Abstract
Proline is an efficient source of both carbon and nitrogen for many bacterial species. In Bacillus subtilis, the proline utilization pathway, encoded by the putBCP operon, is inducible by proline. Here we show that this induction is mediated by PutR, a proline-responsive transcriptional activator of the PucR family. When other amino acids are present in the medium, proline utilization is prioritized through transient repression by CodY, a global transcriptional regulator in gram-positive bacteria that responds to amino acid availability. CodY-mediated repression of the putBCP operon has two novel features. First, repression requires the cooperative binding of CodY to at least two adjacent motifs. Second, though CodY binds to the region that overlaps the putB promoter, repression is due to displacement of PutR rather than competition with RNA polymerase.
Keywords: Bacillus subtilis, proline utilization, CodY-mediated repression, PucR family activator, gene expression
INTRODUCTION
CodY is a global transcriptional regulator of metabolic genes in Bacillus subtilis1; 2; 3; 4; 5. CodY homologs are present in most low G+C gram-positive bacteria and in many species have been shown to coordinate expression of virulence-associated functions with expression of metabolic genes [see3; 6; 7; 8 and references therein].
CodY is a dimeric DNA-binding protein whose activity is increased by interaction with two types of effectors, branched-chain amino acids [isoleucine, leucine, and valine (ILV)]9; 10; 11; 12 and, in some species, GTP2; 11; 13; 14. The highest activity of CodY is observed in cells growing in media containing multiple amino acids15; 16. Many CodY-regulated genes are involved in amino acid or, more generally, nitrogen metabolism1; 2; 3; 4; 5. One of the main functions of CodY appears to be establishing the hierarchy of utilization of various nitrogen-containing compounds under conditions of nutrient-replete growth (the same is likely true for utilization of some carbon sources). Thus, CodY either activates or has no effect on expression of the pathways for utilization of some nitrogen sources, allowing early consumption of such com pounds, and represses the pathways for utilization of those nutrients that are less preferred2; 5. As a result, CodY serves as a master regulator for cell metabolism under conditions of nutrient excess.
Proline (Pro) is an important source of carbon and nitrogen in bacterial cells. In addition, in B. subtilis, the presence of Pro in the medium can modulate the activities of important metabolic regulators such as TnrA, GlnR, GltC, and RocR17; 18; 19 (our unpublished data). Though redundant enzymes exist for some steps of Pro utilization18; 20; 21, the putBCP operon (Fig. 1), encoding Pro dehydrogenase (oxidase)(EC 1.55.99.8), Δ1-pyrroline-5-carboxylic acid dehydrogenase (EC 1.5.1.12), and a Pro transporter, is essential for Pro utilization under normal growth conditions22 (S. Moses, T. Sinner, N. Stökeven, and E. Bremer, manuscript in preparation).
Fig. 1.
Schematic map of the putBCPR chromosomal locus and the putB regulatory region. The locations of the transcription start point and predicted transcription terminator of the putBCP operon are indicated by the bent arrow and inverted dotted triangle, respectively. The putative promoters of the putP and putR genes are indicated by dotted bent arrows. The PutR-binding site and CodY-binding motifs are shown as rectangles above and below the line, respectively. The coordinates indicate the boundaries of the putB210-lacZ fusion with respect to the transcription start point. The vertical arrow indicates the position where the putB210-lacZ fusion was truncated to create the putB100-lacZ fusion.
As detected by microarray analysis, putB is repressed 6-fold by CodY in amino acid-containing medium. Moreover, based on ChIP-to-chip experiments, putB has been suggested to be a direct target of CodY2. In fact, even before CodY was identified, Pro oxidase enzymatic activity (the presumed product of the putB gene) was shown to be a target of repression by a mixture of amino acids that was possibly mediated by CodY23.
In this work, we sought to analyze the molecular mechanism of putBCP regulation. Our results indicate that PutR, a protein encoded by a gene downstream of putBCP, is an activator essential for expression of the operon and that CodY represses the operon by displacing this activator from DNA.
RESULTS
PutR is an activator of the putBCP operon
Expression of the putBCP operon (formerly also known as ycgMNO) is induced by Pro22; 23. A gene, ycgP, located 155 bp downstream of the putP gene and transcribed in the same orientation (Fig. 1), putatively encodes a 411-amino acid transcriptional regulator that is distantly related to B. subtilis PucR, a transcriptional activator of the genes of purine catabolism24 (23% id entity over C-terminal 311 amino acids). A putative DNA-binding helix-turn-helix region can be identified within YcgP from positions 361 to 382. A deletion-insertion mutation in ycgP was constructed and introduced into the chromosome of B. subtilis as described in Materials and Methods. Strain BB3330 (ycgP::cat) lost completely the ability to utilize Pro as either sole nitrogen or sole carbon source, a phenotype identical to that of a putBCP null mutant22. Thus, YcgP appears to be an activator of the putBCP operon and was therefore renamed PutR. No induction of the transcriptional fusion, putB210-lacZ, containing a 210-bp DNA fragment that includes the entire 193-bp intergenic region upstream of putB, was detected in the putR null mutant (Table 1, strains BB3328 and BB3335). Expression of putB- or putC- or putP-lacZ transcriptional fusions, constructed previously by the integration of the lacZ-containing pMutin2 plasmid25 into the corresponding genes26(http://bacillus.genome.ad.jp/), was also dependent on activity of PutR (Table 2).
Table 1.
PutR- and CodY-dependent regulation of the putB210-lacZ fusion
| Strain | Relevant genotype | Fusiona type | Additions to the medium | β-galactosidase activity (MU) | β-galactosidase activity (%)b |
|---|---|---|---|---|---|
| BB3328 | wild-type | putB210p+ | none | 0.16 | 0.04 |
| Pro | 364.0 | 100 | |||
| Pro + ILV | 298.0 | 82 | |||
| Pro + 13 aa | 96.9 | 27 | |||
| Pro + 16 aa | 13.1 | 3.6 | |||
| BB3335 | putR | none | 0.09 | 0.02 | |
| Pro | 0.20 | 0.05 | |||
| Pro + 16 aa | 0.04 | 0.01 | |||
| BB3336 | codY | Pro | 356.0 | 98 | |
| Pro + 16 aa | 167.0 | 46 | |||
| BB3349 | putR codY | Pro | 0.16 | 0.04 | |
| Pro + 16 aa | 0.17 | 0.04 | |||
| BB3396 | wild-type | putB100p+ | Pro | 0.16 | |
| BB3335 | wild-type | putB210p6 | Pro | 0.65 | 100 |
| Pro + 16 aa | 0.03 | 4.6 |
Cells were grown in TSS glucose-ammonium medium with or without Pro and a mixture of 16 amino acids (aa) or the same mixture without ILV (13 aa) or ILV only (see Materials and Methods). β-Galactosidase activity was assayed and expressed in Miller units (MU). All values are averages of at least two experiments, and the mean errors did not exceed 30%.
The putB-lacZ fusions were integrated at the ectopic amyE locus.
β-Galactosidase activity of each fusion in the Pro-containing medium in a wild-type strain was normalized to 100%.
Table 2.
Expression of put::pMutin2 lacZ fusions
| Strain | Relevant genotype | Fusiona type | Additions to the medium | β-galactosidase activity (MU) | β-galactosidase activity (%)b |
|---|---|---|---|---|---|
| BB3331 | wild-type | putB::pMutin2 | none | 0.21 | 0.03 |
| Pro | 817.0 | 100 | |||
| Pro + 16 aa | 7.29 | 0.9 | |||
| BB3346 | putR | Pro | 0.40 | 0.05 | |
| BB3357 | codY | Pro + 16 aa | 188.0 | 23 | |
| BB3332 | wild-type | putC::pMutin2 | none | 0.23 | 0.03 |
| Pro | 849.0 | 100 | |||
| Pro + 16 aa | 7.82 | 0.9 | |||
| BB3347 | putR | Pro | 0.41 | 0.05 | |
| BB3358 | codY | Pro + 16 aa | 75.1 | 8.8 | |
| BB3333 | wild-type | putP::pMutin2 | none | 13.0 | 8.5 |
| Pro | 153.0 | 100 | |||
| Pro + 16 aa | 15.2 | 9.9 | |||
| BB3348 | putR | Pro | 20.4 | 13 | |
| BB3359 | codY | Pro + 16 aa | 83.4 | 55 |
Cells were grown and β-galactosidase was determined as described in Table 1.
The put::pMutin2 lacZ fusions were integrated at the put locus. Integration at the putB and putC genes are apparently polar on the expression of the downstream genes of the putBCP operon.
β-Galactosidase activity of each fusion in the Pro-containing medium in a wild-type strain was normalized to 100%.
Expression of the putP gene was partly independent of Pro and PutR (Table 2, strains BB3333 and BB3348). Apparently, it reflects the presence of an additional internal putP promoter. Existence of such a promoter was suggested by Northern blot experiments (http://bacillus.genome.ad.jp/Images/ycgP.tma.GIF).
CodY-dependent regulation of the putBCP operon
Expression of the putB210-lacZ fusion was reduced 28-fold if a mixture of 16 amino acids was added to the Pro-containing medium but restored to half of the maximal level in a codY null mutant (Table 1, strains BB3328 and BB3336). The 16-amino acid mixture, containing all common proteinaceous amino acids but for glutamine, histidine, asparagines, and tyrosine (see Materials and Methods), is known to make CodY highly active 16. Thus, CodY, as predicted in earlier experiments2, represses expression from the putB promoter. The incomplete restoration of putB expression when CodY was inactive may be due to the negative effect of one or more components of the amino acid mixture on the uptake of Pro, leading to the diminished induction of the operon. Similar roles for CodY-mediated repression and inhibition of the inducer uptake were reported in the amino acid control of histidine utilization genes16; 27.
Although the previously performed DNA microarray experiment2 did not show CodY-dependent regulation of the putC and putP genes, putC::pMutin2 and putP::pMutin2 lacZ fusions were repressed 5.5- to 9.6-fold by CodY in the 16 amino acid-containing medium, as expected if putBCP is a single transcriptional unit (Table 2).
putR expression
Northern blot experiments indicated that putR is not a part of the putBCP operon, though some expression of putR may originate from the putP promoter (http://bacillus.genome.ad.jp/Images/ycgP.tma.GIF). A putative Rho-independent transcriptional terminator can be found immediately downstream of the putP gene. A lacZ fusion containing the entire 155-bp intergenic region between the putP and putR genes was expressed in all media tested indicating the presence of a putR-specific promoter (Table 3). No regulation of the putR-lacZ fusion by Pro or PutR or CodY was detected, suggesting that expression from the putR promoter is constitutive (Table 3).
Table 3.
Expression of the putR-lacZ fusion
| Strain | Relevant genotype | Additions to the medium | β-galactosidase activity (MU) |
|---|---|---|---|
| BB3329 | wild-type | none | 27.7 |
| Pro | 36.2 | ||
| Pro + 16 aa | 30.5 | ||
| BB3341 | putR | Pro | 41.9 |
| BB3342 | codY | Pro + 16 aa | 26.1 |
Cells were grown and β-galactosidase was determined as described in Table 1.
Binding of CodY and PutR to the putB regulatory region
In gel-shift experiments, purified CodY bound in the presence of the effectors ILV and GTP to a DNA fragment containing the entire putB regulatory region with high affinity (apparent KD≈4 nM) (Fig. 2a). (The equilibrium dissociation constant was estimated as the protein concentration needed to shift 50% of DNA fragments under conditions of vast protein excess over DNA.) Purified PutR bound the same fragment in the presence of its putative effector Pro with a similarly high affinity (apparent KD≈4 nM) (Fig. 3a); the absence of Pro reduced the affinity of PutR about 10-fold (Fig. 3b).
Fig. 2. Binding of CodY to the putB regulatory region.
Radioactively labeled putB210p+ (a), putB210p2 (b), putB210p4 (c), putB100p+ (d), and putR (e) DNA fragments were incubated with increasing amounts of purified CodY in the presence of 10 mM ILV and 2 mM GTP. CodY monomer concentrations used (nM) are indicated below each lane. The arrows indicate complexes in which CodY is likely bound to only one motif, as opposed to two motifs simultaneously.
KD, the apparent equilibrium dissociation constant, reflects the protein concentration needed to shift 50% of DNA fragments under conditions of vast protein excess over DNA.
Fig. 3. Binding of PutR to the putB regulatory region.
Radioactively labeled putB210p+ (a, b), putB210p6 (c), putB100p+ (d), and putR (e) DNA fragments were incubated with increasing concentrations of purified PutR. All reactions and the gel and gel-running buffer, other than for part (b), contained 20 mM Pro (no ILV or GTP was included in any of the experiments). PutR monomer concentrations used (nM) are indicated below each lane. KD was defined as in Fig. 3.
Neither CodY nor PutR was able to bind to the putR regulatory region, consistent with the lack of regulation in vivo (Figs. 2e and 3e).
PutR as a Pro-responsive transcriptional activator in vitro
Very low expression from the putBp+ promoter in the absence PutR was detected in in vitro transcription experiments using purified σA-containing B. subtilis RNA polymerase. Addition of PutR together with Pro greatly stimulated transcription from the putB promoter (Fig. 4a). The size of the main putB transcript had a mobility corresponding to a DNA fragment of 131 n (data not shown), in good correlation with the expected transcript size of 132 n (see below). Thus, PutR is a direct activator of putB. Addition of PutR in the absence of Pro caused only a small increase in putB transcription (Fig. 4b); therefore, Pro is the co-activator of PutR. Addition of arginine, that induces the putBCP operon in vivo due to its conversion to Pro, did not activate PutR in vitro (data not shown). We note that the requirement for PutR and Pro in vitro, though very strong, was less stringent than in our in vivo experiments, indicating that the conditions of our in vitro experiments do not fully mimic the in vivo situation. Additionally, though Pro concentrations, as low as 0.8 mM, were able to activate PutR in vitro, higher concentrations of Pro (~16 mM), that were found in cells in glucose-ammonium medium in the absence of exogenous Pro28, did not cause induction of the putB promoter (Tables 1 and 2).
Fig. 4. In vitro transcription from the putB promoter.
The putB210p+ PCR fragment was transcribed at 37°C for 20 min using purified B. subtilis σA-containing RNA polymerase holoenzyme.
(a) All reactions contained 20 mM Pro and increasing amounts of PutR. PutR monomer concentrations used (nM) are indicated below each lane.
(b) Reactions contained no PutR or 100 nM PutR and various concentrations of Pro as indicated below each lane.
(c) The putB210p+ PCR fragment was preincubated at 37°C first with RNA polymerase and with or without 100 nM PutR or CodY for 15 min (as indicated above the lanes) and then incubated for additional 15 min in the presence of increasing amounts of either CodY or PutR (nM of monomer as indicated below each lane). Finally, transcription was initiated by addition of the nucleotide mixture. All reactions contained 20 mM Pro, 10 mM ILV, and 0.2 mM GTP.
Identification of CodY- and PutR-binding sites
A primer extension experiment established that the transcription start point of putB appears to be 40 bp upstream of the initiation codon (Fig. 5a and 5c). The sequences TTGTGA and TACAAT with two and one mismatches to the −35 and −10 regions of σA-dependent promoters, respectively, and a non-canonical 18-bp spacer region can be found upstream of the transcription start point (Fig. 5c).
Fig. 5. Determination of the putB transcription start point and CodY-binding region.
(a) Primer extension analysis of putB mRNA. Primer oBB102 annealing to the lacZ gene of the putB-lacZ fusion was extended with reverse transcriptase using as template total RNA from fusion-containing strains BB3328 (wt) and BB3336 (codY) grown in the 16-amino acid-containing medium. The A + G sequencing ladders obtained for the temp late strand of a putB PCR-generated fragment primed with labeled oBB102 are shown to the right and to the left. The apparent transcription start site of the putB gene is in boldface and marked by the +1 notation. The bent arrow indicates the direction of transcription.
(b) DNase I footprinting analysis of CodY binding to the putB regulatory region. The putB210p+ DNA fragment labeled on the tem plate strand was incubated with increasing amounts of purified CodY in the presence of 10 mM ILV and 2 mM GTP and then with DNase I. The corresponding A + G sequencing ladder is shown to the right. The apparent transcription start site and direction of putB transcription are shown by the bent arrow. The protected areas are indicated by the vertical lines. CodY monomer concentrations used (nM) are indicated above each lane.
(c) The sequence of the coding (non-template) strand of the putB regulatory region used to construct the putB210-lacZ fusions. The likely initiation codon, −10 and −35 promoter regions, the transcription start site and the position of the putBp6 mutation are in bold face. The direction of transcription and translation is indicated by the arrows. The CodY-binding motifs are boxed. The sequences protected by CodY on the template strand in DNase I footprinting experiments are underlined. The sequences protected by PutR are italicized. The coordinates of the 5′ and 3′ ends of the sequence with respect to the transcription start point are shown in parentheses. The boundaries of DNA fragments used to construct various lacZ fusions are indicated by vertical arrows.
A DNase I footprinting experiment showed that, in the presence of ILV and GTP, CodY protects an extended region of DNA, punctuated by some unprotected nucleotides. This region extends from positions −67 to −32 with respect to the transcription start point and overlaps by 5 bp the −35 region of the promoter (Fig. 5b and 5c). The putB CodY-binding site encompasses two non-overlapping 15-bp sequences similar to the CodY-binding consensus motif, AATTTTCWGAAAATT29; 30; 31; motif 1 (from positions −66 to −52) has 4 mismatches and motif 2 (from positions −49 to −35) has 3 mismatches with respect to the consensus (Fig. 5c and Table 4).
Table 4.
Mutations in the CodY-binding motifs
| putB allele | sequence ofa motif 1/motif 2 | number of mismatches in motif 1/motif 2 | score of motif 1/motif 2b |
|---|---|---|---|
| putBp+ | AATaaTCAGAAtcTT/tATTTTgAGAAtATT | 4/3 | 6.5/8.9 |
| putBp1 | AATaaTCAGAAtcTT/tATTTTCAGAAtATT | 4/2 | 6.5/11.6 |
| putBp2 | AATaaTCAGAAtcTT/tATTTTgAGcAtATT | 4/4 | 6.5/5.1 |
| putBp3 | AATaaTCAGAAtATT/tATTTTgAGAAtATT | 3/3 | 10.2/8.9 |
| putBp4 | AATaaTCAGcAtcTT/tATTTTgAGAAtATT | 5/3 | 2.7/8.9 |
| putBp7 | AATaaTCAGAAtcTT/tATTTTgAcAAtATT | 4/4 | 6.5/4.9 |
| putBp8 | AATaaTgAGAAtcTT/tATTTTgAGAAtATT | 5/3 | 3.8/8.9 |
Mismatches to the proposed CodY-binding consensus are indicated by lowercase letters. Mutations are in boldface
The scores for individual CodY-binding motifs have been generated using the position-specific weight matrix as described in31.
In a separate DNase I footprinting experiment, PutR protected a region extending from positions −93 to −45 with respect to the transcription start point, consistent with the general location of binding sites for positive regulators (Fig. 5c and Fig. 6a). Two sites hypersensitive to DNase I digestion indicate that binding of PutR leads to distortion (bending) of DN A. The presence of Pro increased affinity for DNA but did not affect the pattern of DNase I protection (Fig. 6a).
Fig. 6. Mapping of the PutR-binding region and analysis of competition between CodY and PutR.
(a) DNase I footprinting analysis of PutR binding to the putB regulatory region. The putB210p+ DNA fragment labeled on the template strand was incubated with increasing amounts of purified PutR in the presence or absence of 20 mM Pro and then with DNase I. The corresponding A + G sequencing ladder is shown to the right. The apparent transcription start site and direction of putB transcription are shown by the bent arrow. The protected area is indicated by the vertical line. PutR monomer concentrations used (nM) are indicated above each lane.
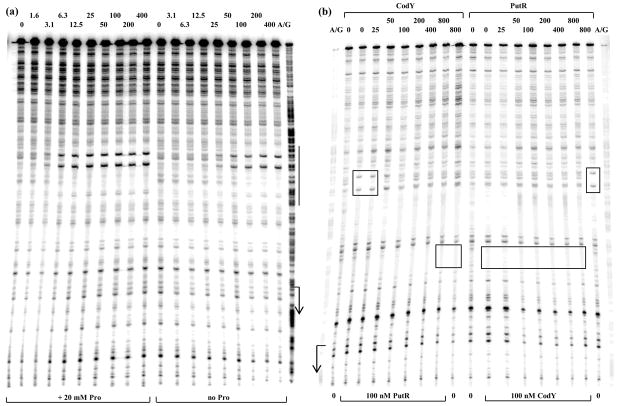
(b) CodY dispaces PutR from the putB regulatory region.
The putB210p+ DNA fragment labeled on the template strand was preincubated for 15 min with or without 100 nM PutR or 100 nM CodY in the presence of 20 mM Pro, 10 mM ILV, and 2 mM GTP, then incubated for additional 15 min with increasing amounts of either CodY or PutR, and finally treated with DNase I. The corresponding A + G sequencing ladder is shown to the left and to the right. The apparent transcription start site and direction of putB transcription are shown by the bent arrow. Parts of the protected areas characteristic for PutR or CodY binding are indicated by rectangles. For PutR, the boxed region corresponds to two hypersensitive sites within a protected region. Protein concentrations (nM of monomer) used in the first and the second stages of the incubation are indicated below or above each lane, respectively.
Competition between CodY and PutR
The CodY- and PutR-binding sites overlap over the region of 24 bp from positions −67 to −45 (Fig. 5C). Thus, a possible mechanism for CodY-mediated repression of the putB promoter would be competition with PutR for DNA binding. Indeed, CodY is able to efficiently displace prebound PutR from its complex with putB DNA (Fig. 6b). Interestingly, the reverse was not the case at the concentrations of CodY and PutR used (Fig. 6b).
The ability of CodY and PutR to compete was confirmed in in vitro transcription experiments (Fig. 4c). Even when the promoter DNA and RNA polymerase were preincubated with PutR, a 2-fold excess of CodY was sufficient to block PutR-dependent transcription almost completely. A 2-fold excess of PutR, on the other hand, was not able to overcome fully repression by prebound CodY, though even at much lower concentration (25 nM) PutR was able to compete with prebound CodY (Fig. 4c).
Though in vitro PutR was not fully competent to displace CodY, addition of Pro to cells pregrown with a mixture of amino acids that lacked Pro and arginine efficiently induced expression of the putB promoter to the level similar to that in cells pregrown with the amino acid mixture and Pro (Fig. 7 and Table 1, strain BB328). Similarly, addition of the amino acid mixture to cells pregrown with Pro led to the apparent repression of the putB promoter and a subsequent decrease of β-galactosidase activity due to cell growth and resulting dilution of enzymatic activity (data not shown).
Fig. 7.
Induction of the CodY-repressed putB promoter. Cells containing the putB210p+-lacZ fusion (strain BB3328) were pregrown in the medium containing the 16-amino acid mixture but without Pro and arginine. The culture was split at the moment indicated by an arrow, and Pro was added to half of the culture. β-Galactosidase activity was expressed in Miller units. Diamonds – no Pro, squares – plus Pro.
Roles of individual CodY-binding motifs in the regulation of putB expression
To determine the extent to which the two CodY-binding motifs contribute to regulation of putB, we constructed lacZ fusions containing mutated versions of the putB regulatory region with single substitutions introduced separately into motif 1 or motif 2 (Table 4). The p2, p4, p7, and p8 down mutations that decreased the similarity of either motif 1 or motif 2 to the CodY-binding consensus led to highly reduced repression by CodY in the 16 amino acid-containing medium (≤2-fold versus 13-fold)(Table 5). In contrast, mutant binding sites with an improved match in either motif (up mutations p1 and p3) caused 8- to 55-fold higher efficiency of putB repression by CodY (Table 5).
Table 5.
Effect of mutations in the CodY-binding motifs on expression of the putB-lacZ fusion
| Strain | Relevant genotype | Fusion type | β-galactosidase activity (MU) | β-galactosidase activity (%)a |
|---|---|---|---|---|
| BB3328 | wild-type | putB210p+ | 13.1 | 7.8 |
| BB3336 | codY | 167.0 | 100 | |
| BB3397 | wild-type | putB210p1up | 0.26 | 0.14 |
| BB3410 | codY | 187.0 | 100 | |
| BB3398 | wild-type | putB210p2down | 220.0 | 69 |
| BB3412 | codY | 319.0 | 100 | |
| BB3512 | wild-type | putB210p3up | 1.85 | 1.0 |
| BB3520 | codY | 177.0 | 100 | |
| BB3513 | wild-type | putB210p4down | 38.1 | 75 |
| BB3521 | codY | 50.9 | 100 | |
| BB3516 | wild-type | putB210p2down/p4down | 149.0 | 101 |
| BB3525 | codY | 148.0 | 100 | |
| BB3561 | wild-type | putB210p7down | 81.0 | 57 |
| BB3567 | codY | 141.0 | 100 | |
| BB3562 | wild-type | putB210p8down | 72.9 | 50 |
| BB3568 | codY | 146.0 | 100 | |
| BB3593 | wild-type | putB210p1up/p8down | 1.24 | 0.73 |
| BB3595 | codY | 171.0 | 100 | |
| BB3594 | wild-type | putB210p2down/p3up | 51.5 | 16 |
| BB3596 | codY | 323.0 | 100 |
Cells were grown in TSS glucose-ammonium medium with Pro and a mixture of 16 aa. β-Galactosidase activity was determined as described in Table 1.
β-Galactosidase activity of each fusion in a strain containing a codY null mutation was normalized to 100%.
We also combined mutations in motifs 1 and 2. The two down mutations, p2 and p4, together decreased the efficiency of putB repression more than either mutation alone, leading to the complete relief of repression (Table 5). On the other hand, a defect in either one of motifs due to down mutations p2 or p8 could be partially compensated by an up mutation in the other motif, p3 or p1, respectively (Table 5).
Small effects of the p2 and p4 down mutations on expression of the putB-lacZ fusion were detected even in the absence of CodY (Table 5). This could be due to their direct effect on either the strength of the promoter or the ability of PutR to activate the promoter. In any case, in gel-shift experiments, these mutations reduced the affinity of CodY for the putB promoter region about 7-fold, confirming that both motif 1 and motif 2 are important for CodY binding (Fig. 2b and c). Deletion of most of motif 1, as in the putB100 construct that leaves only 59 bp upstream of the transcription start point (Figs. 1 and 5c), had an effect on CodY affinity very similar to that of the p4 mutation (Fig. 2d).
Preliminary analysis of the PutR binding site
Deletion of most of the PutR protected region in the putB100-lacZ fusion completely prevented induction of this fusion by Pro (Table 1, strain BB3396) and prevented binding of PutR to putB DNA in vitro (Fig. 3d). Alignment of regulatory regions upstream of the putB gene from seven Bacillus species (all genomes contained the putR gene downstream of putB) revealed several highly conserved regions (Fig. 8). Two of them corresponded to parts of the CodY-binding motifs 1 and 2. Another region corresponded to the upstream part of the PutR binding site identified in this work. A 17-bp conserved sequence with partial dyad symmetry, TTGTGGNAAAMCCACAA, could be discerned in this region, 1 bp upstream of the CodY-binding motif 1. Introduction of a single mutation, p6, in one of the conserved residues at position −82 with respect to the transcription start point (G3 to C within the dyad) almost completely abolished expression from the putB promoter (Table 1, strain BB3335), indicating that the dyad symmetry sequence may be essential for the PutR-mediated activation of transcription.
Fig. 8. Alignment of putB regulatory regions from different Bacillus species.
Nucleotides sequences upstream of and including the translation initiation codons of the putB homologs from seven Bacillus strains were aligned using the CLUSTAL W2.1 software (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Identical nucleotides are indicated by asterisks. Initiation codons and putative −10 and −35 promoter regions of B. subtilis putB are in bold. The CodY-binding motifs are underlined and the dyad-symmetry sequence within the PutR binding site is shown by arrows. The accession numbers for the sequences analyzed are: B. subtilis subsp. subtilis (B.s.sub.) - AL009126.3, B. subtilis BSn5 (B.s.Bsn5) - CP002468.1, B. subtilis subsp. spizizenii (B.s.sp.) - CP002183.1, B. amyloliquefaciens (B.amyl.) -FN597644.1, B. atrophaeus (B.atr.) - CP002207.1, B. licheniformis (B.lich.) - NC_006270, B. pumilus (B.pum.) - NC_009848.1. The coordinates are with respect to the translation initiation codons.
In accord with the expression analysis in vivo, only very poor binding of PutR to the putB210p6 fragment was detected in vitro (Fig. 3c).
DISCUSSION
Multiple mechanisms of gene regulation by CodY
CodY regulates directly or indirectly several hundred B. subtilis genes, most of them negatively2. Direct effects are mediated via CodY binding to regulatory regions of target genes. In all cases of direct negative or positive regulation analyzed until now, CodY binds within or in the immediate vicinity of the promoter regions or at a significant distance (>60 bp) downstream of the transcription start point31; 32 Binding of CodY at sites overlapping with the promoter apparently affects the ability of RNA polymerase to initiate transcription as shown in in vitro experiments13; 33; 34; 35. Binding of CodY to the downstream sites inhibits RNA polymerase progression, i.e., represses transcription by a roadblock mechanism35. Thus, in these cases, CodY acts as a direct regulator of gene expression interfering with the activity of RNA polymerase.
The putB CodY-binding site overlaps the binding site for the operon-specific regulator, PutR, and the role of CodY in putBCP repression is to reduce activation of the operon by competition with PutR. The putB CodY-binding site also overlaps the −35 region and is thus in a perfect location for CodY to prevent binding of RNA polymerase to the promoter or otherwise interfere with initiation of transcription. Indeed, in the absence of PutR, CodY is able to repress residual putB transcription both in vivo (Table 1, strains BB3335 and BB3349) and in vitro (Fig. 4c). However, the PutR-independent putB promoter activity is barely detectable in vitro and is >2,000-fold lower in cells lacking PutR or grown without Pro than in wild-type cells under induced conditions. Therefore, the ability of CodY to interfere directly with RNA polymerase activity is realized only when putB expression is unlikely to have any physiological significance, and the only activity of CodY that matters in wild-type cells is its ability to displace PutR. Thus, CodY, is a direct repressor of some genes but an indirect repressor of putB.
It is likely that most CodY-regulated genes are also regulated by other global or operon-specific regulators. In several cases, CodY-binding sites overlap the binding sites for other regulators, such as the global repressor AbrB36; 37, CcpC, a repressor of the citB and citZ genes36, the carbon metabolism regulator CcpA34, and the competence transcription factor ComK33. However, in the latter two cases, no competition between CodY and the second regulator was observed and only mild competition was suggested between CodY and CcpC. A claim38 that an important aspect of CodY-dependent repression of the ilvB operon is com petition with CcpA, which serves as a positive regulator, is probably incorrect39; 40. Other examples of competition between repressors and activators have been noted in prokaryotes. However, interplay between PutR and CodY appears to be a rare case in which activator displacement is the sole basis for negative regulation (see also Ref.41; 42; 43).
PutR as a Pro-responsive activator
PutR is the second characterized member of the PucR family of transcriptional regulators, COG2508 (http://www.ncbi.nlm.nih.gov/COG/). PucR regulates several B. subtilis transcription units involved in purine utilization24. PucR serves as a positive regulator of some genes and as a negative regulator of others and is autoregulated. PucR and PutR both respond to nitrogen-containing metabolites as their effectors24. The PucR consensus binding site was suggested based on sequence alignment of PucR-regulated genes and extensive mutational analysis of the regulatory region of one of these genes44. There are no obvious similarities between this sequence and the PutR binding site. PutR has no similarity to PutA, the hybrid multifunctional protein that catalyzes both steps of Pro degradation and serves as a positive transcriptional regulator of its own expression in Escherichia coli and other proteobacteria45.
PutR requires Pro as a co-activator. The presence of Pro increases the affinity of PutR for DNA but does not affect the pattern of PutR binding to the putB promoter. It is likely that interaction with Pro also causes a conform ational change in PutR that allows PutR to productively interact with RNA polymerase.
Expression of the putBCP operon was reported to be highly induced in the absence of polynucleotide phosphorylase46 and positively regulated by Spo0A and SpoIIID47; 48. Additionally, a putative binding site for CcpA was identified within the putC gene49. The exact mechanisms for these forms of regulation and their possible interactions with the PutR- and CodY-mediated regulation described here are not known.
Roles of multiple CodY-binding motifs within a single CodY-binding site
In previous work, we have indicated that the CodY-binding sites of several B. subtilis genes, such as dppA and ybgE, are associated with 3 to 4 adjacent or overlapping versions of the CodY-binding motif, AATTTTCWGAAAATT31; 35. Weak motifs with 4 to 6 mismatches with respect to the consensus that overlap with or are adjacent to stronger CodY-binding motifs can be found in the regulatory regions of many other CodY-dependent genes and appear to be a common theme (our unpublished analysis). However, in no case had the functional importance of multiple motifs within the same site been demonstrated experimentally. The putB regulatory region contains two easily recognizable CodY-binding motifs, separated by 2 bp and with 3 or 4 mismatches each, and the location of these motifs exactly corresponds to the actual CodY-binding site as detected by DNase I protection experiments. Both of these motifs are required for regulation and for efficient CodY binding. We hypothesize that each of these sequences is responsible for binding of one CodY dimer to the putB DNA and that binding occurs in the cooperative manner. This model was confirmed by the observation of faster migrating complexes of CodY with some putB DNA fragments that apparently reflects binding of CodY to one of the motifs only (Fig. 2). In any case, the tandem organization of two relatively weak motifs appears to be responsible for the high affinity of CodY bind in to putB DNA. Interestingly, an additional sequence with 5 mismatches to the CodY-binding consensus overlaps putB motif 2 (from positions −40 to −26) and another similar sequence can be found from positions −16 to −2 with respect to the transcription start point.
It should be noted that the apparent interaction of CodY molecules bound to two adjacent motifs within a single site is in contrast to previously characterized independent CodY binding to multiple sites that are separated by more than 50 bp in the bcaP or ybgE regulatory regions32; 35.
It is likely that weak CodY-binding motifs that are adjacent to or overlap with other motifs also serve the purpose of increasing CodY affinity for the regulatory regions of other genes. It is also possible that weaker motifs are required to be in groups of at least two to provide effective binding and regulation by CodY. Recently, experimental data that show the requirement of sequences outside a single 15-bp motif within a CodY-binding site were obtained also for hutP, yufN, and bcaP genes (L. Wray, S. Fisher, submitted )(Belitsky and Sonenshein, submitted).
MATERIALS AND METHODS
Bacterial strains and culture media
The B. subtilis strains constructed in this study were all derivatives of strain SMY50 and are described in Table 6 or in the text and were grown at 37°C in DS nutrient broth medium or TSS minimal medium with 0.5% glucose as the carbon source and 0.2% NH4Cl as nitrogen source51. The same media with addition of agar were used for growth of bacteria on plates. The TSS medium was supplemented as indicated with 2 mg/ml Pro and a mixture of 16 amino acids23. This mixture contained all amino acids commonly found in proteins except for glutamine, asparagine, histidine, and tyrosine; the mixture included isoleucine, leucine, and valine each at 200 μg/ml and Pro at 100 μg/ml (when Pro and 16-amino acid mixture were add ed together, the final concentration of Pro was 2.1 mg/ml). In some experiments ILV were omitted from the amino acid-containing medium (leaving a 13-amino acid mixture) or added separately to TSS. E. coli strain JM10752 was used for isolation of plasmids and was grown in L broth or on L agar plates53. The following antibiotics were used when appropriate: tetracycline, 15 μg/ml; spectinomycin, 50 μg/ml; chloram phenicol, 2.5 μg/ml, or the combination of erythromycin, 0.5 μg/ml, and lincomycin, 12.5 μg/ml, for B. subtilis strains; ampicillin, 50 μg/ml, for E. coli strains.
Table 6.
B. subtilis strains used
| Strain | Genotype | Source or reference |
|---|---|---|
| SMY | prototroph | 50 |
| PS251 | codY::(erm::spc) trpC2 | P. Serror |
| YCGMd | putB::pMutin2 (lacZ erm) trpC2 | NBRPa |
| YCGNd | putC::pMutin2 (lacZ erm) trpC2 | NBRPa |
| YCGOd | putP::pMutin2 (lacZ erm) trpC2 | NBRPa |
| BB1888 | lacA ::tet | 31 |
| BB2511 | ΔamyE::spc lacA ::tet | 31 |
| BB3328 | ΔamyE::[erm Φ (putB210p+-lacZ)] lacA ::tet | BB2511xpBB1662 |
| BB3329 | ΔamyE::[erm Φ (putRp+-lacZ)] lacA ::tet | BB2511xpBB1663 |
| BB3330 | ΔputR::cat | SMYxpBB1669 |
| BB3331 | putB::pMutin2 (lacZ erm) lacA ::tet | BB1888xDNA(YCGMd) |
| BB3332 | putC::pMutin2 (lacZ erm) lacA ::tet | BB1888xDNA(YCGNd) |
| BB3333 | putP::pMutin2 (lacZ erm) lacA ::tet | BB1888xDNA(YCGOd) |
| BB3396 | ΔamyE::[erm Φ (putB100p+-lacZ)] lacA ::tet | BB2511xpBB1671 |
| BB3397 | ΔamyE::[erm Φ (putB210p1-lacZ)] lacA ::tet | BB2511xpBB1672 |
| BB3398 | ΔamyE::[erm Φ (putB210p2-lacZ)] lacA ::tet | BB2511xpBB1673 |
| BB3512 | ΔamyE::[erm Φ (putB210p3-lacZ)] lacA ::tet | BB2511xpBB1708 |
| BB3513 | ΔamyE::[erm Φ (putB210p4-lacZ)] lacA ::tet | BB2511xpBB1709 |
| BB3514 | ΔamyE::[erm Φ (putB210p5-lacZ)] lacA ::tet | BB2511xpBB1710 |
| BB3515 | ΔamyE::[erm Φ (putB210p6-lacZ)] lacA ::tet | BB2511xpBB1711 |
| BB3516 | ΔamyE::[erm Φ (putB210p2/p4-lacZ)] lacA ::tet | BB2511xpBB1712 |
| BB3561 | ΔamyE::[erm Φ (putB210p7-lacZ)] lacA ::tet | BB2511xpBB1715 |
| BB3562 | ΔamyE::[erm Φ (putB210p8-lacZ)] lacA ::tet | BB2511xpBB1716 |
| BB3593 | ΔamyE::[erm Φ (putB210p1/p8-lacZ)] lacA ::tet | BB2511xpBB1721 |
| BB3594 | ΔamyE::[erm Φ (putB210p2/p3-lacZ)] lacA ::tet | BB2511xpBB1722 |
The strains were obtained from the National BioResource Project (NIG, Japan): B. subtilis (http://www.shigen.nig.ac.jp/bsub/).
General molecular genetics methods
Methods for DNA manipulations, transformation, primer extension, gel-shift experiments, DNase I footprinting, and sequence analysis were as previously described31; 32; 54. The G+A sequencing ladder was generated according to a published procedure by boiling the samples for 20 min55. All oligonucleotides used in this work are described in Table 7. Chromosomal DNA of B. subtilis strain SMY or plasmids constructed in this work were used as templates for PCR. All cloned PCR-generated fragments were verified by sequencing.
Table 7.
Oligonucleotides used
| Name | Sequencea | Specificity |
|---|---|---|
| Flanking forward primers | ||
| oBB67 | 5′-GCTTCTAAGTCTTATTTCC | pHK23 |
| oBB480 | 5′-AGAAGTCTAGAAGGATGGAGGAAGC | putB210 |
| oBB482 | 5′-GCTGTTCTAGACCAGTTCAACGAC | putR, 5′-flank |
| oBB484 | 5′-GCAATaAGCTTAGAATTCCATATGAATATGC | putR, 3′-flank |
| oBB488 | 5′-CAAAAATAATCTAGAATCTTTGTATTTT | putB100 |
| oBB506 | 5′-TTTATGAATTCAGGAGATATACCATGGAAGAGCTTTTAG | putR (overexpression) |
| Flanking reverse primers | ||
| oBB102 | 5′-CACCTTTTCCCTATATAAAAGC | pHK23 |
| oBB481 | 5′-TGTGAAAGCTTCCAACCTCAACAC | putB210 |
| oBB483 | 5′-TCAATAAGCTTATCAACATCTG | putR, 5′-flank |
| oBB485 | 5′-ATCAAcTCGAGATAGAGCTGG | putR, 3′-flank |
| oBB507 | 5′-TGGTTTCTAGATTAATGGTGATGGTGATGGTGTTTCTTTTTCATCAGC | putR (overexpression) |
| Internal mutagenic forward primers | ||
| oBB490 | 5′-CTTTGTATTTTCAGAATATTGTG | putB210p1 |
| oBB492 | 5′-GTATTTTGAGcATATTGTGAAC | putB210p2 |
| oBB549 | 5′-CAGAATATTTGTATTTTGAG | putB210p3 |
| oBB551 | 5′-GGCAAACCCACAAAAATAATCAGcATCTTTG | putB210p4 |
| oBB555 | 5′-AACTATCTGATTcTGGCAAACCC | putB210p6 |
| oBB562 | 5′-GTATTTTGAcAATATTGTGAACGC | putB210p7 |
| oBB564 | 5′-GGCAAACCCACAAAAATAATgAGAATCTTTG | putB210p8 |
| Internal mutagenic reverse primers | ||
| oBB489 | 5′-CACAATATTCTGAAAATACAAAG | putB210p1 |
| oBB491 | 5′-CACAATATgCTCAAAATACAAAG | putB210p2 |
| oBB548 | 5′-CTCAAAATACAAATATTCTG | putB210p3 |
| oBB550 | 5′-CAAAATACAAAGATgCTGATTATTTTTG | putB210p4 |
| oBB554 | 5′-GGGTTTGCCAgAATCAGATAG | putB210p6 |
| oBB561 | 5′-CACAATATTgTCAAAATACAAAG | putB210p7 |
| oBB563 | 5′-ACAAAGATTCTcATTATTTTTGTGG | putB210p8 |
The altered nucleotides are in boldface; those conferring an up mutation are in uppercase, those conferring down mutations are in lowercase. The restriction sites and His codons are underlined.
Construction of the putR null mutant
A deletion-insertion mutation within the putR gene was constructed by inserting the 1.14-kb cat-cassette, excised from pJPM1256 with Hind III and Eco RI, between two fragments flanking the putR gene in the chromosome that were synthesized by PCR (see Table 7 for oligonucleotides used) and cloned in the integrative plasmid pBB544 (Neor)57. The orientation of the cat gene in the resulting ΔputR::cat plasmid, pBB1669, is opposite that of the putR gene. pBB1669 was introduced into B. subtilis SMY, and Catr Neos transform ants, arising from double-crossover homologous recombination events, were selected. The replacement of the chromosomal putR gene by the ΔputR::cat allele in strain BB3330 was confirmed by comparing sizes of the PCR fragments from the wild-type and mutant putR chromosomal loci.
Construction of transcriptional lacZ fusions
Plasmid pBB1662 carrying the putB210p+-lacZ fusion was constructed by cloning a 210-bp PCR fragment (see Table 7) containing the entire regulatory region upstream of the putB gene between the XbaI and HindIII sites of the integrative plasmid pHK2331. Plasmid pBB1663 (putRp+-lacZ) carrying a 242-bp PCR fragment containing the entire intergenic region between putP and putR was constructed in a manner similar to pBB1662. Plasmid pBB1671 (putB100p+-lacZ) carrying a version of the putB regulatory region truncated at the 5′-end was constructed in a manner similar to pBB1662 using a 100-bp PCR product (see Table 7).
B. subtilis strains carrying various lacZ fusions at the amyE locus (Table 6) were isolated after transforming strain BB2511 (amyE::spc lacA) with the appropriate plasmids, selecting for resistance to erythromycin conferred by the plasmids, and screening for loss of the spectinomycin-resistance phenotype, which indicated a double crossover, homologous recombination event. Strain BB2511 and all its derivatives have very low endogenous β-galactosidase activity due to a null mutation in the lacA gene58.
Mutations in the CodY-binding and PutR-binding sites and the putB promoter
Mutations in the 210-bp putB regulatory region were introduced by two-step overlapping PCR. In the first step, PCR products containing the 5′-part of the putB regulatory region were synthesized by using oligonucleotide oBB480 as the forward primer and mutagenic oligonucleotides specified in Table 7 as the reverse primer. In a similar manner, PCR products containing the 3′-part of the putB regulatory region were synthesized by using mutagenic oligonucleotides specified in Table 7 as the forward primer and oligonucleotide oBB481 as the reverse primer. In the case of the double mutations p2/p4, p2/p3, and p1/p8, plasmid pBB1673 (putBp2-lacZ) or pBB1672 (putBp1-lacZ) was used as template for PCR. The appropriate pairs of PCR products were used in a second, splicing step of PCR mutagenesis as overlapping templates to generate modified fragments containing the entire putB regulatory region; oligonucleotides oBB480 and oBB481 served as the forward and reverse PCR primers, respectively.
The spliced PCR products were digested with XbaI and HindIII and cloned in pH K23, as described above, to construct pBB1672 (putB210p1-lacZ), pBB1673 (putB210p2-lacZ), pBB1708 (putB210p3-lacZ), pBB1709 (putB210p4-lacZ), pBB1711 (putB210p6-lacZ), pBB1712 (putB210p2/p4-lacZ), pBB1714 (putB210p7-lacZ), pBB1715 (putB210p8-lacZ), pBB1721 (putB210p1/p8-lacZ), and pBB1722 (putB210p2/p3-lacZ).
Purification of CodY
CodY-His5 was purified to near homogeneity as described previously31.
Overexpression and purification of PutR
The 1.27-kb modified putR gene containing a 6xHis codontag at the 3′-terminus was synthesized by PCR (see Table 7) and cloned in the expression vector pBAD18 between the EcoRI and XbaI sites59. The resulting plasmid, pBB1683, was introduced in strain LMG19459 and expression of PutR was induced in L broth (at A600 = 0.3) by addition of 0.2% L-arabinose and incubation for an additional 4 hours. PutR-His6 was purified to more than 80% purity as described previously for CodY-His531. Elution from the Ni2+-affinity column (His·Bind resin; Novagen) was with a buffer containing 385 mM imidazole.
PutR-His6, if expressed in B. subtlis cells from a modified putR gene integrated at the putR locus, had activity indistinguishable from that of the wild-type PutR (data not shown).
Labeling of DNA fragments
The PCR products containing the regulatory regions of the putB and putR genes were synthesized using vector-specific oligonucleotide oBB67 as the forward primer and oligonucleotide oBB102 as the reverse primer and plasmids containing relevant putB-lacZ or putR-lacZ fusions as template. oBB102 (which would prime synthesis of the template strand of the PCR product) was labeled using T4 polynucleotidekinase and [γ-32P]-ATP. oBB67 starts 96 bp upstream of the XbaI site used for cloning, and oBB102 starts 36 bp downstream of the HindIII site that serves as a junction between the promoters and the lacZ part of the fusions.
In vitro transcription
Experiments were performed as described in35 and in Figure legends in the presence of various amounts of PutR and CodY. His10-tagged RNAP and the unmodified σA factor were purified from B. subtilis cells as described60; 61. 20 mM Pro and 10 mM ILV were added to the reactions when specified. A 349-bp putB promoter fragment, synthesized using pBB1662 as a PCR template and oligonucleotides oBB67 and oBB102 as primers, was used as template for in vitro transcription.
Enzyme assays
β-Galactosidase specific activity was determined as described previously62.
Acknowledgments
I am grateful to A. L. Sonenshein for encouragement, helpful discussions, and careful reading of the manuscript, S. Picossi for purification of B. subtilis RNA polymerase and the σA factor, E. Bremer for useful discussions, and the National BioResource Project (NIG, Japan): B. subtilis for the gift of strains. This work was supported by a research grant (GM042219) from the U. S. Public Health Service to A. L. Sonenshein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Slack FJ, Serror P, Joyce E, Sonenshein AL. A gene required for nutritional repression of the Bacillus subtilis dipeptide permease operon. Mol Microbiol. 1995;15:689–702. doi: 10.1111/j.1365-2958.1995.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 2.Molle V, Nakaura Y, Shivers RP, Yamaguchi H, Losick R, Fujita Y, Sonenshein AL. Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. J Bacteriol. 2003;185:1911–1922. doi: 10.1128/JB.185.6.1911-1922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonenshein AL. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr Opin Microbiol. 2005;8:203–207. doi: 10.1016/j.mib.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Sonenshein AL. Control of key metabolic intersections in Bacillus subtilis. Nat Rev Microbiol. 2007;5:917–927. doi: 10.1038/nrmicro1772. [DOI] [PubMed] [Google Scholar]
- 5.Fisher SH. Regulation of nitrogen metabolism in Bacillus subtilis: vive la difference! Mol Microbiol. 1999;32:223–232. doi: 10.1046/j.1365-2958.1999.01333.x. [DOI] [PubMed] [Google Scholar]
- 6.Dineen SS, McBride SM, Sonenshein AL. Integration of metabolism and virulence by Clostridium difficile CodY. J Bacteriol. 2010;192:5350–5362. doi: 10.1128/JB.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majerczyk CD, Dunman PM, Luong TT, Lee CY, Sadykov MR, Somerville GA, Bodi K, Sonenshein AL. Direct targets of CodY in Staphylococcus aureus. J Bacteriol. 2010;192:2861–2877. doi: 10.1128/JB.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stenz L, Francois P, Whiteson K, Wolz C, Linder P, Schrenzel J. The CodY pleiotropic repressor controls virulence in Gram-positive pathogens. FEMS Immunol Med Microbiol. 2011;62:123–139. doi: 10.1111/j.1574-695X.2011.00812.x. [DOI] [PubMed] [Google Scholar]
- 9.Guedon E, Serror P, Ehrlich SD, Renault P, Delorme C. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol Microbiol. 2001;40:1227–1239. doi: 10.1046/j.1365-2958.2001.02470.x. [DOI] [PubMed] [Google Scholar]
- 10.Petranovic D, Guedon E, Sperandio B, Delorme C, Ehrlich D, Renault P. Intracellular effectors regulating the activity of the Lactococcus lactis CodY pleiotropic transcription regulator. Mol Microbiol. 2004;53:613–621. doi: 10.1111/j.1365-2958.2004.04136.x. [DOI] [PubMed] [Google Scholar]
- 11.Shivers RP, Sonenshein AL. Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Mol Microbiol. 2004;53:599–611. doi: 10.1111/j.1365-2958.2004.04135.x. [DOI] [PubMed] [Google Scholar]
- 12.Levdikov VM, Blagova E, Joseph P, Sonenshein AL, Wilkinson AJ. The structure of CodY, a GTP- and isoleucine-responsive regulator of stationary phase and virulence in gram-positive bacteria. J Biol Chem. 2006;281:11366–11373. doi: 10.1074/jbc.M513015200. [DOI] [PubMed] [Google Scholar]
- 13.Ratnayake-Lecamwasam M, Serror P, Wong KW, Sonenshein AL. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev. 2001;15:1093–1103. doi: 10.1101/gad.874201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handke LD, Shivers RP, Sonenshein AL. Interaction of Bacillus subtilis CodY with GTP. J Bacteriol. 2008;190:798–806. doi: 10.1128/JB.01115-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slack FJ, Mueller JP, Sonenshein AL. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J Bacteriol. 1993;175:4605–4614. doi: 10.1128/jb.175.15.4605-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher SH, Rohrer K, Ferson AE. Role of CodY in regulation of the Bacillus subtilis hut operon. J Bacteriol. 1996;178:3779–3784. doi: 10.1128/jb.178.13.3779-3784.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belitsky BR, Wray LV, Jr, Fisher SH, Bohannon DE, Sonenshein AL. Role of TnrA in nitrogen source-dependent repression of Bacillus subtilis glutamate synthase gene expression. J Bacteriol. 2000;182:5939–5947. doi: 10.1128/jb.182.21.5939-5947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belitsky BR, Sonenshein AL. Modulation of activity of Bacillus subtilis regulatory proteins GltC and TnrA by glutamate dehydrogenase. J Bacteriol. 2004;186:3399–3407. doi: 10.1128/JB.186.11.3399-3407.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardan R, Rapoport G, Debarbouille M. Role of the transcriptional activator RocR in the arginine-degradation pathway of Bacillus subtilis. Mol Microbiol. 1997;24:825–837. doi: 10.1046/j.1365-2958.1997.3881754.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang TC, Huang YW, Hung HJ, Ho CT, Wu ML. Delta 1-pyrroline-5-carboxylic acid formed by proline dehydrogenase from the Bacillus subtilis ssp. nat to expressed in Escherichia coli as a precursor for 2-acetyl-1-pyrroline. J Agric Food Chem. 2007;55:5097–5102. doi: 10.1021/jf0700576. [DOI] [PubMed] [Google Scholar]
- 21.von Blohn C, Kempf B, Kappes RM, Bremer E. Osmostress response in Bacillus subtilis: characterization of a proline up take system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma B. Mol Microbiol. 1997;25:175–187. doi: 10.1046/j.1365-2958.1997.4441809.x. [DOI] [PubMed] [Google Scholar]
- 22.Bremer E. Adaptation to changing osmolarity. In: Sonenshein AL, Hoch JA, Losick R, editors. Bacillus subtilis and its closeset relatives: from genes to cells. American Society for Microbiology; Washington, D.C: 2002. pp. 385–391. [Google Scholar]
- 23.Atkinson MR, Wray LV, Jr, Fisher SH. Regulation of histidine and proline degradation enzymes by amino acid availability in Bacillus subtilis. J Bacteriol. 1990;172:4758–4765. doi: 10.1128/jb.172.9.4758-4765.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultz AC, Nygaard P, Saxild HH. Functional analysis of 14 genes that constitute the purine catabolic pathway in Bacillus subtilis and evidence for a novel regulon controlled by the PucR transcription activator. J Bacteriol. 2001;183:3293–3302. doi: 10.1128/JB.183.11.3293-3302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vagner V, Dervyn E, Ehrlich SD. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 1998;144:3097–3104. doi: 10.1099/00221287-144-11-3097. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi K, Ehrlich SD, Albertini A, Amati G, Andersen KK, Arnaud M, Asai K, Ashikaga S, Aymerich S, Bessieres P, Boland F, Brignell SC, Bron S, Bunai K, Chapuis J, Christiansen LC, Danchin A, Debarbouille M, Dervyn E, Deuerling E, Devine K, Devine SK, Dreesen O, Errington J, Fillinger S, Foster SJ, Fujita Y, Galizzi A, Gardan R, Eschevins C, Fukushima T, Haga K, Harwood CR, Hesker M, Hosoya D, Hullo MF, Kakeshita H, Karamata D, Kasahara Y, Kawamura F, Koga K, Koski P, Kuwana R, Imamura D, Ishimaru M, Ishikawa S, Ishio I, Le Coq D, Masson A, Mauel C, Meima R, Mellado RP, Moir A, Moriya S, Nagakawa E, Nanamiya H, Nakai S, Nygaard P, Ogura M, Ohanan T, O’Reilly M, O’Rourke M, Pragai Z, Pooley HM, Rapoport G, Rawlins JP, Rivas LA, Rivolta C, Sadaie A, Sadaie Y, Sarvas M, Sato T, Saxild HH, Scanlan E, Schumann W, Seegers JF, Sekiguchi J, Sekowska A, Seror SJ, Simon M, Stragier P, Studer R, Takamatsu H, Tanaka T, Takeuchi M, Thomaides HB, Vagner V, van Dijl JM, Watabe K, Wipat A, Yamamoto H, Yamamoto M, Yamamoto Y, Yamane K, Yata K, Yoshida K, Yoshikawa H, Zuber U, Ogasawara N. Essential Bacillus subtilis genes. Proc Natl Acad Sci U S A. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wray LV, Jr, Fisher SH. Analysis of Bacillus subtilis hut operon expression indicates that histidine-dependent induction is mediated primarily by transcriptional antitermination and that amino acid repression is mediated by two mechanism s: regulation of transcription initiation and inhibition of histidine transport. J Bacteriol. 1994;176:5466–5473. doi: 10.1128/jb.176.17.5466-5473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whatmore AM, Chudek JA, Reed RH. The effects of osmotic up shock on the intracellular solute pools of Bacillus subtilis. J Gen Microbiol. 1990;136:2527–2535. doi: 10.1099/00221287-136-12-2527. [DOI] [PubMed] [Google Scholar]
- 29.Guedon E, Sperandio B, Pons N, Ehrlich SD, Renault P. Overall control of nitrogen metabolism in Lactococcus lactis by CodY, and possible models for CodY regulation in Firmicutes. Microbiology. 2005;151:3895–3909. doi: 10.1099/mic.0.28186-0. [DOI] [PubMed] [Google Scholar]
- 30.den Hengst CD, van Hijum SA, Geurts JM, Nauta A, Kok J, Kuipers OP. The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J Biol Chem. 2005;280:34332–34342. doi: 10.1074/jbc.M502349200. [DOI] [PubMed] [Google Scholar]
- 31.Belitsky BR, Sonenshein AL. Genetic and biochemical analysis of CodY-binding sites in Bacillus subtilis. J Bacteriol. 2008;190:1224–1236. doi: 10.1128/JB.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belitsky BR, Sonenshein AL. Contributions of multiple binding sites and effector-independent binding to CodY-mediated regulation in Bacillus subtilis. J Bacteriol. 2011;193:473–484. doi: 10.1128/JB.01151-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smits WK, Hoa TT, Hamoen LW, Kuipers OP, Dubnau D. Antirepression as a second mechanism of transcriptional activation by a minor groove binding protein. Mol Microbiol. 2007;64:368–381. doi: 10.1111/j.1365-2958.2007.05662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shivers RP, Dineen SS, Sonenshein AL. Positive regulation of Bacillus subtilis ackA by CodY and CcpA: establishing a potential hierarchy in carbon flow. Mol Microbiol. 2006;62:811–822. doi: 10.1111/j.1365-2958.2006.05410.x. [DOI] [PubMed] [Google Scholar]
- 35.Belitsky BR, Sonenshein AL. Road block repression of transcription by Bacillus subtilis CodY. J Mol Biol. 2011 doi: 10.1016/j.jmb.2011.06.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HJ, Kim SI, Ratnayake-Lecamwasam M, Tachikawa K, Sonenshein AL, Strauch M. Complex regulation of the Bacillus subtilis aconitase gene. J Bacteriol. 2003;185:1672–1680. doi: 10.1128/JB.185.5.1672-1680.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serror P, Sonenshein AL. Interaction of CodY, a novel Bacillus subtilis DNA-binding protein, with the dpp promoter region. Mol Microbiol. 1996;20:843–852. doi: 10.1111/j.1365-2958.1996.tb02522.x. [DOI] [PubMed] [Google Scholar]
- 38.Shivers RP, Sonenshein AL. Bacillus subtilis ilvB operon: an intersection of global regulons. Mol Microbiol. 2005;56:1549–1559. doi: 10.1111/j.1365-2958.2005.04634.x. [DOI] [PubMed] [Google Scholar]
- 39.Tojo S, Satomura T, Morisaki K, Deutscher J, Hirooka K, Fujita Y. Elaborate transcription regulation of the Bacillus subtilis ilv-leu operon involved in the biosynthesis of branched-chain amino acids through global regulators of CcpA, CodY and TnrA. Mol Microbiol. 2005;56:1560–1573. doi: 10.1111/j.1365-2958.2005.04635.x. [DOI] [PubMed] [Google Scholar]
- 40.Brinsmade SR, Kleijn RJ, Sauer U, Sonenshein AL. Regulation of CodY activity through modulation of intracellular branched-chain amino acid pools. J Bacteriol. 2010;192:6357–6368. doi: 10.1128/JB.00937-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nasser W, Robert-Baudouy J, Reverchon S. Antagonistic effect of CRP and KdgR in the transcription control of the Erwinia chrysanthemi pectinolysis genes. Mol Microbiol. 1997;26:1071–1082. doi: 10.1046/j.1365-2958.1997.6472020.x. [DOI] [PubMed] [Google Scholar]
- 42.Vitale E, Milani A, Renzi F, Galli E, Rescalli E, de Lorenzo V, Bertoni G. Transcriptional wiring of the TOL plasmid regulatory network to its host involves the submission of the sigma54-promoter Pu to the response regulator Para. Mold Microbiol. 2008;69:698–713. doi: 10.1111/j.1365-2958.2008.06321.x. [DOI] [PubMed] [Google Scholar]
- 43.Ishida Y, Kori A, Ishihama A. Participation of regulator AscG of the beta-glucoside utilization operon in regulation of the propionate catabolism operon. J Bacteriol. 2009;191:6136–6144. doi: 10.1128/JB.00663-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beier L, Nygaard P, Jarmer H, Saxild HH. Transcription analysis of the Bacillus subtilis PucR regulon and identification of a cis-acting sequence required for PucR-regulated expression of genes involved in purine catabolism. J Bacteriol. 2002;184:3232–3241. doi: 10.1128/JB.184.12.3232-3241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Zhu W, Bellur PS, Rewinkel D, Becker DF. Direct linking of metabolism and gene expression in the proline utilization A protein from Escherichia coli. Amino Acids. 2008;35:711–718. doi: 10.1007/s00726-008-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deikus G, Babitzke P, Bechhofer DH. Recycling of a regulatory protein by degradation of the RNA to which it binds. Proc Natl Acad Sic U S A. 2004;101:2747–2751. doi: 10.1073/pnas.0307343101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molle V, Fujita M, Jensen ST, Eichenberger P, Gonzalez-Pastor JE, Liu JS, Losick R. The Spo0A regulon of Bacillus subtilis. Mol Microbiol. 2003;50:1683–1701. doi: 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- 48.Eichenberger P, Fujita M, Jensen ST, Conlon EM, Rudner DZ, Wang ST, Ferguson C, Haga K, Sato T, Liu JS, Losick R. The program of gene transcription for a single differentiating cell type during sporulation in Bacillus subtilis. PLoS Biol. 2004;2:e328. doi: 10.1371/journal.pbio.0020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lulko AT, Buist G, Kok J, Kuipers OP. Transcriptome analysis of temporal regulation of carbon metabolism by CcpA in Bacillus subtilis reveals additional target genes. J Mol Microbiol Biotechnol. 2007;12:82–95. doi: 10.1159/000096463. [DOI] [PubMed] [Google Scholar]
- 50.Zeigler DR, Pragai Z, Rodriguez S, Chevreux B, Muffler A, Albert T, Bai R, Wyss M, Perkins JB. The origins of 168, W23, and other Bacillus subtilis legacy strains. J Bacteriol. 2008;190:6983–6995. doi: 10.1128/JB.00722-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fouet A, Sonenshein AL. A target for carbon source-dependent negative regulation of the citB promoter of Bacillus subtilis. J Bacteriol. 1990;172:835–844. doi: 10.1128/jb.172.2.835-844.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 53.Miller JH. Experiments in molecular genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1972. [Google Scholar]
- 54.Sambrook J, Fritsch EF, Maniatis TJ. Molecular cloning: a laboratory manual. 2. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1989. [Google Scholar]
- 55.Liu ST, Hong GF. Three-minute G + A specific reaction for DNA sequencing. Anal Biochem. 1998;255:158–159. doi: 10.1006/abio.1997.2457. [DOI] [PubMed] [Google Scholar]
- 56.Mueller JP, Bukusoglu G, Sonenshein AL. Transcript ional regulation of Bacillus subtilis glucose starvation-inducible genes: control of gsiA by the ComP-ComA signal transduction system. J Bacteriol. 1992;174:4361–4373. doi: 10.1128/jb.174.13.4361-4373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belitsky BR, Gustafsson MC, Sonenshein AL, Von Wachenfeldt C. An lrp-like gene of Bacillus subtilis involved in branched-chain amino acid transport. J Bacteriol. 1997;179:5448–5457. doi: 10.1128/jb.179.17.5448-5457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daniel RA, Haiech J, Denizot F, Errington J. Isolation and characterization of the lacA gene encoding beta-galactosidase in Bacillus subtilis and a regulator gene, lacR. J Bacteriol. 1997;179:5636–5638. doi: 10.1128/jb.179.17.5636-5638.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qi Y, Hulett FM. PhoP-P and RNA polymerase sigmaA holoenzyme are sufficient for transcription of Pho regulon promoters in Bacillus subtilis: PhoP-P activator sites within the coding region stimulate transcription in vitro. Mol Microbiol. 1998;28:1187–1197. doi: 10.1046/j.1365-2958.1998.00882.x. [DOI] [PubMed] [Google Scholar]
- 61.Helmann JD. Purification of Bacillus subtilis RNA polymerase and associated factors. Methods Enzymol. 2003;370:10–24. doi: 10.1016/S0076-6879(03)70002-0. [DOI] [PubMed] [Google Scholar]
- 62.Belitsky BR, Sonenshein AL. Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J Bacteriol. 1998;180:6298–6305. doi: 10.1128/jb.180.23.6298-6305.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]