal.ppat.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii K, Munshi UM, Ablan SD, Demirov DG, Soheilian F, Nagashima K, Stephen AG, Fisher RJ, Freed EO. Functional role of Alix in HIV-1 replication. Virology. 2009;391(2):284–292. doi: 10.1016/j.virol.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrlich LS, Medina GN, Khan MB, Powell MD, Mikoshiba K, Carter CA. Activation of the Inositol (1,4,5)-Triphosphate Calcium Gate Receptor is Required for HIV Gag Release. J Virol. 2010;84(13):6438–6451. doi: 10.1128/JVI.01588-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grigorov B, Arcanger F, Roingeard P, Darlix J-L, Muriaux D. Assembly of infectious HIV-1 in human epithelial and T-lymphoblastic cell lines. J Mol Biol. 2006;20(4):1–15. doi: 10.1016/j.jmb.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Perlman M, Resh MD. Identification of an intracellular trafficking and assembly pathway for HIV-1 Gag. Traffic. 2006;7(6):731–745. doi: 10.1111/j.1398-9219.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 15.Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem. 2007;102(5):1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- 16.Patterson RL, Boehning D, Snyder SH. Inositol 1, 4, 5-triphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 17.Vermassen E, Parys JB, Mauger JP. Subcellular distribution of the inositol1,4,5-triphosphate receptors: functional relevance and molecular determinants. Biol Cell. 2004;96(1):3–17. doi: 10.1016/j.biolcel.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Rhee S. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh P, Park JI, Manzoli L, Cocco L, Peak JC, Katan M, Fukami K, Kataoka T, Yun S, Ryu SH. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 2008;41(6):415–34. doi: 10.5483/bmbrep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- 20.Smyth JT, Hwang SY, Tomita T, DeHaven WI, Mercer JC, Putney JW. Activation and regulation of store-operated calcium entry. J Cell Mol Med. 2010;14(10):2337–2349. doi: 10.1111/j.1582-4934.2010.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaca L. SOCIC: the store-operated calcium influx complex. Cell Calcium. 2010;47(3):199–209. doi: 10.1016/j.ceca.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Bootman MD, Lipp P, Berridge MJ. The organization and functions of local Ca2+ signals. J Cell Sci. 2001;114(Pt 12):2213–2222. doi: 10.1242/jcs.114.12.2213. [DOI] [PubMed] [Google Scholar]
- 23.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Demirov DG, Orenstein JM, Freed EO. The late domain of human immunodeficiency virus type 1 p6 promotes virus release in a cell type-dependent manner. J Virol. 2002;76(1):105–117. doi: 10.1128/JVI.76.1.105-117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medina G, Pincetic A, Ehrlich LS, Zhang Y, Tang Y, Leis J, Carter CA. Tsg101 can replace Nedd4 function in ASV Gag release but not membrane targeting. Virology. 2008;377(1):30–38. doi: 10.1016/j.virol.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wojcikiewicz RJH. Type I, II, and III inositol 1,4,5-triphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem. 1995;270(19):11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 27.Wilsher NE, Court WJ, Ruddle R, Newbatt YM, Aherne W, Sheldrake PW, Jones NP, Katan M, Eccles SA, Raynaud FI. The phosphoinositide-specific phospholipase C inhibitor U73122 (1-(6-((17beta-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione) spontaneously forms conjugates with common components of cell culture medium. Drug MetabDispos. 2007;35(7):1017–22. doi: 10.1124/dmd.106.014498. [DOI] [PubMed] [Google Scholar]
- 28.Jones NP, Peak J, Brader S, Eccles SA, Katan M. PLCgamma1 is essential for early events in integrin signalling required for cell motility. J Cell Sci. 2005;118(Pt 12):2695–706. doi: 10.1242/jcs.02374. [DOI] [PubMed] [Google Scholar]
- 29.Bae Y, Lee TG, Park JC, Hur JH, Kim Y, Heo K, Kwak JY, Suh PG, Ryu SH. Identification of a compound that directly stimulates phopholipase C activity. Mol Pharmacol. 2003;63(5):1043–1050. doi: 10.1124/mol.63.5.1043. [DOI] [PubMed] [Google Scholar]
- 30.Fang YC, Kuo DH, Shieh P, Chen FA, Kuo CC, Jan CR. Effect of m-3M3FBS on Ca(2+) movement in Madin-Darby canine renal tubular cells. Hum Exp Toxicol. 2009;28(10):655–663. doi: 10.1177/0960327109106972. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard K, Hepler JR. Cell signaling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal. 2006;18(2):135–50. doi: 10.1016/j.cellsig.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Bence K, Ma W, Kozasa T, Huang XY. Direct stimulation of Bruton’s tyrosine kinase by G(q)-protein alpha-subunit. Nature. 1997;389(6648):296–299. doi: 10.1038/38520. [DOI] [PubMed] [Google Scholar]
- 33.Mueller H, Stadtmann A, Van Aken H, Hirsch E, Wang D, Ley K, Zarbock A. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways. Blood. 2010;115(15):3118–3127. doi: 10.1182/blood-2009-11-254185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson P, Young KW, Ennion SJ, Kew JN, Nahorski SR, Challiss RA. Altered expression of G(q/11alpha) protein shapes mGlu1 and mGlu5 receptor-mediated single cell inositol 1,4,5-trisphosphate and Ca(2+) signaling. Mol Pharmacol. 2006;69(1):174–184. doi: 10.1124/mol.105.014258. [DOI] [PubMed] [Google Scholar]
- 35.Harmon B, Ratner L. Induction of the Galpha(q) signaling cascade by the human immunodeficiency virus envelope is required for virus entry. J Virol. 2008;82(18):9191–9205. doi: 10.1128/JVI.00424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeHaven W, Smyth JT, Boyles RR, Bird GS, Putney JW., Jr Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J Biol Chem. 2008;2839(28):19265–19273. doi: 10.1074/jbc.M801535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu S, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol. 2005;145(4):405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J Physiol. 2008;586(Pt 22):5383–5401. doi: 10.1113/jphysiol.2008.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ono A, Ablan SD, Lockett SJ, Nagashima K, Freed EO. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc Natl Acad Sci U S A. 2004;101(41):14889–14894. doi: 10.1073/pnas.0405596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saad JS, Miller J, Tai J, Kim A, Ghanam RH, Summers MF. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc Natl Acad Sci USA. 2006;103(30):11364–11369. doi: 10.1073/pnas.0602818103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shkriabai N, Datta SA, Zhao Z, Hess S, Rein A, Kvaratskhelia M. Interactions of HIV-1 Gag with assembly cofactors. Biochemistry. 2006;45(13):4077–4083. doi: 10.1021/bi052308e. [DOI] [PubMed] [Google Scholar]
- 42.Chan R, Uchil PD, Jin J, Shui G, Ott DE, Mothes W, Wenk MR. Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. J Virol. 2008;82(22):11228–11238. doi: 10.1128/JVI.00981-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saad JS, Ablan SD, Ghanam RH, Kim A, Andrews K, Nagashima K, Soheilian F, Freed EO, Summers MF. Structure of the myristylated human immunodeficiency virus type 2 matrix protein and the role of phosphatidylinositol-(4,5)-bisphosphate in membrane targeting. J Mol Biol. 2008;382(2):434–447. doi: 10.1016/j.jmb.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamard-Peron E, Juillard F, Saad JS, Roy C, Roingeard P, Summers MF, Darlix JL, Picart C, Muriaux D. Targeting of murine leukemia virus gag to the plasma membrane is mediated by PI(4,5)P2/PS and a polybasic region in the matrix. J Virol. 2010;84(1):503–515. doi: 10.1128/JVI.01134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehrlich LS, Medina GN, Carter CA. Sprouty2 Regulates PI(4,5)P2/Ca2+ Signaling and HIV-1 Gag Release. J Mol Biol. 2011 doi: 10.1016/j.jmb.2011.04.069. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malli R, Frieden M, Hunkova M, Trenker M, Graier WF. Ca2+ refilling of the endoplasmic reticulum is largely preserved albeit reduced Ca2+ entry in endothelial cells. Cell Calcium. 2007;41(1):63–76. doi: 10.1016/j.ceca.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bootman MD, Berridge MJ, Llewelyn Roderick H. Calcium signalling: more messengers, more channels, more complexity. Current Biology. 2002;12(16):R563–R565. doi: 10.1016/s0960-9822(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 48.Putney JW., Jr Inositol lipids and TRPC channel activation. Biochem Soc Symp. 2007;74:37–45. doi: 10.1042/BSS0740037. [DOI] [PubMed] [Google Scholar]
- 49.Shuttleworth T. Arachidonic acid, ARC channels, and Orai proteins. Cell Calcium. 2009;45(6):602–610. doi: 10.1016/j.ceca.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316(5833):1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 51.Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, Sundquist WI. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007;26(19):4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDonald B, Martin-Serrano J. No strings attached: the ESCRT machinery in viral budding and cytokinesis. J Cell Sci. 2009;122(Pt 13):2167–2177. doi: 10.1242/jcs.028308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitsuyama F, Sawai T. The redistribution of Ca2+ stores with inositol 1,4,5-triphosphate receptor to the cleavage furrow in a microtubule-dependent manner. Int J Dev Biol. 2001;45(8):861–868. [PubMed] [Google Scholar]
- 54.Wong R, Hadjiyanni I, Wei HC, Polevoy G, McBride R, Sem KP, Brill JA. PIP2 hydrolysis and calcium release are required for cytokinesis in Drosophila spermatocytes. Curr Biol. 2005;15(15):1401–1406. doi: 10.1016/j.cub.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 55.Mitsuyama F, Futatsugi Y, Okuya M, Karagiozov K, Kato Y, Kanno T, Sano H, Koide T, Sawai T. Microinjected F-actin into dividing newt eggs moves toward the next cleavage furrow together with Ca2+ stores with inositol 1,4,5-trisphosphate receptor in a microtubule-and microtubule motor-dependent manner. Ital J Anat Embryol. 2008;113(3):143–151. [PubMed] [Google Scholar]
- 56.Li W, Webb SE, Chan CM, Miller AL. Multiple roles of the furrow deepening Ca2+ transient during cytokinesis in zebrafish embryos. Dev Biol. 2008;316(2):228–248. doi: 10.1016/j.ydbio.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 57.Arredouani A, Yu F, Sun L, Machaca K. Regulation of store-operated Ca2+ entry during the cell cycle. J Cell Sci. 2010;123(Pt 13):2155–2162. doi: 10.1242/jcs.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hicks S, Jezyk MR, Gershburg S, Seifert JP, Harden TK, Sondek J. General and versatile autoinhibition of PLC isozymes. Mol Cell. 2008;31(3):383–94. doi: 10.1016/j.molcel.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Everett K, Buehler A, Bunney TD, Margineanu A, Baxendale RW, Vatter P, Retlich M, Walliser C, Manning HB, Neil MA, Dunsby C, French PM, Gierschik P, Katan M. Membrane environment exerts an important influence on rac-mediated activation of phospholipase Cγ2. Mol Cell Biol. 2011;31(6):1240–1251. doi: 10.1128/MCB.01408-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandes F, Chen K, Ehrlich LS, Jin J, Chen MH, Medina GN, Symons M, Montelaro R, Donaldson J, Tjandra N, Carter CA. Phosphoinositides direct equine infectious anemia virus Gag trafficking and release. Traffic. 2011;12(4):438–451. doi: 10.1111/j.1600-0854.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sagi S, Seasholtz TM, Kobiashvili M, Wilson BA, Toksoz D, Brown JH. Physical and functional interactions of Galphaq with Rho and its exchange factors. J Biol Chem. 2001;276(18):15445–15452. doi: 10.1074/jbc.M008961200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goff A, Ehrlich LS, Cohen SN, Carter CA. Tsg101 control of Human Immunodeficiency Virus type 1 Gag trafficking and release. J Virol. 2003;77(17):9173–9182. doi: 10.1128/JVI.77.17.9173-9182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hattori M, Suzuki AZ, Higo T, Miyauchi H, Michikawa T, Nakamura T, Inoue T, Mikoshiba K. Distinct roles of inositol 1,4,5-triphosphate receptor types 1 and 3 in Ca2+ signaling. J Biol Chem. 2004;279(12):11967–11975. doi: 10.1074/jbc.M311456200. [DOI] [PubMed] [Google Scholar]
- 64.Ehrlich LS, Krausslich HG, Wimmer E, Carter C. Expression in Escherichia coli and purification of human immunodeficiency virus type 1 capsid protein (p24) AIDS Res Hum Retroviruses. 1990;6(10):1169–1175. doi: 10.1089/aid.1990.6.1169. [DOI] [PubMed] [Google Scholar]
- 65.Manders EMM, Verbee FJ, Aten JA. Measurement of colocalization of objects in dual color confocal images. J Microsc. 1993;169:375–82. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.