Abstract
The emergence of resistance against most current drugs emphasizes the need to develop new approaches to control bacterial pathogens, particularly Staphylococcus aureus. Bacterial fatty acid synthesis is one such target that is being actively pursued by several research groups to develop anti-Staphylococcal agents. Recently, the wisdom of this approach has been challenged based on the ability of a Gram-positive bacterium to incorporate extracellular fatty acids and thus circumvent the inhibition of de novo fatty acid synthesis. The generality of this conclusion has been challenged, and there is enough diversity in the enzymes and regulation of fatty acid synthesis in bacteria to conclude that there isn’t a single organism that can be considered typical and representative of bacteria as a whole. We are left without a clear resolution to this ongoing debate and await new basic research to define the pathways for fatty acid uptake and that determine the biochemical and genetic mechanisms for the regulation of fatty acid synthesis in Gram-positive bacteria. These crucial experiments will determine whether diversity in the control of this important pathway accounts for the apparently different responses of Gram-positive bacteria to the inhibition of de novo fatty acid synthesis in presence of extracellular fatty acid supplements.
Introduction
Membrane biogenesis is a vital facet of bacterial physiology. Bacterial survival depends on membrane lipid homeostasis and the ability to adjust their lipid composition to acclimatize the cell to a variety of environments [1]. Bacterial membranes consist of proteins embedded in a lipid matrix that closely approximates a phospholipid bilayer. Although there is considerable diversity of phospholipid structures in the bacterial world, the majority of membrane phospholipids are glycerolipids containing two fatty acid chains. These phospholipid acyl chains determine the viscosity of the membrane, which in turn influence many crucial membrane-associated functions, such as the passive permeability of hydrophobic molecules, active solute transport and protein–protein interactions. The essential role for fatty acids in membrane structure has focused attention on targeting this pathway for the development of novel antibacterial therapeutics.
Overview of bacterial fatty acid synthesis
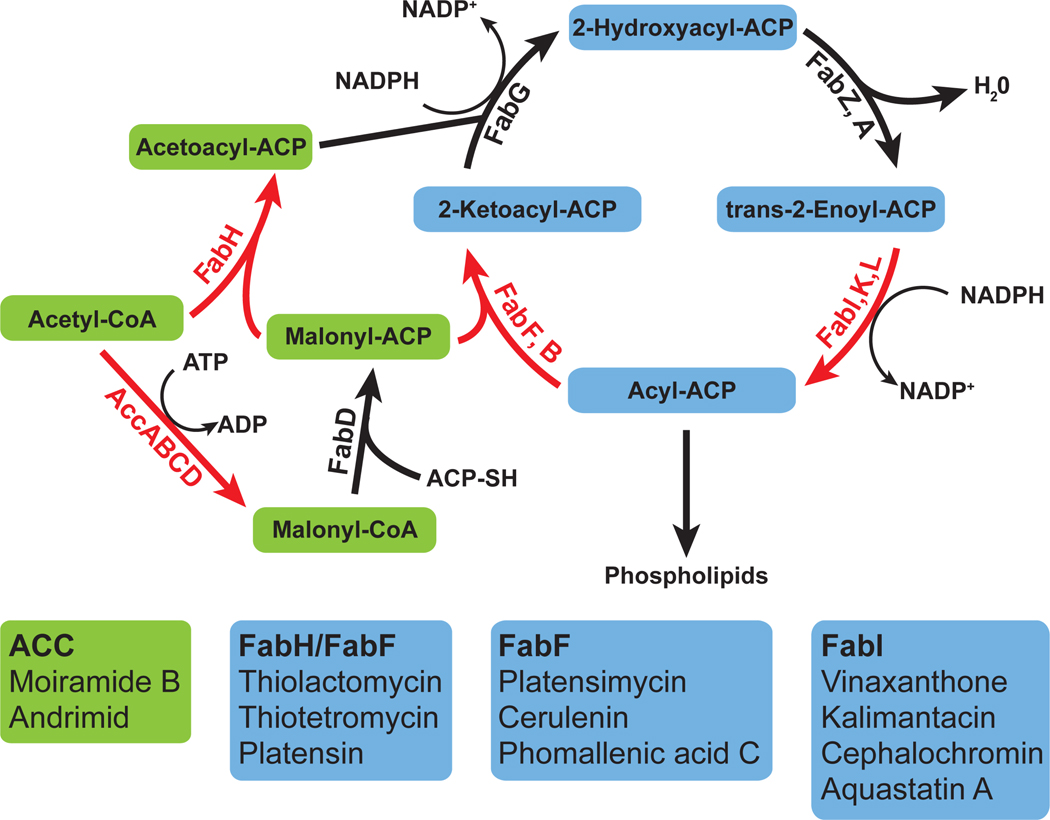
Type II fatty acid synthesis (FASII) is the process used by bacteria to generate the fatty acid components of phospholipids. Unlike the multifunctional mammalian type I fatty acid synthase, each of the reactions is performed by a separate enzyme (Figure 1a). The first commtted step reaction is performed by the acetyl-CoA carboxylase complex (ACC). The resulting malonyl-CoA is used to prime the elongation module which extends the growing fatty acid with consecutive reduction, dehydration, reduction and condensation reactions by the Fatty acid biosynthesis (Fab) enzymes. Two FabI (enoyl-ACP reductase) inhibitors, the anti-mycobacterial drug isoniazid and triclosan, were in wide use before their mechanism of action was elucidated [2]. The clinical significance of these compounds has fueled the development of some promising new FabI inhibitors through structure-based drug design [3,4] that target drug-resistant S. aureus infections.
Figure 1.
(a) Bacterial FASII cycle: Green indicates initiation module and blue elongation module. Growth of a new acyl chain is initiated by the ACC complex. Malonyl-CoA produced is converted to malonyl-ACP (acyl-carrier protein) where it is condensed with acyl-CoA by FabH. The subsequent acetoacyl-ACP feeds into the elongation module where it is extended by 2 carbons with each round of the cycle by a condensation reaction with malonyl-ACP via FabF. The resulting acyl-ACP can be used by glycerol-3-phosphate acyltransferases to synthesize phospholipids. Key regulatory reactions are indicated in red. (b) Examples of natural products proven to specifically target FASII.
Natural Product Inhibitors of FASII
Natural product inhibitors that specifically target FASII have been identified from a diverse collection of microorganisms (see Figure 1b for examples). These natural products commonly target the key regulatory points in FASII, reflecting Nature’s own identification of the most effective antimicrobial targets. The ACC and condensation reactions are key regulatory steps in FASII and FabI catalyzes the rate-limiting step in the elongation cycle. Natural products have been identified that target ACC [5], the condensing enzymes (FabF/FabH) [6,7] or the enoyl-ACP reductase (FabI) [8–11]. Natural products have proven in vivo efficacy in Gram-positive and Gram-negative murine infection models [5,12–14].
A promising natural product with regard to development of a clinically effective antimicrobial agent is platensimycin (FabF inhibitor). This secondary metabolite isolated from Streptomyces platensis shows in vivo efficacy in murine S. aureus infection models with no reported toxicity [13]. The major drawback to most natural products, including platensimycin, is inferior pharmacokinetic properties and poor oral bioavailability. Only the continuous infusion of a high platensimycin dose proved effective in mice infected with S. aureus [13]. The complex tetracyclic moiety of platensimycin has presented challenge to medicinal chemists attempting total syntheses of the compound. Total synthesis has been accomplished by numerous groups in as little as 10–20 linear steps, although with a poor yield of just a few milligrams [15]. A handful of analogs housing either a modified aromatic or tetracyclic domain have been produced. Not one of the compounds created has improved activity or more desirable bioavailability, emphasizing the importance of each functional group of platensimycin [15,16]. This roadblock is consistently encountered in attempts to improve any of the reported FASII-targeted natural products (cerulenin, thiolactomycin, and platensin) [15,17,18]. These disappointing results point to the need to identify new chemically tractable scaffolds as starting points for the development of fatty acid synthesis inhibitors.
The problem and promise of bacterial diversity
In the early days of bacteria metabolic research, the fatty acid biosynthetic pathways were thought to be shared by all bacteria, and E. coli lipid metabolism became the paradigm. However, the advent of whole genome sequencing coupled with discoveries in the laboratory over the last 10 years has revealed significant diversity in the enzymology of lipid metabolism. In FASII, the basic catalytic steps are conserved; however, there are distinctly different protein isoforms that carry out the reactions. Bacillus subtilis contains the enoyl-ACP reductase FabL in addition to a FabI, and Streptococcus pneumoniae uses the flavoprotein FabK instead of a FabI [19,20]. Most Gram-positive bacteria also utilize alternative glycerol-PO4 acyltransferase machinery (PlsX/PlsY) to construct their phospholipids, differing from the PlsB enzyme characterized in E. coli and eukaryotes [21].
This diversity suggests that developing FASII inhibitors that target a broad range of bacteria is unlikely and that drug discovery efforts should focus on specific disease targets. To some, this may make FASII a less desirable drug candidate. On the other hand, diversity opens the door to designing highly effective, genus-specific inhibitors against major pathogens rather than designing less potent molecules that affect a larger diversity of protein structures. The fact that there are no FASII inhibitors currently deployed in the clinic suggests that targeting FASII would be particularly useful in controlling drug-resistant strains by targeting a completely different cellular process. In particular, targeting FASII shows promise in counteracting the spread of multi-drug resistant S. aureus, an easily identifiable pathogen that causes a significant mortality and morbidity in hospitals [22]. This realization has focused recent efforts on optimizing natural products and synthetic compounds against the FASII machinery of S. aureus, a bacterium that is acquiring resistance to the most commonly used broad-spectrum antibiotics.
Gram-negative bacteria
The idea of targeting FASII evolved in E. coli where FASII is essential for cell growth even though these organisms incorporate exogenous fatty acids into membrane phospholipids [23]. In E. coli, extracellular fatty acids cross the outer membrane through the FadL porin and translocate to the inner aspect of the inner membrane where they are activated by acyl-CoA synthetase (FadD). Acyl-CoAs are used by the PlsB/PlsC acyltransferases to completely support phospholipid synthesis. The essentiality of the FASII genes in Gram-negative bacteria arises from the requirement for β-hydroxy-fatty acids to assemble the lipid A core structure of outer membrane lipopolysaccharides. Fatty acid supplementation cannot support lipid A synthesis because there is no mechanism to transfer acyl chains from CoA to the acyl carrier protein (ACP) of FASII so that the hydroxyl group can be introduced. The gene called acyl-ACP synthetase (aas) in E. coli actually encodes a lysophospholipid acyltransferase with a bound ACP subunit, and the acyl-ACP intermediate does not dissociate from the enzyme [24,25]. Supplementation with hydroxy-fatty acids is also ineffective because the acyltransferases of lipid A biosynthesis only use ACP thioester substrates [26]. Thus, there is little doubt that suitably designed FASII inhibitors would be effective against Gram-negative bacteria.
Gram-positive bacteria
The E. coli model for bacterial lipid metabolism was used to establish FabI as the target for triclosan [27,28]. The observation that triclosan has rather potent activity against virtually all groups of bacteria suggested at the time that the E. coli model could be extended to all bacteria and that FabI inhibitors would be universal antibacterials. However, the advent of genome sequencing showed that the E. coli paradigm for FASII did not extend to many Gram-positive bacteria. Members of the Lacetobacillales instead contain a flavoprotein enoyl-ACP reductase that is resistant to triclosan inhibition [20] and has no structural resemblance to FabI [29], Thus, unlike Gram-negative bacteria where FabI was the target, the antibacterial effect of triclosan against Gram-positive bacteria is due a different, and still unknown mechanism. This important fact is not widely appreciated, and triclosan is still being used as an FASII-specific inhibitor in bacteria that lack FabI, although its inhibition of fatty acid synthesis is not direct [30,31]. In contrast, other Gram-positive bacteria, including the important pathogen S. aureus, do contain a FabI, and in light of the importance of this pathogen the next generation FabI inhibitors were developed focused on optimizing their potency against S. aureus FabI [2].
Recently, the wisdom of targeting FASII in Gram-positive pathogens was questioned by Brinster et al. [30] based on the finding that FASII is not essential in Streptococcus agalactiae (Lacetobacillales) if the cultures are supplemented with fatty acids or human serum. Whether the findings with S. agalactiae can be reasonably extended to all Gram-positive bacteria has spurred a vigorous debate. Balemans et al. [31] were unable to show fatty acids rescued S. aureus treated with a FabI inhibitor, whereas Brinster et al. [32] have countered by showing that S. aureus can incorporate exogenous fatty acids and that they have constructed a S. aureus fabI knockout that is a fatty acid auxotroph. Important challenges to the position of Brinster et al. are the numerous reports of the efficacy of fatty acid synthesis inhibitors against S. aureus in mouse models [5,12,13,31,33–35]. Brinster et al. [30] suggest that these experiments may be flawed because the compounds were administered to the animals before S. aureus became adapted to the presence of exogenous fatty acids in the host. However, there is no definition of what this adaptation process may entail, and without some experimental evidence for a specific genetic or biochemical response in S. aureus that governs its utilization of extracellular fatty acids for phospholipid synthesis, it is hard to argue against the consistent finding that FASII inhibitors are effective in animals against this pathogen. Although the fallacy of using E. coli as a model for all bacteria is apparent there has been little research on the pathway(s) for the uptake and utilization of exogenous fatty acids by Gram-positive bacteria except in B. subtilis where extracellular fatty acids are converted to acyl-CoAs and degraded by a β-oxidation system [36]. It is clear that Gram-positive bacteria neither use the same acyltransferase enzyme nor the same acyl donor to initiate phospholipid synthesis [21], therefore the pathway for the uptake and incorporation of extracellular fatty acids into phospholipids in Gram-positive bacteria must also be different. These bacteria must use a system that converts exogenous fatty acids to either acyl-ACP or acyl-PO4 (not acyl-CoA), but experimental evidence to substantiate these suppositions is lacking. It is also not clear whether the rate of exogenous fatty acid uptake occurs at a rate that matches de novo biosynthesis and would support the same growth rate as FASII.
Targeting Acetyl-CoA carboxylase (ACC)
Formation of malonyl-CoA is essential to support FASII, and in light of the discovery of natural products that target acetyl-CoA carboxylase [37], researchers have made headway in developing small molecules that inhibit this enzyme [5,38–40]. Perhaps one can make a stronger argument that the ability of Gram-positive bacteria to incorporate exogenous fatty acids into their phospholipids would make ACC an unattractive target, but there are experiments with animal models showing that ACC inhibitors are effective against S. aureus [5]. Here also there is a dearth of basic research on membrane lipid homeostasis in Gram-positive bacteria that may explain this apparent paradox. For example, Lacetobacillales, like S. pneumoniae, synthesize straight-chain saturated and monounsaturated fatty acids that are almost identical to the major fatty acids in a mammalian host. Therefore, replacement of endogenous fatty acids with host-derived fatty acids may be predicted to have little impact on membrane biophysical properties. However, S. aureus and other Bacillales synthesize branched-chain, saturated fatty acids that are not found in mammals. The importance of fatty acid structure to membrane function is well established [1], and it is not known whether bacteria like S. aureus can cope with its normal complement of fatty acids being replaced by the structurally unrelated mammalian fatty acids.
Targeting the acyltransferase systems
Another attractive target for Gram-positive antibacterial drug discovery are the acyltransferases required for the synthesis of membrane phospholipids. Most of these bacteria rely on the PlsX/PlsY system to initiate phospholipid synthesis by converting acyl-ACP to acyl-PO4 by PlsX followed by the transfer of the fatty acid to the 1-position of glycerol-PO4 by the PlsY acyltransferase [21]. The unique acyl-PO4 intermediate is not found in mammals making the Gram-positive acyltransferase system a desirable target. One approach developed acyl-PO4 analogs as potentially dual function product inhibitors PlsX and substrate inhibitors of PlsY [41], however, these hydrophobic molecules will need to be significantly improved before useful compounds will emerge. One strength of targeting these steps in lipid synthesis is that there are no mammalian PlsX/PlsY counterparts and acyltransferase inhibitors cannot be circumvented by supplementation with extracellular fatty acids.
Conclusions
There is no consensus about the wisdom of developing FASII inhibitors as antibacterial drugs against Gram-positive pathogens. One view is that S. aureus cannot be effectively treated with FASII inhibitors in the presence of exogenous fatty acids, while another interpretation argues that FASII inhibitors can be effective clinical agents. The latter position is supported by numerous animal models that show effective control of S. aureus infections using a number of different FASII inhibitors. There are no examples of animal infection models using FASII inhibitors against Lacetobacillales like S. pneumonia, so we do not know if the in vitro experiments suggesting FASII inhibitors will not work against these organisms in vivo is in fact the case. It seems that we are left with an embarrassing lack of basic scientific understanding of the diversity in the regulation of lipid metabolism in Gram-positive pathogens that would resolve these conflicting views with data. Basic research in this area is sorely needed to define the pathway(s) for exogenous fatty acid incorporation into membrane lipids in Gram-positive bacteria, and whether these pathways differ between groups of Gram-positive bacteria. We do not know whether the distinct differences in genetic regulation of FASII gene expression contribute to the response of Gram-positive bacteria to extracellular fatty acids. The Bacillales (i.e. S. aureus) control FASII gene expression via the FapR repressor that is released from DNA by the first committed pathway intermediate, malonyl-CoA [42]. The Lacetobacillales (i.e. S. pneumoniae) use FabT, a repressor that requires long-chain acyl-ACP end-products of FASII to bind to DNA [43]. Almost nothing is known about the biochemical regulation of FASII in Gram-positive bacteria and differences in this key feature may underlie the differences in the response of organisms to extracellular fatty acids. The idea that Gram-positive bacteria replace all their endogenous fatty acids with host-derived fatty acids during infection has not been directly tested by determining the fatty acid composition of bacterial phospholipids recovered from infected animals. So we are left with a confusing debate over the utility of FASII inhibitors in S. aureus that will only be solved by a deeper molecular understanding of the diversity of FASII biochemistry and regulation among Gram-positive bacteria. In the meantime, the effectiveness of fatty acid synthesis inhibitors in S. aureus infection models cannot be ignored and highlights the importance of obtaining a basic understanding the regulation of lipid metabolism in clinically important pathogens.
Highlights.
Bacterial lipid synthesis is a desirable focus for drug discovery because this pathway is not targeted by existing drugs, and is therefore likely to produce compounds that attack bacteria resistant to known antibiotics.
Although fatty acid synthesis inhibitors may not be effective against some Gram-positive bacteria that are supplemented with extracellular fatty acids; there is compelling evidence that such inhibitors may be useful against many Gram-positive and all Gram-negative organisms even in the presence of extracellular fatty acids.
The diversity in bacterial lipid metabolism indicates that it will be difficult to design inhibitors that block the growth of all bacteria under all environmental conditions.
Targeting the unique acyltransferases of Gram-positive bacteria is an unexplored area that holds promise to find effective inhibitors that cannot be overcome by fatty acid supplements.
Acknowledgements
This research was supported by National Institutes of Health grant GM034496, Cancer Center (CORE) Support Grant CA21765 and the American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Zhang Y-M, Rock CO. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol. 2008;6:222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- 2.Lu H, Tonge PJ. Inhibitors of FabI, an enzyme drug target in the bacterial fatty acid biosynthesis pathway. Acc Chem Res. 2008;41:11–20. doi: 10.1021/ar700156e. [DOI] [PubMed] [Google Scholar]
- 3.Karlowsky JA, Kaplan N, Hafkin B, Hoban DJ, Zhanel GG. AFN-1252, a FabI inhibitor, demonstrates a Staphylococcal-specific spectrum of activity. Antimicrob Agents Chemother. 2009;53:3544–3548. doi: 10.1128/AAC.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdanovich T, Clark C, Kosowska-Shick K, Dewasse B, McGhee P, Appelbaum PC. Antistaphylococcal activity of CG400549, a new experimental FabI inhibitor, compared with that of other agents. Antimicrob Agents Chemother. 2007;51:4191–4195. doi: 10.1128/AAC.00550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freiberg C, Pohlmann J, Nell PG, Endermann R, Schuhmacher J, Newton B, Otteneder M, Lampe T, Habich D, Ziegelbauer K. Novel bacterial acetyl-coenzyme A carboxylase inhibitors with antibiotic efficacy in vivo. Antimicrob Agents Chemother. 2006;50:2707–2712. doi: 10.1128/AAC.00012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young K, Jayasuriya H, Ondeyka JG, Herath K, Zhang C, Kodali S, Galgoci A, Painter R, Brown-Driver V, Yamamoto R, Silver LL, Zheng Y, Ventura JI, Sigmund J, Ha S, Basilio A, Vicente F, Tormo JR, Pelaez F, Youngman P, Cully D, Barrett JF, Schmatz D, Singh SB, Wang J. Discovery of FabH/FabF inhibitors from natural products. Antimicrob Agents Chemother. 2006;50:519–526. doi: 10.1128/AAC.50.2.519-526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Agnolo G, Rosenfeld IS, Awaya J, Omura S, Vagelos PR. Inhibition of fatty acid biosynthesis by the antibiotic cerulenin. Specific inactivation of b-ketoacyl-acyl carrier protein synthetase. Biochim Biophys Acta. 1973;326:155–166. doi: 10.1016/0005-2760(73)90241-5. [DOI] [PubMed] [Google Scholar]
- 8. Mattheus W, Masschelein J, Gao LJ, Herdewijn P, Landuyt B, Volckaert G, Lavigne R. The kalimantacin/batumin biosynthesis operon encodes a self-resistance isoform of the FabI bacterial target. Chem Biol. 2010;17:1067–1071. doi: 10.1016/j.chembiol.2010.07.015. • Discovery of a FabI-directed natural product inhibitor and its cognate resistance protein.
- 9.Zheng CJ, Sohn MJ, Kim WG. Vinaxanthone, a new FabI inhibitor from Penicillium sp. J Antimicrob Chemother. 2009;63:949–953. doi: 10.1093/jac/dkp058. [DOI] [PubMed] [Google Scholar]
- 10.Zheng CJ, Sohn MJ, Lee S, Hong YS, Kwak JH, Kim WG. Cephalochromin, a FabI-directed antibacterial of microbial origin. Biochem Biophys Res Commun. 2007;362:1107–1112. doi: 10.1016/j.bbrc.2007.08.144. [DOI] [PubMed] [Google Scholar]
- 11.Kwon YJ, Fang Y, Xu GH, Kim WG. Aquastatin A, a new inhibitor of enoyl-acyl carrier protein reductase from Sporothrix sp. FN611. Biol Pharm Bull. 2009;32:2061–2064. doi: 10.1248/bpb.32.2061. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti SL, Herath K, Cummings R, Salazar O, Gonzalez I, Basilio A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully DF, Singh SB. Discovery of platencin, a dual FabF and FabH inhibitor with in vivo antibiotic properties. Proc Natl Acad Sci U S A. 2007;104:7612–7616. doi: 10.1073/pnas.0700746104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio A, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature (London) 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- 14.Miyakawa S, Suzuki K, Noto T, Harada Y, Okazaki H. Thiolactomycin a new antibiotic. IV. Biological properties and chemotherapeutic activity in mice. J Antibiot (Tokyo) 1982;35:411–419. doi: 10.7164/antibiotics.35.411. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Sintim HO. Dialkylamino-2,4-dihydroxybenzoic acids as easily synthesized analogues of platensimycin and platencin with comparable antibacterial properties. Chemistry. 2011;17:3352–3357. doi: 10.1002/chem.201002410. [DOI] [PubMed] [Google Scholar]
- 16.Palanichamy K, Kaliappan KP. Discovery and syntheses of "superbug challengers"-platensimycin and platencin. Chem Asian J. 2010;5:668–703. doi: 10.1002/asia.200900423. [DOI] [PubMed] [Google Scholar]
- 17.Ohno T, Awaya J, Kesado T, Nomura S, Omura S. Mechanism of action of CM-55, a synthetic analogue of the antilipogenic antibiotic cerulenin. Antimicrob Agents Chemother. 1974;6:387–392. doi: 10.1128/aac.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim P, Zhang Y-M, Shenoy G, Nguyen Q-A, Boshoff HI, Manjunatha UH, Goodwin M, Lonsdale JT, Price AC, Miller DJ, Duncan K, White SW, Rock CO, Barry CE, III, Dowd CS. Structure-activity relationships at the 5-position of thiolactomycin: an intact (5R)-isoprene unit is required for activity against the condensing enzyme from Mycobacterium tuberculosis and Escherichia coli. J Med Chem. 2006;49:159–171. doi: 10.1021/jm050825p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heath RJ, Su N, Murphy CK, Rock CO. The enoyl-[acyl-carrier-protein] reductases FabI and FabL from Bacillus subtilis. J Biol Chem. 2000;275:40128–40133. doi: 10.1074/jbc.M005611200. [DOI] [PubMed] [Google Scholar]
- 20.Heath RJ, Rock CO. A triclosan-resistant bacterial enzyme. Nature (London) 2000;406:145–146. doi: 10.1038/35018162. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y-J, Zhang Y-M, Grimes KD, Qi J, Lee RE, Rock CO. Acyl-phosphates initiate membrane phospholipid synthesis in gram-positive pathogens. Molec Cell. 2006;23:765–772. doi: 10.1016/j.molcel.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cronan JE, Jr, Rock CO. Chapter 3.6.4. Biosynthesis of membrane lipids. In: Böck I, Curtis R III, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, Ussery D, editors. Eco-Sal-Escherichia coli and Salmonella typhimurium: cellular and molecular biology. [Online.] Washington, DC: ASM Press; 2008. http://www.ecosal.org. [Google Scholar]
- 24.Cooper CL, Hsu L, Jackowski S, Rock CO. 2-Acylglycerolphosphoethanolamine acyltransferase/acyl-acyl carrier protein synthetase is a membrane-associated acyl carrier protein binding protein. J Biol Chem. 1989;264:7384–7389. [PubMed] [Google Scholar]
- 25.Hsu L, Jackowski S, Rock CO. Isolation and characterization of Escherichia coli K-12 mutants lacking both 2-acyl-glycerophosphoethanolamine acyltransferase and acyl-acyl carrier protein synthetase activity. J Biol Chem. 1991;266:13783–13788. [PubMed] [Google Scholar]
- 26.Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in Gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy CW, Roujeinikova A, Sedelnikova S, Baker PJ, Stuitje AR, Slabas AR, Rice DW, Rafferty JB. Molecular basis of triclosan activity. Nature (London) 1999;398:383–384. doi: 10.1038/18803. [DOI] [PubMed] [Google Scholar]
- 28.Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J Biol Chem. 1999;274:11110–11114. doi: 10.1074/jbc.274.16.11110. [DOI] [PubMed] [Google Scholar]
- 29.Marrakchi H, DeWolf WE, Jr, Quinn C, West J, Polizzi BJ, So CY, Holmes DJ, Reed SL, Heath RJ, Payne DJ, Rock CO, Wallis NG. Characterization of Streptococcus pneumoniae enoyl-[acyl carrier protein] reductase (FabK) Biochem J. 2003;370:1055–1062. doi: 10.1042/BJ20021699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brinster S, Lamberet G, Staels B, Trieu-Cuot P, Gruss A, Poyart C. Type II fatty acid synthesis is not a suitable antibiotic target for Gram-positive pathogens. Nature (London) 2009;458:83–86. doi: 10.1038/nature07772. •• This manuscript challenges the wisdom of targeting fatty acid biosynthesis for therapeutics development against all Gram-positive pathogens.
- 31. Balemans W, Lounis N, Gilissen R, Guillemont J, Simmen K, Andries K, Koul A. Essentiality of FASII pathway for Staphylococcus aureus. Nature (London) 2010;463:E3. doi: 10.1038/nature08667. •• Reports that a FabI-directed fatty acid synthesis inhibitor cannot be circumvented by the supplementation with extracellular fatty acids.
- 32. Brinster S, Lamberet G, Staels B, Trieu-Cuot P, Gruss A, Poyart C. Brinster et al. reply. Nature (London) 2010;463:E4. doi: 10.1038/nature07772. •• S. aureus is shown to incorporate extracellular fatty acids, and that a fabI knockout was constructed in S. aureus that is a fatty acid auxotroph.
- 33.Payne DJ, Miller WH, Berry V, Brosky J, Burgess WJ, Chen E, DeWolf JW, Jr, Fosberry AP, Greenwood R, Head MS, Heerding DA, Janson CA, Jaworski DD, Keller PM, Manley PJ, Moore TD, Newlander KA, Pearson S, Polizzi BJ, Qiu X, Rittenhouse SF, Slater-Radosti C, Salyers KL, Seefeld MA, Smyth MG, Takata DT, Uzinskas IN, Vaidya K, Wallis NG, Winram SB, Yuan CC, Huffman WF. Discovery of a novel and potent class of FabI-directed antibacterial agents. Antimicrob Agents Chemother. 2002;46:3118–3124. doi: 10.1128/AAC.46.10.3118-3124.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller WH, Seefeld MA, Newlander KA, Uzinskas IN, Burgess WJ, Heerding DA, Yuan CC, Head MS, Payne DJ, Rittenhouse SF, Moore TD, Pearson SC, Berry V, DeWolf WE, Jr, Keller PM, Polizzi BJ, Qiu X, Janson CA, Huffman WF. Discovery of aminopyridine-based inhibitors of bacterial enoyl-ACP reductase (FabI) J Med Chem. 2002;45:3246–3256. doi: 10.1021/jm020050+. [DOI] [PubMed] [Google Scholar]
- 35. Miller JR, Dunham S, Mochalkin I, Banotai C, Bowman M, Buist S, Dunkle B, Hanna D, Harwood HJ, Huband MD, Karnovsky A, Kuhn M, Limberakis C, Liu JY, Mehrens S, Mueller WT, Narasimhan L, Ogden A, Ohren J, Prasad JV, Shelly JA, Skerlos L, Sulavik M, Thomas VH, VanderRoest S, Wang L, Wang Z, Whitton A, Zhu T, Stover CK. A class of selective antibacterials derived from a protein kinase inhibitor pharmacophore. Proc Natl Acad Sci U S A. 2009;106:1737–1742. doi: 10.1073/pnas.0811275106. • Description of a novel class of acetyl-CoA carboxylase inhibitors.
- 36.Matsuoka H, Hirooka K, Fujita Y. Organization and function of the YsiA regulon of Bacillus subtilis involved in fatty acid degradation. J Biol Chem. 2007;282:5180–5194. doi: 10.1074/jbc.M606831200. [DOI] [PubMed] [Google Scholar]
- 37.Freiberg C, Brunner NA, Schiffer G, Lampe T, Pohlmann J, Brands M, Raabe M, Habich D, Ziegelbauer K. Identification and characterization of the first class of potent bacterial acetyl-CoA carboxylase inhibitors with antibacterial activity. J Biol Chem. 2004;279:26066–26073. doi: 10.1074/jbc.M402989200. [DOI] [PubMed] [Google Scholar]
- 38.Pohlmann J, Lampe T, Shimada M, Nell PG, Pernerstorfer J, Svenstrup N, Brunner NA, Schiffer G, Freiberg C. Pyrrolidinedione derivatives as antibacterial agents with a novel mode of action. Bioorg Med Chem Lett. 2005;15:1189–1192. doi: 10.1016/j.bmcl.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Santoro N, Brtva T, Roest SV, Siegel K, Waldrop GL. A high-throughput screening assay for the carboxyltransferase subunit of acetyl-CoA carboxylase. Anal Biochem. 2006;354:70–77. doi: 10.1016/j.ab.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Kay E, Humair B, Denervaud V, Riedel K, Spahr S, Eberl L, Valverde C, Haas D. Two GacA-dependent small RNAs modulate the quorum-sensing response in Pseudomonas aeruginosa. J Bacteriol. 2006;188:6026–6033. doi: 10.1128/JB.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimes KD, Lu Y-J, Zhang Y-M, Luna VA, Hurdle JG, Carson EI, Qi J, Kudrimoti S, Rock CO, Lee RE. Novel acylphosphate mimetics target PlsY, an essential acyltransferase in gram-positive bacteria. Chem Med Chem. 2008;3:1936–1945. doi: 10.1002/cmdc.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schujman GE, Paoletti L, Grossman AD, de Mendoza D. FapR, a bacterial transcription factor involved in global regulation of membrane lipid biosynthesis. Dev Cell. 2003;4:663–672. doi: 10.1016/s1534-5807(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 43. Jerga A, Rock CO. Acyl-acyl carrier protein regulates transcription of fatty acid biosynthetic genes via the FabT repressor in Streptococcus pneumoniae. J Biol Chem. 2009;284:15364–15368. doi: 10.1074/jbc.C109.002410. • Identifies long-chain acyl-ACP as the ligand that regulates the DNA binding of FabT, the transcription factor that controls fatty acid synthesis gene expression in S. pneumoniae.