Abstract
Background
Antiretroviral therapy access with successful outcomes for children is expanding in resource limited countries. The aim of this study was to determine treatment responses of children in a routine setting where first line therapy with lopinavir/ritonavir is routinely included for young children.
Methods
Outpatient records of children who initiated ART between April 2004 and March 2008 at a government clinic in Soweto were reviewed. Children <3 years initiated ART with lopinavir/ritonavir- and those ≥ 3 years efavirenz-containing regimens
Results
ART was initiated at median age 4.3 years, 28.6% also received TB treatment. During 3155 child-years of follow-up (median follow-up 17 months), 132 (6%) children died giving a mortality rate of 4.2 (95%CI: 3.5, 5.0) deaths per 100 child-years. By 12 and 24 months, 84% and 96% of children achieved virologic suppression. The proportion of children with viral rebound rose from 5.4% to 16.3% at 24 and 36 months from start of ART. Younger children (receiving lopinavir/ritonavir-based first-line therapy) with higher viral loads, suppressed more slowly and were more likely to die. Children given TB treatment at the time of viral suppression were more likely to have virologic rebound.
Conclusion
Despite good treatment outcomes overall, children with advanced disease at ART initiation had poorer outcomes, particularly those < 3 years of age, most of whom were treated with LPV/r-containing therapy. The increasing risk of viral rebound over time for the whole cohort is concerning given currently limited available treatment options for children.
Keywords: Children, HIV treatment outcomes, South Africa
INTRODUCTION
More than 2 million children 15 years or younger live with the human immunodeficiency virus (HIV) worldwide, most of these in Southern Africa.(1) By 2009 about 360,000 children were receiving antiretroviral therapy (ART).(2) South Africa has more than 5 million HIV-infected people,(1) of whom it is estimated that more than 250 000 are children and approximately 54% of those in need to receive treatment. While the gap between those on treatment and those in need is closing, monitoring therapeutic responses as a measure of quality of care is essential to guide programs.
Standard of care for children in lower middle income countries (LMIC) has been to receive non-nucleoside transcriptase inhibitor-based (NNRTI) therapy. WHO guidelines now recommend that protease inhibitor (PI),usually lopinavir/ritonavir (LPV/r)-based ART be started as first-line treatment for children less than 2 years of age who have been exposed to nevirapine for prevention of mother-to-child HIV transmission (PMTCT).(3) Recent evidence from IMPAACT P1060 reinforces that LPV/r-based treatment is more potent than nevirapine (NVP) after NVP exposure for PMTCT.(4) Reports on outcomes of children receiving ART in LMIC demonstrate favorable immunologic and growth outcomes, (5) and pooled data estimate viral suppression rates of about 70% by 12 months on ART. (6) However, there are few reports from large pediatric cohorts accessing treatment at facilities where LPV/r-based therapy is routinely used in young children as first-line treatment, and where routine viral load monitoring occurs facilitating reporting on virologic response rates over time.
The aim of this study was to assess treatment outcomes including virologic, immunologic and clinical responses to ART among the children receiving routine clinical care who initiated treatment in the Harriet Shezi Children’s clinic (HSCC), an outpatient pediatric HIV clinic at Chris Hani Baragwanath Hospital (CHBH) in Soweto, South Africa from April 2004 through March 2008.
METHODS
Site, clinical procedures and data collection
The HSCC is a routine government-funded public service outpatient facility partially supported by private grant funding. Medical care, drugs and laboratory testing (including viral load testing) are provided at no cost to patients. Most children access care at HSCC after referral from the pediatric wards of CHBH although referralsfrom other sites and services occur. Routine medical care includes a clinical assessment at the initial visit, determination of clinical stageusing World Health Organization (WHO) criteria, which transitioned from a three stage to a four stage system (with Stage III and IV indicating moderate to severe disease) in late 2005.(7) ART was initiated in children who met the local criteria (an adaptation from the WHO guidelines for ART in children)(8) which at the time included; recurrent (>2 admissions per year) or prolonged (> 4 weeks) hospitalization, WHO clinical stage III&IV, or CD4 percentage (CD4%) <20% in children under 18 months and <15% for older children. The first-line regimen consisted of stavudine, lamivudine, and LPV/r for children younger than 3 years of age or stavudine, lamivudine and efavirenz (EFV) for those over 3 years or over 10kg of weight. Abacavir was available for single drug substitution for suspected stavudine toxicity, and second-line regimens which recommended didanosine, zidovudine with either LPV/r or NVP/EFV depending on age and prior regimen, were available for children failing first-line therapy. Virologic suppression was defined as viral load <400 copies/ml after ART initiation in children who had at least one viral load subsequent to the start of ART. Viral rebound was defined as viral load >1000 copies/ml on two subsequent occasions after viral suppression had occurred.
Tuberculosis (TB) was diagnosed and treated if there was bacteriological evidence from body fluids or a positive PPD >5mm in a child <5 years of age.(9). Clinical signs including; severe failure to thrive, prolonged (more than two weeks) cough, suspicious chest radiograph with or without a positive contact history or bacteriological diagnosis, often formed the basis of TB diagnosis. Children presenting with one or more of these features were usually commenced on TB treatment. Standard treatment for TB included rifampin (Rif), isoniazid (INH) and pyrazinamide for 2 months, and INH and Rif continued for a further 4 months. Complicated TB was treated for longer periods. For children less than 3 years or receiving a LPV/r-based regimen, clinical practice for TB co-treated children was to increase the dose of ritonavir (super-boosted LPV/r) or to double the LPV/r dose.(10) The dose of EFV for children taking EFV-based therapy with TB treatment was not modified.
Children started on ART were clinically reevaluated after 1 month and then 3-monthly, monitoring blood tests (CD4, viral load, complete blood counts, alanine-aminotransferase) were performed pre-treatment and 6-monthly or when clinically needed. The pharmacy provided a maximum of 2 months supply of medication at a time, pharmacy visits were not always coordinated with clinical visits. Treatment readiness and adherence counseling was conducted with patients on an individual basis by members of a team of experienced counselors. An important eligibility criterion for ART initiation was to have at least one caregiver able to supervise drug administration to the child. Prior to ART initiation, disclosure to another adult living in the same house who could help with the child’s ART was encouraged. Although patients had to check in with counselors at all visits and adherence was queried, there was no consistent system for assessing adherence. Nevertheless intensive counseling and home visits were conducted where poor adherence was detected
Laboratory tests were conducted at the central laboratory by the National Health Laboratory Services. HIV diagnosis was based on two HIV antibody tests for children 18 months or older or a single polymerasechain reaction (PCR) test to detect HIV-1 proviral DNA for younger children. Plasma viral load was initially measured using the AMPLICOR HIV-1 MONITOR Test, v1.5 (Roche) with lower limits of detection at 400 RNA copies/ml and the upper bound at 750,000 RNA copies/ml and subsequently a NASBA based viral load assay (Nuclisens EasyQ, Biomerieux) with lower and upper limits of detection at 25 and 3 million RNA copies/ml. CD4 cell counts and percents were measured using flow cytometry (Beckman Coulter). (11)
To track program performance indicators and participant appointment-keeping, an electronic database was developed for the clinic. At the end of each clinic day, an on-site team of data capturers entered designated elementsfrom standardized clinical forms into the database. A list of patients who missed clinic appointments was generated from this database and given to a full-time on-site defaulter tracer who contacted them by phone (about 75% of patients have a phone). For patients who could not be contacted by phone a home visit was scheduled. The outcome of the tracing and reasons for defaulting were recorded in the electronic record.
Main outcomes
The main outcomes for this analysis include: (1) mortality, (2) virologic suppression defined as viral load below 400 RNA copies/ml, (3) virologic rebound defined as having at least 2 viral load measurements above 1000 RNA copies/ml after initial viral suppression, (4) CD4 cell counts and percents, and (5) weight-, and height-for-age z scores. Gender-specific weight-for-age Z-score (WAZ) and height-for-age Z-scores (HAZ) were calculated based on WHO growth charts for children younger than 5 years and on US Centers for Disease Control and Prevention (CDC) charts for children 5 years or older, because WHO charts were not yet available for older children at the time this analysis was initiated. (12, 13)
Statistical analysis
We calculated mortality rates within the first 90 days, and thereafter,(early and late mortality respectively) consistent with prior publications (14–16). Kaplan Meier life-tables were used to estimate the cumulative probability of attaining a viral load <400 copies/ml with follow-time calculated from initiation of therapy, or the cumulative probability of having virologic rebound after achieving viral suppression. The follow-up time was censored at loss to follow-up, death, last clinic visit, or at 36 months, whichever occurred first for all time-to-event analyses.
For immunologic and growth response, we modeled the change in the means of CD4, CD4%, WAZ, and HAZ over time since ART initiation using mixed models with maximum likelihood estimation.(17) Fixed effect for time from ART initiation to repeat measurement of CD4 cell count, weight, or height was included in the model as well as a random intercept for each child. To account for the anticipated nonlinear effects, polynomials for time to measurement were also included as fixed effects and their contribution to the model evaluated using the likelihood ratio test from the nested models. Fixed effect of age at ART initiation and interactions terms with time from ART initiation to repeat measurement were also included in the model and their contribution to the model fit assessed. The estimated means were obtained and plotted over time since ART initiation.
Cox proportional hazard models were used to estimate the crude and adjusted Hazard Ratios (HR) of the association of each baseline characteristic and each of the 3 time-to-event outcomes. To assess whether these associations differed by the initial ART regimen, stratified analyses were conducted for children 3 years or younger at ART initiation (LPV/r regimen) and for those older (efavirenz regimen). The proportional hazards assumption for each of the covariates included in each of the models was formally evaluated using the Kolmogorov-type supremum test.(18) All analyses were conducted using SAS 9.1 (SAS Institute, Cary, NC) and all tests were conducted using a 2-sided 0.05 significance level, without correction for multiple comparisons and model selection.
RESULTS
Description of the cohort
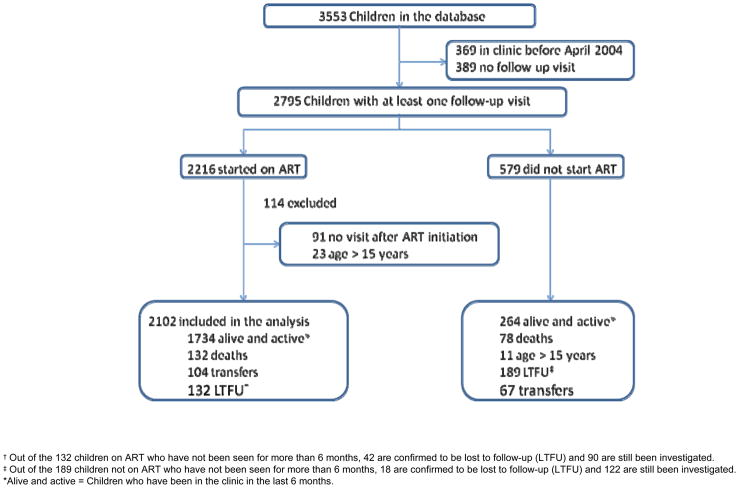
As of March 31st 2008, 3553 HIV-infected children had attended HSCC, 369 before April 2004 and 389 had no follow-up visit after their initial visit. Of the remaining 2795, 2216 ever initiated ART and 2102 were younger than 15 years (figure 1).
Figure 1.
Description of the cohort of HIV infected children included in the HSCC’s database
At ART initiation, 24% (495) were younger than 18 months, 16% (337), and 17% (347) between 18 to 35 months, 36 to 59 months, respectively. The median age was 4.27 [interquartile range (IQR): 1.61 to 7.45] years (see table, supplemental digital content 1 which illustrates characteristics of HIV-infected children 15 or younger years at the time of Antiretroviral Therapy initiation at HSCC (April, 2004 – March, 2008).. Fifty one percent (1068) of children were male, 78% (1402) had WHO clinical stage III or IV disease, and 29% (602) were on TB treatment. Median CD4% was 11.45% and most were severely underweight for age: 31% (601) with WAZ score below −3 standard deviations (SD) and 50% HAZ score below −2.6. More than half of children 18 months or younger had WAZ- and HAZ-scores below −3 SD. The median time from the first visit at the clinic to ART initiation was 6 weeks (IQR: 2 to 15) and the median follow-up time on ART was 17 months (IQR: 6 to 29). Two hundred and seventeen (10%) children had follow-up longer than 36 months. During follow-up, 104 (5%) transferred out (transferred-out rate: 3.3 per 100 child-years), and 132 (6%) were lost-to-follow-up (not seen at clinic for more than 6 months; loss-to-follow-up rate 4.2 per 100 child years).
Mortality
Of 2102 children started on ART, 132 (6%) died during 3155 child-years of follow-up: mortality rate 4.2 (95%CI: 3.5, 5.0) deaths per 100 child-years. Seventy died within 90 days after ART initiation (early mortality rate: 14.65 (95% CI: 11.59, 18.51); and 62 after 90 days (late mortality rate: 2.3 (95% CI: 1.8, 3.0) deaths/100 child-years) (Table 1). Of note is the fact that 91 children who started ART did not come for any follow-up visit and their whereabouts was unknown at the time of this analysis.
Severe underweight for age at time of ART initiation was the strongest predictor of death and the only statistically significant predictor of early death. Children with WAZ scores < −3SD compared with those with WAZ scores ≥ −2SD had 8.56-fold (95% CI: 3.49, 20.99) increased hazard for death during the first 90 days of treatment, and 4.12-fold (95% CI: 1.81, 9.35) increased hazard for later mortality after adjusting for other baseline characteristics. The adjusted hazard ratio (aHR) comparing children with≥ 5 log HIV RNA copies/ml to those with higher pre-treatment viral load for late mortality was 0.38 (0.16, 0.87). TB co-treatment was also statistically associated with hazard of late mortality (aHR; 1.97 (1.08, 3.60). Compared with children <18 months, those aged 5 years or older had an aHR for late mortality of 0.40 (0.19, 0.85). Stratifying analyses by age (LPV/r vs NNRTI-based regimen) did not change these results substantially. Overall mortality rate per year did not change substantially over the time period.
Immunologic and growth responses
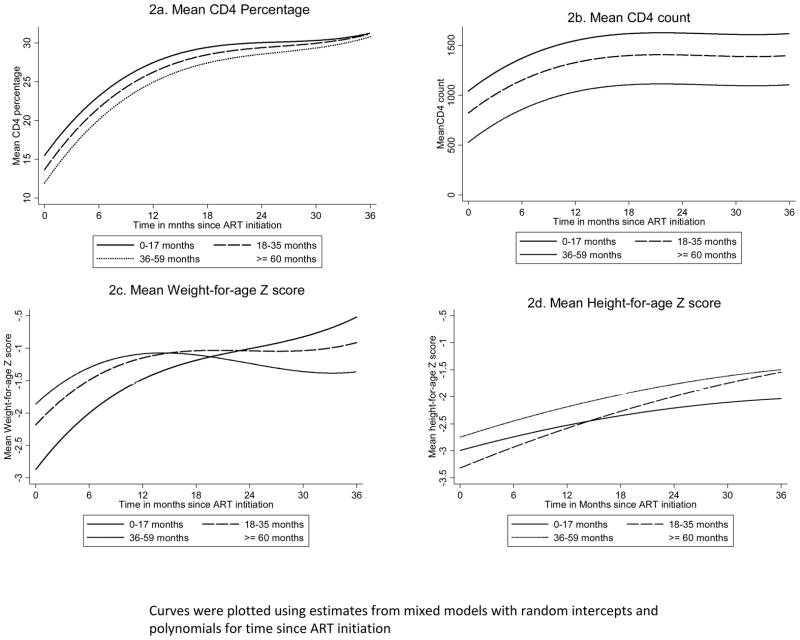
After ART initiation, the mean CD4% doubled within 12 months: rising from 12.7% (95% CI: 11.9%, 13.4%) baseline, to 25.1% (95% CI: 24.4%, 25.8%). After this initial “catch up” phase, the mean CD4 cell percentage continued to rise at a slow pace to reach 27.9% (95% CI: 27.2%, 28.7%), and 30.6% (95% CI: 29.7%, 31.6%) by 24 and 36 months respectively (figure 2a). The same pattern was observed with the mean CD4 count (figure 2b). The baseline mean of 520 (95% CI: 487, 552) cells/ml doubled by 12 months to 1023 (95% CI: 994, 1052) and reached 1120 (95% CI: 1063, 1176) by 36 months. The change in CD4% was faster among children <18 months (p-value for interaction <0.01). The change in mean absolute CD4 count did not vary statistically by age categories (p-value for interaction =0.09).
Figure 2.
Change in mean CD4 percentage, absolute CD4 cell count, weight-, and height-for-age z scores over time among children initiating antiretroviral therapy at HSCC by age groups
The mean WAZ also rose rapidly from −2.40 (95% CI: −2.48, −2.33) at baseline, to −1.40 (95% CI: −1.46, −1.33) at 12 months and continued to improve overtime to reach −1.18 (95% CI: −1.29, −1.07) at 36 months (figure 2c). The improvement in the mean HAZ was less pronounced and almost constant overtime (figure 3d): rising from −2.69 (95% CI: −2.76, −2.64) at baseline to −2.26 (95% CI: −2.32, −2.20) at 12 months, to −1.76 (95% CI: −1.85, −1.66) at 36 months (figure 2d). Younger children gained weight and height more rapidly than older children (p-value for the interaction terms <0.001 for both WAZ and HAZ).
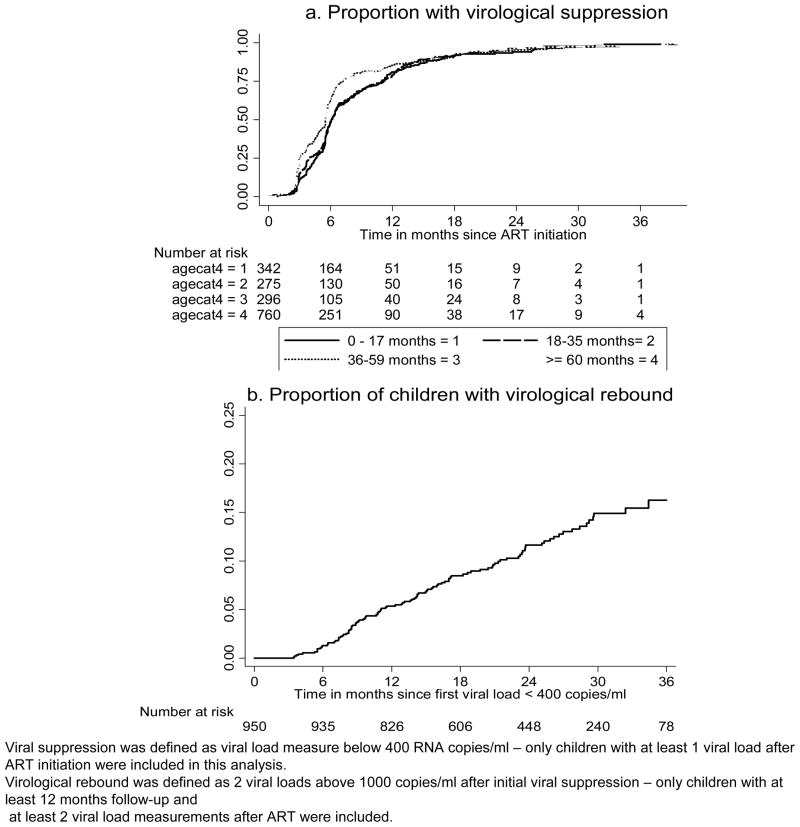
Figure 3.
Kaplan Meier estimate of the proportion of children with viral suppression or virologic failure over follow-up time on ART among children receiving ART at Harriet Shezi Children’s clinic between April 2004 and March 2008
Virologic response
Among the 2102 children, 1673 (79.6%) had at least one viral load measurement after ART initiation (supplemental digital content 2, table illustrating characteristics at ART initiation associated with virologic suppression and virologic rebound, among children initiating antiretroviral therapy at HSCC (April, 2004 to March, 2008) The cumulative probability of achieving a viral load < 400 copies/ml was 59.4% (95% CI: 57.0%, 61.8%) 6 months after ART initiation, 84.0% (95% CI: 82.6%, 86.2%) by 12 months, 96.2% (95% CI: 94.4%, 96.7%) by 24 months (figure 3a).
Children greater than 3 years at ART initiation (correlating with those who started EFV- -based regimens) were more likely to achieve virologic suppression early compared with children younger than 3 years (on LPV/r-containing regimens); HR = 1.33 (1.09, 1.63), and 1.35 (1.14, 1.59) for 36–59, and >=60 months, respectively compared to those <18 months (supplemental digital content 2, table illustrating characteristics at ART initiation associated with virologic suppression and virologic rebound, among children initiating antiretroviral therapy at HSCC (April, 2004 to March, 2008)). After stratification by age at ART initiation, none of the baseline characteristics was statistically associated with viral suppression among children <3 years. Among older children, WAZ < −3SD (aHR=1.22 95% CI: 1.01, 1.46) and lower CD4% (aHR 0.71 95% CI: 0.51, 0.98) were associated with higher likelihood of viral suppression.
Virologic rebound after suppression
Of those who achieved viral suppression, 950 (56.8%) had at least two viral load measurement after achieving suppression. The proportion of children who had viral rebound, increased progressively from 1.3% (95% CI: 0.7%, 2.2%) at 6 months, to 5.4% (95% CI: 4.1%, 7.0%) at 12 months, to 11.6% (95% CI: 9.5%, 14.2%) at 24 month, to reach 16.3% (95% CI: 13.2%, 20.0%) at 36 months after initial viral suppression (figure 3b). None of the characteristics measured pre-ART was associated with viral rebound (supplemental digital content 2, table). However, when they were considered at time of viral suppression, being on TB treatment, particularly in young children was the only factor found to be statistically associated with increased hazard of viral rebound: aHR 2.31 (95% CI 1.01, 5.29) and 1.29 (95% CI 0.72, 2.32) among < 3 years (LPV/r-based regimen) and ≥ 3 years (EFV-based regimen) old, respectively.
DISCUSSION
Our findings demonstrate excellent virologic suppression rates and immunologic and somatic growth responses achieved among children receiving ART in a busy routine urban service in South Africa. The mortality rate within 90 days of treatment was high, particularly in the youngest age groups. This pattern of mortality is similar to rates reported for children in the region.(5, 14, 19) and may even be higher, given that some children who did not return for a visit after starting ART are likely to have died. Younger children in this cohort had the most advanced disease. As has been previously reported in both HIV-infected adult and child cohorts,(14–16, 20) mortality was strongly correlated with advanced disease, indicated by lower CD4%, likelihood of receiving TB therapy and low weight for age at the time of treatment initiation. These findings reflect the fact that infants and young children are not being diagnosed and treated early, and access services only when they are in an advanced stage of illness.
The virologic response rate is comparable with that reported in smaller cohorts in the region. (5, 21, 22) The 60% viral suppression rate at 6 months in our cohort, however, is probably an underestimate as children who had not yet had a follow-up viral load measurement by 6 months were considered to have not yet achieved virologic suppression. Children who started ART before 3 years of age (on LPV/r-based regimen) or with high viral load at time of ART initiation tended to suppress more slowly than older children although viral suppression rates reached similar levels to the older children after 6 months. This may be due to the fact that the younger children have higher viral loads at baseline or it may be an effect of the unpleasant flavor of LPV/r liquid formulation which may have contributed to poor adherence, although it would be expected that this would be sustained beyond 6 months. Older children on the other hand may have had better adherence (23) and EFV is more pleasant to take. The lack of adherence data for this cohort makes it difficult to interpret this data with any certainty.
A high proportion of children (29%) were receiving TB treatment at the time of starting ART. There is a high burden of TB co-infection with HIV in South Africa,(24) Whilst we did not have information on the number of children with bacteriologically confirmed TB in our cohort, the fact that a large proportion were co-treated for TB may have important implications for drug interactions. Rifampin is a strong inducer of cytochrome p450 enzymes, (25, 26) resulting in accelerated clearance of PI’s and NNRTI’s.(27–30) Co-treatment for TB was common especially in the youngest age group and drug interaction between LPV/r and rifampin-containing TB treatment may explain some of the slower virologic suppression and the higher risk of virologic rebound. LPV/r dosing was modified to accommodate co-treatment for TB.(31) We have recently reported a separate analysis focusing on virologic and toxicity outcomes among TB/HIV co-treated children with super-boosted LPV/r or double-dose LPV/r. Findings from this analysis demonstrate that viral suppression rates by 6 months in children who have been TB co-treated with modified doses of LPV/r may be reduced, although after TB treatment is stopped and by a year after starting ART, virologic outcomes among children with altered doses of LPV/r and TB treatment were similar to those who were taking LPV/r– containing ART and did not have TB treatment.(32)
LPV/r has also been demonstrated in one study from Cape Town, South Africa, to result in good viral suppression rates, even when adherence levels are lower than for children who were taking nevirapine-based ART.(33) The poor palatability and potential treatment interactions associated with LPV/r-based regimens must, however, be considered in the plans for using this treatment as part of first-line therapy. Options are, however, currently limited as nevirapine-based therapy has been shown to be less effective than LPV/r in babies when used first line.(4) Newer treatment agents and classes of drugs have been developed, some being investigated in pediatric trials, may prove more effective and durable, especially for use together with TB treatment. Expedited study for this purpose should occur.
Improvement in CD4 and growth were good and we have reported elsewhere that in this cohort gains in weight, CD4 count and C4% correlate well with subsequent clinical and virologic responses.(34).
The loss-to-follow-up rate of children taking ART was low, because of the active defaulter tracer program at the clinic. Considerable efforts and resources are required to maintain children on treatment once they have started. Of concern is the attrition of children who accessed the clinic services briefly but never returned and were excluded from our analysis. The mortality among this group was likely high.
This study has several limitations;. 1) the study was conducted retrospectively and there is irretrievable missing information, 2) our analysis was censored at 36 months of follow-up because of the limited number of children with longer follow-up information, 3) adherence was not consistently measured and recorded and therefore could not be used in our analysis. Nevertheless, this is one of the largest cohorts being treated in a single location. These operational findings inform practice, particularly since a subset of children received LPV/r as part of first line treatment, a regimen which is becoming more widely used among young children in under resourced countries.
Our results emphasize that although ART outcomes for children in large programs are generally favorable, there are delays in identification and access to treatment resulting in high mortality even after ART is commenced. Maintaining children with successful ART outcomes over prolonged periods requires considerable resources as cumulative proportions of children with viral rebound over time suggests that poor adherence or treatment interactions may diminish the long term success of treatment. New pediatric formulations, fixed dose combinations and simplified strategies for improving durability of treatment which take into account drug interactions with commonly used agents such as TB drugs must continue to be investigated.
Supplementary Material
Supplemental digital content 1: table that illustrates characteristics of HIV-infected children 15 or younger years at the time of Antiretroviral Therapy initiation at HSCC (April, 2004 –March, 2008).
Supplemental digital content 2, table illustrating characteristics at ART initiation associated with virologic suppression and virologic rebound, among children initiating antiretroviral therapy at HSCC (April, 2004 to March, 2008)
Acknowledgments
We thank Dr. Annelies van Rie for her helpful and insightful comments.
Funding Support:TM is a Fogarty Fellow sponsored by grants 5U2RTW007370 and 5U2RTW007373. The Harriet Shezi Children’s Clinic received funding from the United States PEPFAR programme UNICEF, the Elizabeth Glaser Pediatric AIDS Foundation and, Rockefeller Brother’s Fund, South African business sector, private donations, individuals, schools and churches.
MY was an NIH Fogarty Fellow sponsored by grant. DHHS/NIH/FIC 5 D43 TW01039-08 and had received support from the UNC Center for Global Initiative.
References
- 1.UNAIDS. AIDS epidemic update: December 2007. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); Dec, 2007. [Google Scholar]
- 2.WHO. Towards Universal Access: Scaling up priority HIV/AIDS interventions in the health sector –Progress Report 2010. Geneva: 2010. [Google Scholar]
- 3.WHO. Recommendations for a public health approach 2010 revision. 2010. Antiretroviral therapy for HIV infection in infants and children: towards universal access. [PubMed] [Google Scholar]
- 4.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral Treatment for Children with Peripartum Nevirapine Exposure. New England Journal of Medicine. 2010;363(16):1510–20. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutcliffe CG, van Dijk JH, Bolton C, et al. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008 Aug;8(8):477–89. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 6.Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009 Dec 15;49(12):1915–27. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Recommendations for a public health approach (2005 revision) Geneva, Switzerland: World Health Organisation; 2005. Antiretroviral drugs and the prevention of mother-to-child transmission of HIV infection in resource-limited settings. [Google Scholar]
- 8.Meyers T, Eley B, Leoning W. Health. 1. Jacana Media; 2005. Guidelines for the management of HIV-infected children - 2005. [Google Scholar]
- 9.Department of Health, South Africa. Guidelines for the Management of HIV infected Children in South Africa. 2005. [Google Scholar]
- 10.la Porte CJ, Colbers EP, Bertz R, et al. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob Agents Chemother. 2004 May;48(5):1553–60. doi: 10.1128/AAC.48.5.1553-1560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glencross D, Scott LE, Jani IV, et al. CD45-assisted PanLeucogating for accurate, cost-effective dual-platform CD4+ T-cell enumeration. Cytometry. 2002 Apr 15;50(2):69–77. doi: 10.1002/cyto.10068. [DOI] [PubMed] [Google Scholar]
- 12.CDC. A SAS program for CDC growth charts. Center for Disease Control and Prevention; 2000. [updated May 22, 2007; cited 2008 December 31]; Available from: http://www.cdc.gov/NCCDPHP/dnpa/growthcharts/resources/sas.htm. [Google Scholar]
- 13.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height, and Body Mass Index-for-Age: Methods and Development. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- 14.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. Jama. 2007 Oct 24;298(16):1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 15.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama. 2006 Aug 16;296(7):782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 16.Zachariah R, Fitzgerald M, Massaquoi M, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. Aids. 2006 Nov 28;20(18):2355–60. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 17.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997 Oct 30;16(20):2349–80. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18.Lin DY, Wei LJ, Ying Z. Checking the Cox Model with Cumulative Sums of Martingale-Based Residuals. Biometrika. 1993;80(3):557–72. [Google Scholar]
- 19.Davies MA, Keiser O, Technau K, et al. Outcomes of the South African National Antiretroviral Treatment Programme for children: the IeDEA Southern Africa collaboration. S Afr Med J. 2009 Oct;99(10):730–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006 Mar 11;367(9513):817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 21.Eley B, Davies MA, Apolles P, et al. Antiretroviral treatment for children. S Afr Med J. 2006 Sep;96(9 Pt 2):988–93. [PubMed] [Google Scholar]
- 22.Reddi A, Leeper SC, Grobler AC, et al. Preliminary outcomes of a pediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr. 2007;7:13. doi: 10.1186/1471-2431-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibb DM, et al. Adherence to prescribed antiretroviral therapy in human immunodeficiency virus-infected children in the PENTA 5 trial. Pediatr Infect Dis J. 2003;22(1):56–62. doi: 10.1097/00006454-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 24.WHO. Global Tuberculosis control 2010. Geneva: 2010. [Google Scholar]
- 25.Vishnuvardhan DML, Richert C. Lopinavir: acute exposure inhibits P-glycoprotein; extended exposure induces P-glycoprotein. AIDS. 2003;17:1092–4. doi: 10.1097/01.aids.0000060380.78202.b5. [DOI] [PubMed] [Google Scholar]
- 26.Sham HL, Kempf DJ, Molla A, et al. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob Agents Chemother. 1998 Dec;42(12):3218–24. doi: 10.1128/aac.42.12.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burger DMAS, Child M, Been-Tiktak A, et al. Effect of rifampin on steady-state pharmacokinetics of atazanavir with ritonavir in healthy volunteers. Antimicrob Agents Chemother. 2006;50:3336–42. doi: 10.1128/AAC.00461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton ME, Shaw LM, Schentag JJ, Evans WE. Applied pharmacokinetics and pharmakodynamics, Principles of therapeutic drug monitoring. 4. Lippincott: Williams and Wilkins; 2005. [Google Scholar]
- 29.La Porte CJCE, Bertz R. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob Agents Chemother. 2004;48(5):1553–60. doi: 10.1128/AAC.48.5.1553-1560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIlleron H, Ren Y, Nuttall J, et al., editors. Double-dose lopinavir/ritonavir provides insufficient lopinavir exposure in children receiving rifampicin-based anti-TB treatment; Conf Retroviruses Opportunistic Infect; 2009 Feb 8–11; Montreal. 2009. [Google Scholar]
- 31.Maartens G, Decloedt E, Cohen K. Effectiveness and safety of antiretrovirals with rifampicin: crucial issues for high-burden countries. Antivir Ther. 2009;14(8):1039–43. doi: 10.3851/IMP1455. [DOI] [PubMed] [Google Scholar]
- 32.Frohoff C, et al. Antiretroviral therapy outcomes in HIV-infected children after adjusting protease inhibitor dosing during tuberculosis treatment. PLoS One. 2011;6(2):e17273. doi: 10.1371/journal.pone.0017273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller AD, Myer L, Jaspan H. Virologic suppression achieved with suboptimal adherence levels among South African children receiving boosted protease inhibitor-based antiretroviral therapy. Clin Infect Dis. 2009 Jan 1;48(1):e3–5. doi: 10.1086/595553. [DOI] [PubMed] [Google Scholar]
- 34.Yotebieng M, Van Rie A, Moultrie H, Meyers T. Six-month gain in weight, height, and CD4 predict subsequent antiretroviral treatment responses in HIV-infected South African children. AIDS. 2010 Jan 2;24(1):139–46. doi: 10.1097/QAD.0b013e328332d5ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content 1: table that illustrates characteristics of HIV-infected children 15 or younger years at the time of Antiretroviral Therapy initiation at HSCC (April, 2004 –March, 2008).
Supplemental digital content 2, table illustrating characteristics at ART initiation associated with virologic suppression and virologic rebound, among children initiating antiretroviral therapy at HSCC (April, 2004 to March, 2008)