Abstract
Most gastrointestinal stromal tumors (GISTs) are driven by KIT or PDGFRA activating mutations, but a small subset is associated with loss function of the succinate dehydrogenase (SDH) complex of mitochondrial inner membrane proteins. This occurs via germline mutations of the SDH subunit genes and hitherto unknown mechanisms. SDH-deficient GISTs especially include pediatric GISTs and those associated with Carney triad (CT) or Carney-Stratakis syndromes (CSS); the latter two also include paraganglioma as a component. SDH-deficient GISTs were identified in this study based on immunohistochemical loss of SDHB, which signals functional loss of the SDH complex. We found 66 SDH-deficient GISTs among 756 gastric GISTs, with an estimated frequency of 7.5% of unselected cases. Nearly all gastric GISTs in patients <20 years and a substantial percentage of those in patients <40 years belonged to this group, but only rare GISTs in older adults were SDH-deficient. There was an over 2:1 female predominance. Two patients each had either pulmonary chondroma or paraganglioma (CT), but none of the examined cases had SDH germline mutations (CSS) or somatic KIT/PDGFRA or BRAF mutations. SDH-deficient GISTs were often multiple and typically showed plexiform muscularis propria involvement and epithelioid hypercellular morphology. They were consistently KIT- and DOG1/Ano 1-positive and almost always SMA-negative. Tumor size and mitotic activity varied, and the tumors were somewhat unpredictable with some with low mitotic rates developing metastases. Gastric recurrences occurred in 11 patients and peritoneal and liver metastases 8 and 10 patients. Lymph node metastases were detected in 5 patients, but lymphovascular invasion was present in >50% of cases studied; these two were not related to adverse outcome. Seven patients died of disease, but many had long survivals, even with peritoneal or liver metastases. All 378 non-gastric GISTs and 34 gastric non-GIST mesenchymal tumors were SDHB-positive. SDH-deficient GISTs constitute a small subgroup of gastric GISTs, usually occur in children and young adults, and often have a chronic course similar to that of pediatric and Carney triad GISTs and potential association with paraganglioma, necessitating long-term follow-up.
Keywords: succinate dehydrogenase subunit B, SDHB, succinate dehydrogenase, gastrointestinal stromal tumor, prognosis
INTRODUCTION
Most gastrointestinal stromal tumors (GISTs) occur in older adults and are driven by alternative KIT or PDGFRA receptor signaling activating mutations. However, 10–15% GISTs lack such mutations (are wild type in relation to those mutations). 12,14,20 These include three clinicopathologic subgroups: pediatric GISTs, those associated with neurofibromatosis type 1, and sporadic wild-type GISTs. 19,25,32 Rare examples of wild-type GISTs, mostly intestinal ones, have been reported to harbor BRAF mutations.2,16
A newly described pathogenetic mechanism for GIST is the intratumoral loss- of-function of the succinate dehydrogenase (SDH) complex.17 In Carney-Stratakis syndrome (GIST and paraganglioma) and familial paraganglioma syndromes, loss-of-function SDHB, C, or D germline mutations are associated with a somatic loss of function alteration causing bi-allelic inactivation of SDH in tumor cells typical of classic tumor suppressor genes.22,28,35 In the non-hereditary Carney triad (GIST, paraganglioma and pulmonary chondroma) and most pediatric GISTs, the same end result occurs via currently unknown mechanisms. 35 Activation of pseudohypoxia signaling by overexpression of hypoxia inducible factor 1 alpha (HIF1α) is understood to be among the central events in the pathogenesis of SDH-deficient tumors. 17,18,31 It has also been shown that silencing of SDHB expression induces tumor-like phenotypic traits in cell cultures. 6
Succinate dehydrogenase (succinate-coenzyme Q reductase or mitochondrial complex 2) consists of four subunit proteins: SDHA, SDHB, SDHC, and SDHD localized in the inner mitochondrial membrane. This complex acts at the interphase of the tricarboxylic acid cycle and electron transport chain. One of the subunits, SDHB, is a hydrophilic iron-sulphur protein, encoded by chromosomal DNA. 34
SDHB is normally ubiquitously expressed with granular cytoplasmic immunostaining reflecting its mitochondrial location. 9,10 However, GISTs from patients with hereditary paraganglioma syndromes, Carney-Stratakis syndrome, Carney triad, and pediatric GISTs in general, have been found to be SDHB deficient based on small number of cases studied. 8,10,17 Loss of any subunit protein leads to the loss of SDHB expression due to destabilization of the SDH complex, and therefore SDHB loss is a marker for any bi-allelic loss of function of the succinate dehydrogenase complex (SDH-deficient GIST). 11,17 A similar role for SDH loss was previously discovered in paragangliomas. Many of them are also SDHB-negative generally reflecting loss-of-function germline mutations in one of the SDH-genes, which in combination with somatic loss-of-function of the other allele lead to silencing of one of the SDH-genes and functional loss of the SDH-complex. 9,26
In this study, we report 66 SDH-deficient gastric GISTs among 1134 GISTs and examine their pathology, prognosis, and genetic background.
MATERIALS AND METHODS
Gastrointestinal stromal tumors from stomach (n = 756), small intestine (n = 265), rectum (n = 43), colon (n = 23), esophagus (n = 7), and of extragastrointestinal locations (n = 40), a total 1134 GISTs, were immunohistochemically examined for succinate dehydrogenase subunit B (SDHB) expression (retention or loss). The cases were derived from different institutions and included a major component of second opinion referrals. In most cases, the immunostaining was performed on multitumor blocks containing 5-60 tumors. However, in 10 cases with limited material, unstained slides, hematoxylin and eosin stained slides, or slides with previous negative immunostains were processed for SDHB immunostaining, especially from young patients. Twenty-six SDHB-negative cases were studied with an independent tissue array or conventional section, and the negative result was confirmed in each case. Some of the pediatric GISTs were previously reported 25, as was the cohort of SDHB-positive NF1-associated GISTs. 37
In addition, 34 non-GIST mesenchymal tumors of the GI tract were also studied for SDHB-expression, including inflammatory myofibroblastic tumors, leiomyomas, leiomyosarcomas, schwannomas, plexiform fibromyxomas, and synovial sarcomas.
The primary mouse monoclonal antibody to SDHB, 21A11 (ABCAM, Cambridge, Massachusetts) was used in a dilution of 1:1000. The immunostaining was performed using the Leica BondMax autostainer (Leica Microsystems, Bannockburn, IL) utilizing the BondMax avidin biotin free polymer-based detection system preceded by heat-induced epitope retrieval with Leica retrieval solution (alkaline buffer). Diaminobenzine was used as the chromogen. Only cases with positive internal control (vascular, lymhpohematopoietic, smooth muscle, or epithelial elements) were considered informative. Only 1% of cases were deemed uninformative and eliminated from further analysis.
Immunostaining for KIT (polyclonal antibody A4502, Dako Cytomation, Carpinteria, CA), diluted 1:250, and DOG1/anoctamin1 (monoclonal antibody, clone K9, Leica), diluted 1:100, Desmin (clone D33, Dako), diluted 1:50, and CD34 (clone QBEnd/10), prediluted (Ventana Medical Systems, Tucson, AZ, were performed following similar epitope retrieval and procedure as described above. Smooth muscle actin (clone 1A4, Sigma Chemicals, St. Louis, MO), diluted 1:1600, and S100 protein (polyclonal, Dako Cytomation) diluted 1:1600, immunohistochemistry was performed similarly without epitope retrieval pretreatment.
The gastrointestinal stromal tumors that were immunohistochemically negative for SDHB (all gastric) were analyzed histologically in detail: mitotic count/50 high power fields (corresponding to 5 mm2), plexiform/multinodular architecture, cellular components (epithelioid vs. spindle), cytologic atypia (especially pleomorphism), tumor necrosis, cytoplasmic vacuolization, nuclear palisading, lymphovascular invasion, and nodal metastases. Prognostic groups were assigned as previously described.24
The gross features were recorded from available data, including tumor size, multiplicity, and possible metastases. Clinical follow-up was obtained from tumor registries, treating physicians, or in some cases from patients or family members. Follow-up information was available for 54 patients (range: <1–44 years, median, 14.7 years).
DNA was obtained from formalin-fixed and paraffin-embedded tumor tissue. Mutational analysis of KIT and PDGFRA genes was performed as previously described.18 KIT exons 9, 11, 13 and 17, and PDGFRA exons 12, 14 and 18, mutational hot spots for GISTs, were PCR-amplified, and the products were sequenced directly. BRAF mutation analysis was performed to detect the previously reported exon 15 mutant. 2,16
Molecular studies of SDH-genes were focused on selected coding and intronic sequences reportedly mutated in SDH-deficient GISTs. 17,28 SDHB exons 1, 3, 4, 6 and 7, SDHC exons 1 and 5, and SDHD exon 1, and intronic boundaries of all analyzed exons were PCR amplified and sequenced directly. These primer sequences and PCR conditions are listed in supplementary table available on-line.
RESULTS
Demographics of the SDHB-negative and positive GISTs
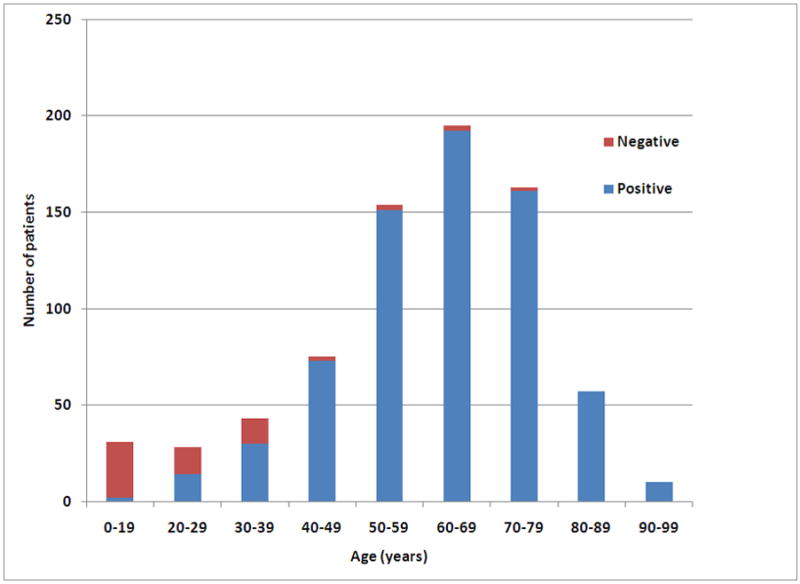
A total of 66 GISTs, all gastric, 8.7% of all 756 gastric GISTs studied, were immunohistochemically SDHB-negative (SDHB deficient GISTs). Because 10 patients were selected by age for analysis, we estimate the true frequency among all gastric GISTs as 7.5%. This patient group consisted mainly of children and young adults but included a small number of older adults (Fig. 1). The patient age range was 8–77 years (median, 22 years; mean, 37 years). Thirty three individuals were 21 years or younger. There was a significant overall female predominance: 47 females and 19 males, more prominent in the age groups < 21 years. However, there was an equal gender distribution in the age group ≥ 30 years (Table 1).
Fig. 1.
Frequency of SDHB-negative and positive gastric GISTs as a function of patient age.
Table 1.
Clinicopathologic summary of the 66 SDH-deficient GISTs
| Case | Age | Sex | Size (cm) | Mitoses/50 HPFs | Prognostic group | Recurrences, metastases and additional findings | Status at last follow-up | Length of follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | F | 8 largest, multiple | 18 | 6a | Abdominal metastases, 18 yrs | AWD | 18 years |
| 2 | 9 | F | 2.5 cm | 1 | 3a | Gastric recurrence, 5.0 years. Regional abdominal metastasis at 10 years, exicised following 6 month of imatinib | ANED | 16 years |
| 3 | 10 | F | 5 + 3 cm, two | 10 | 5 | ANED | 24.1 years | |
| 4 | 10 | F | 5.7 and a smaller nodule | 3 | 3a | ANED | 8.6 years | |
| 5 | 10 | F | 6 + 3 cm, two | 11 | 6a | Lymph node metastasis at presentation | ANED | 1. 8 years |
| 6 | 10 | F | >10 | 8 | 6b | Gastric recurrence, 13 years, gastrectomy | ANED | 29.4 years |
| 7 | 11 | F | 3 | 16 | 5 | Gastric recurrence at 3 years | ANED | 20.8 years |
| 8 | 11 | F | 5 | 0 | 2 | ANED | 7 years | |
| 9 | 12 | F | 2.7 | 6 | 5 | ANED | 9.7 years | |
| 12 | F | 4 | 3 | 2 | ANED | 6.0 years | ||
| 11 | 12 | F | 5 | 18 | 5 | Pulmonary chondroma one year later (Carney triad); Gastric recurrences, 9 and 33 years, total gastrectomy | ANED | 41.2 years |
| 12 | 12 | F | 8.5 | 57 | 6a | Liver metastases at presentation | LTF | |
| 13 | 13 | F | 4.3 | 4 | 2 | Liver and mesocolic metastases, time of diagnosis unknown | DOD | 6.5 years |
| 14 | 14 | F | 3.5 | 2 | 2 | ANED | 28 years | |
| s15 | 14 | F | 4.5 | 7 | 5 | ANED | 13 years | |
| 16 | 14 | F | 9 | 6 | 6a | Pulmonary chondroma at presentation (Carney triad). Gastric recurrence, 4 years | ANED | 9 years |
| 17 | 14 | F | Multiple, 0.5–4.5 cm | 8 | 5 | Liver metastasis, 2.4 years. Received imatinib mesylate for 5 years | AWD | 8.8 years |
| 18 | 14 | M | 7 | 9 | 6a | ANED | 4.9 years | |
| 19 | 15 | F | 3.5, another in fundus | 6 | 5 | Peritoneal micrometastases at presentation Subtotal gastrectomy for residual disease6 months later | ANED | 17.4 years |
| 20 | 16 | F | Unknown, recurrence 4.5 cm | 3 (2nd rec) | * | Gastric recurrences 17, 23, and 24 years later | AWD | 24 years |
| 21 | 16 | M | 8 | 2 | 3a | Liver metastasis, 22 years later | DOD | 23.3 years |
| 22 | 16 | M | Unknown | ND | * | Liver metastasis, 14 years later. Received imatinib for 19 years after primary, for 7 years | DOD | 27 years |
| 23 | 17 | M | 2.7 | 6 | 5 | Liver metastasis at presentation. Gastric recurrence 3 years later | AWD | 18.8 years |
| 24 | 18 | F | 3.5 | 3 | 2 | LTF | ||
| 25 | 18 | M | Unknown | 7 | DOD | 15.8 years | ||
| 26 | 19 | F | 1.5 | 4 | 1 | ANED | 17.5 years | |
| 27 | 19 | F | 10 | 1 | 3a | Liver metastasis, 42 years later | AWD | 44 years |
| 28 | 19 | F | 12 | 3 | 3b | Lymph node metastasis at presentation | ANED | 16.6 years |
| 29 | 19 | F | 4.5, 2.5 and 1.5 cm (three) | 11 | 5 | Received imatinib for 5 years | ANED | 7.0 years |
| 30 | 21 | F | 7 | 13 | 6a | ANED | 10.1 years | |
| 31 | 21 | M | 7 plus smaller ones | 2 | 3a | ANED | 17.3 years | |
| 32 | 21 | F | Unknown | 16 | * | Peritoneal metastases at presentation | LTF | |
| 33 | 21 | F | 11 (rec) | Gastric recurrence, 9 years | AWD | 9 years | ||
| 34 | 22 | F | 5 | 9 | 5 | LTF | ||
| 35 | 22 | F | 5 + smaller nodule | 15 | 5 | Gastric recurrence and peritoneal metastases 22.7 years later. Total gastrectomy. | ANED | 33 years |
| 36 | 24 | F | 2.2 | 0 | 2 | LTF | ||
| 37 | 25 | F | 4 | 5 | 2 | ANED | 8.5 years | |
| 38 | 25 | F | Unknown | 11 | * | LTF | ||
| 39 | 26 | F | 5.8 | 14 | 6a | Liver and lymph node metastasis at presentation | DOD | 3.3 years |
| 40 | 28 | F | Large palpable tumor, multiple gastric nodules | 1 | * | Gastric ulcer diagnosis six years before the GIST | ANED | 31.5 years |
| 41 | 29 | F | 5 | 9 | 5 | ANED | 24.7 years | |
| 42 | 29 | F | 5 + 4.5 cm and two smaller nodules | 14 | 5 | Lymph node metastases and omental nodules at presentation | ANED | 9 years |
| 43 | 29 | M | 10 | 7 | 6a | Gastric recurrence, 5 years and carotid body paraganglioma 25.5 years | ANED | 25.5 years |
| 44 | 30 | M | 7 | 11 | 6a | Recurrence in abdominal soft tissue at 12.8 years | AWD | 14.7 years |
| 45 | 30 | M | 6.1 + 3.2 cm | 11 | 3a | ANED | 7.2 years | |
| 46 | 31 | F | 5 cm, plus smaller separate nodules | 11 | 6a | ANED | 16.3 years | |
| 47 | 32 | F | 6.5 | 30 | 6a | Metastases, not further specified | DOD | 1.3 years |
| 48 | 33 | M | 5 | 2 | ANED | 2.5 years | ||
| 49 | 35 | F | ND | 2 (rec) | * | Liver metastasis, unknown interval | LTF | |
| 50 | 37 | F | 3 | 1 | 2 | ANED | 10.3 years | |
| 51 | 37 | F | 5 | 2 | 2 | LTF | ||
| 52 | 37 | M | 5 | 0 | 2 | DUC | 21.0 years | |
| 53 | 38 | F | 9 | 4 | 3a | Lymph node metastasis at presentation | ATSU | 3.6 years |
| 54 | 39 | F | 3 | 5 | 2 | ANED | 9.9 years | |
| 55 | 39 | M | 6.5 | 4 | 3a | Pheochromocytoma 24 years before GIST (at age of 15 years) | ANED | 5.8 years |
| 56 | 39 | M | ND | 3 | LTF | |||
| 57 | 40 | F | 4.5 | 3 | 2 | ANED | 18.8 years | |
| 58 | 48 | M | ND | 1 | LTF | |||
| 59 | 51 | M | 4 | 1 | 2 | ANED | 16.8 years | |
| 60 | 52 | M | 10 | 3 | 3a | LTF | ||
| 61 | 56 | F | >10, multinodular | 1 | 3b | ANED | 31.0 years | |
| 62 | 61 | M | 1.5 | 3 | 2 | ANED | 9.5 years | |
| 63 | 61 | M | Large, liver metastasis with rupture | Primary tumor not studied | * | DOD | 2.2 years | |
| 64 | 68 | M | 2.5 | 0 | 2 | DOPC | ||
| 65 | 71 | F | 5.5 | 4 | 3a | ANED | 11.0 years | |
| 66 | 77 | F | 3.5 | 0 | 2 | DUC | 14.7 years |
Could not be assessed. rec = based on evaluation of recurrent tumor.
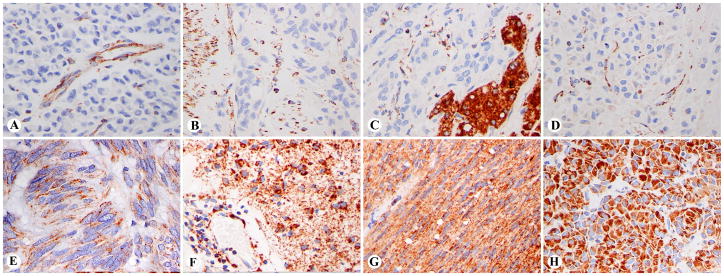
The 66 SDHB-negative GISTs showed SDHB immunoreactivity only in non-neoplastic elements, such as vascular endothelial and smooth muscle cells, lymphohistiocytic cells, residual muscularis propria, or epithelial elements, such as hepatocytes. The tumor cells were negative for cytoplasmic granular staining, although they occasionally showed weak focal cytoplasmic blush of positivity (Fig. 2A–D).
Fig. 2.
Examples of immunohistochemically SDHB-negative and positive gastric GISTs. A-D. SDHB- negative cases with staining limited to blood vessels, lymphohistiocytic infiltration, smooth muscle, or hepatocytes. C. Liver metastasis with positive hepatocytes. Note a faint cytoplasmic blush in panel D. E–H. SDHB- positive spindle cell and epithelioid GISTs with granular cytoplasmic staining of various intensities in tumor cells and vessel walls.
Most gastric GISTs, 690 of the total of 756 cases (91.2%), were immunohistochemically positive for SDHB showing granular cytoplasmic staining of various intensities (Fig. 2E–H). While SDHB-positive GISTs were rare in younger age groups, they overwhelmingly predominated in the older age groups (Fig. 1). There were only two patients with SDHB-positive gastric GISTs under the age of 21 years: a 16-year-old male and a 19-year-old female.
All non-gastric GISTs were SDHB-positive (Table 2). This included all small intestinal GISTs (n = 265), sporadic and NF1-associated, regardless of patient age. All esophageal, colorectal, and extragastrointestinal GISTs were also SDHB-positive. With the exception of NF1-associated small intestinal GISTs (which contained a number of younger patients), the median ages in each site-related group were around 60 years or above.
Table 2.
Demographics of the SDHB-positive gastric and non-gastric GISTs (normal SDHB-expression).
| Site | Total cases | Male: Female | Age range (years) | Median Age (years) | ≤21 years | 22–29 years | 30–39 years |
|---|---|---|---|---|---|---|---|
| Stomach, SDHB-positive | 690 | 375:315 | 16–94 | 62 | 2 | 11 | 31 |
| Small intestine, sporadic | 243 | 136:107 | 21–86 | 60 | 1 | 7 | 20 |
| Small intestine, NF1-associated | 22 | 8:14 | 25–67 | 41 | 0 | 2 | 2 |
| Rectum | 43 | 35:8 | 17–90 | 58 | 1 | 6 | 3 |
| Colon | 23 | 11:12 | 49–88 | 66 | 0 | 0 | 0 |
| Esophagus | 7 | 5:2 | 52–68 | 61 | 0 | 0 | 0 |
| Omentum | 10 | 4:6 | 38–74 | 60 | 0 | 0 | 1 |
| Abdomen, NOS | 22 | 13:9 | 31–82 | 69 | 0 | 0 | 1 |
| Retroperitoneum | 8 | 2:6 | 21–83 | 61 | 1 | 0 | 0 |
| Total, nongastric | 378 | 214:164 | 3 | 15 | 27 |
All 34 non-GIST mesenchymal tumors of the GI tract examined were SDHB positive. These included 9 intramural leiomyomas, 8 leiomyosarcomas, 6 inflammatory myofibroblastic tumors, 7 gastric schwannomas, and two each of gastric plexiform fibromyxomas and synovial sarcomas.
Clinicopathologic features of SDH-deficient GISTs
Clinicopathologic features of the patients with SDH-deficient GISTs are summarized in Table 1. Most patients came to medical attention for symptoms related to gastrointestinal bleeding, and some were diagnosed based on vague abdominal complaints or ulcer symptoms. In one patient with ulcer symptoms prior to the endoscopy era, the GIST diagnosis was delayed by several years until it was detected as a palpable mass. None of the patients had family history of GIST or neurofibromatosis type 1.
When stated, the tumors were located in the antrum (n= 20), lesser curvature (n=11), posterior wall/lesser sac (n = 2), and one each in the greater curvature/fundus, fundus, cardia or upper stomach. Multiple synchronous tumors were present in at least 12 patients.
Other tumors in patients with SDHB-deficient GISTs
Two patients, both children, had pulmonary chondromas diagnosed simultaneously with the GIST in one case and 1 year later in the other case, originally suspected of metastatic disease. Two different patients had paragangliomas. One had an adrenal pheochromocytoma 24 years prior to GIST, and another had a carotid body paraganglioma 25.5 years after the first GIST. One additional patient had renal onkocytoma 40 years after the GIST. None of the patients were diagnosed with adrenal adenoma or esophageal leiomyoma.
Gross features
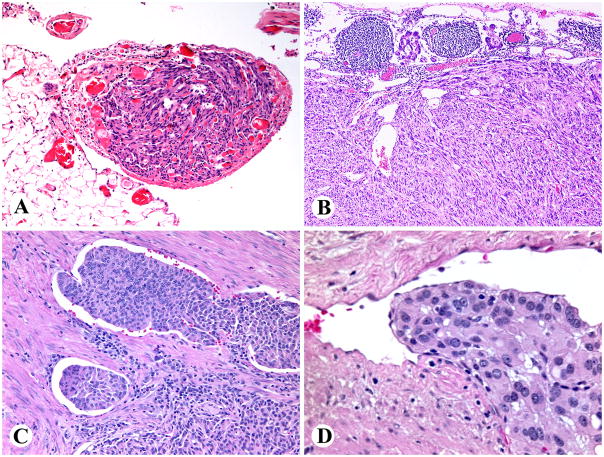
Multiple synchronous GISTs were detected in 12 patients. The size of the dominant tumor varied from 1.5–12 cm (median, 5.0 cm). The GISTs were typically described as multinodular or sometimes bilobed masses, often divided by apparent fibrous septa (Fig. 3). At least 7 larger tumors were centrally cystic. On sectioning the tumors varied from yellowish to pale tan to pink and brown, and many examples contained focal areas of hemorrhage.
Fig. 3.
Subtotal gastrectomy specimen contains a multinodular SDHB-negative GIST with a mucosal ulceration.
Histologic features of SDHB-deficient gastric GISTs
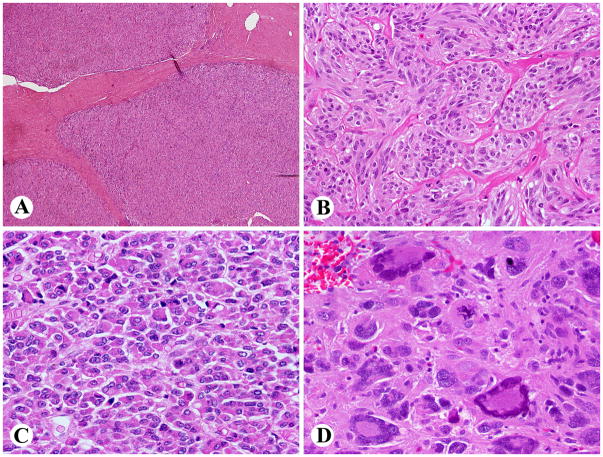
The tumors commonly involved muscularis propria as multiple apparently separate nodules, creating a “microplexiform” pattern (Fig 4A). Such an arrangement was seen in nearly all cases specifically evaluated for this feature (32/34). Ulceration was present in 19 of 45 cases.
Fig. 4.
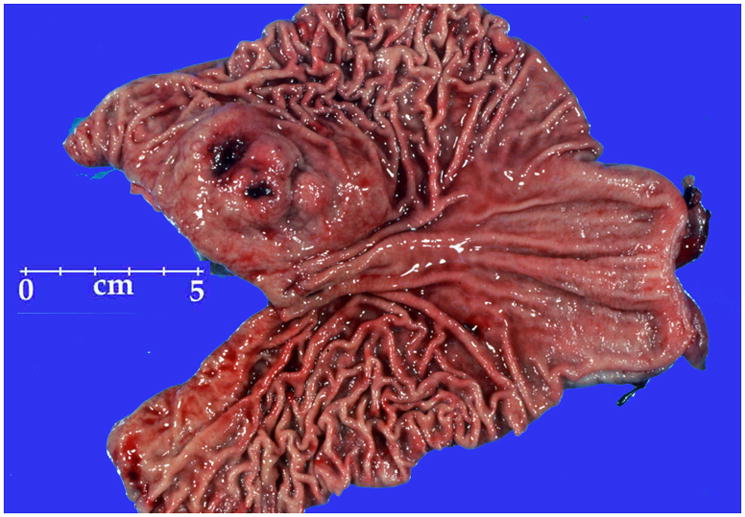
Histologic features typical of SDH-deficient GISTs. A. Multinodular, “plexiform” muscularis propria involvement. B. An example showing an organoid pattern with mixed epithelioid and spindled cytology. C. Epithelioid hypercellular cytology was the predominant finding. D. Marked nuclear pleomorphism was a rare finding. An atypical mitotic figure is seen right to the center.
Epithelioid cytology dominated in 37 cases, 20 tumors had mixed epithelioid and spindle cell, 7 spindle cell, and 1 had extensively pleomorphic cytology. Most commonly the tumors featured the epithelioid hypercellular type characterized by high cellularity and back-to-back cells in dense sheets (n = 27). Although most cases showed rather uniform cytology, four tumors showed significant nuclear pleomorphism, and two of them had atypical mitoses (Fig. 4). Features commonly seen in adult GISTs, such as palisaded-vacuolated or sclerosing (spindle cell or epithelioid) morphology were only rarely encountered. Nuclear palisading (typically vague) was seen in only 9 cases, but vacuolization was detectable in most cases, usually focally. Only two tumors had coagulation necrosis; both patients had long survivals. None of the tumors revealed calcification of mucosal invasion.
Two patients had omental/peritoneal micrometastases at presentation (Fig. 5B). Lymph node metastases were encountered in 5 of 12 cases in which lymph nodes where present and re-examined in this study (Fig. 5B). However, lymphovascular invasion around the tumor nodules was common and was seen in 17 of the 31 cases evaluated for this feature (Fig. 5C, D). In some cases, sharply demarcated micronodules in fact were intravascular tumor deposits, similar to those seen in some gastric glomus tumors.
Fig. 5.
A. Peritoneal GIST micrometastasis. B. Lymph node metastasis of a SDH-deficient GIST. This tumor shows spindle cell morphology, seen in a minority of cases. C, D. Lymphovascular invasion was a common feature seen in half of the cases.
Immumohistochemically nearly all examined cases were extensively positive for KIT (52/53) and DOG1/Ano 1 (31/31), with >50% of tumor cells positive in each case. CD34 was detected in 44/52 cases (usually extensively), but muscle markers only rarely: SMA 1/51 case (focally), and desmin in 1/49 cases (in 3% of tumor cells). None of the 47 examined cases were S100 protein positive.
Mutation analysis
All samples evaluated for KIT exons 9 (n = 30), 11 (n =31), 13 (n = 30), and 17 (n = 31), and PDGFRA exons 12 (n = 29), exon 14 (n = 24), and exon 18 (n = 29) were wild type. This was also true for BRAF exon 15 (n=16). No mutations were found in SDHB exons 1 (n=22), 3 (n=21), 4 (n=22), 6 (n=20) and 7 (n=22), and SDHC exons 1 (n=24) and 5 (n=23), and SDHD exon 1(n=23) coding sequences. All flanking intronic sequences except exon/intron 6 boundary, were evaluated. The latter could not be examined due to the lack of a compatible primer system for assaying formalin-fixed and paraffin-embedded tissue.
Follow-up
Eleven patients developed one or more gastric recurrences or second primary tumors <1–33 years from presentation and underwent subtotal or total gastrectomy. Only one of these patients had liver metastases. Ten patients had liver metastasis, 3 of them at presentation. The longest interval from primary tumor to liver metastasis was 42 years. Eight patients had abdominal soft tissue metastases. Only one of them, who also had liver metastases, died of disease, but three patients survived for 10–17 years after peritoneal metastases. So far, 7 patients had died, all but one with known liver metastases, but 3 have survived for 10–18 years with liver metastases. Four of the 7 patients who died of tumor were males. Two patients who died of disease had mitotic rates < 5/50 HPFs. Four patients were known having received imatinib mesylate for 6 months to 7 years, and one patient underwent excision of abdominal metastases subsequent to imatinib response. No one was known having received sunitib malate.
DISCUSSION
In this study we evaluated clinicopathologically 66 succinate dehydrogenase (SDH) deficient gastric GISTs, defined here by immunohistochemical negativity for SDHB, one of the normally ubiquitously expressed subunits of the SDH complex. Based on knowledge of the impact of loss of any SDH – subunits inactivation of the entire SDH complex in other tumors associated with SDH-deficiency, especially paragangliomas 11,17 – it is appropriate to designate this group as SDH-deficient GISTs.
We found SDH-deficient GISTs exclusively among gastric GISTs (66/756, 8.7%). However, this overestimates the true frequency, as the analysis was in part targeted to GISTs of young patients. Based on an unselected sample, we estimate that the true frequency of SDHB-deficient GISTs among all gastric GISTs is 7.5%. While most GISTs in young patients (≤21 years of age) were SDH-deficient, approximately half of them in patients at ages 21–30 years were SDH-deficient, whereas this was only rarely the case in GISTs of older adults. Previous studies have found SDH-deficient (SDHB-negative) pediatric, Carney Triad or Carney Stratakis syndrome-associated GISTs 8,10,17 along with rare examples of similar adult GISTs (3%) in one study.10 While there was an overall female predominance, the gender distribution was equal in age groups > 30 years in our study.
There are a number of histologic clues to SDH-negative status. These include epithelial hypercellular histology, plexiform growth pattern in the muscularis propria, and lymphovascular invasion and lymph node metastases that are otherwise rare in gastric GISTs. In fact, the “plexiform” muscularis propria involvement resembles that often seen in gastric glomus tumors, in some of the nodules may be intravascular.23 Similar patterns were recently also reported in rare adult GISTs resembling pediatric GISTs, but their SDHB-status was not known.33
Immunohistochemically SDH-deficient GISTs are distinctive for strong KIT expression, in contrast to many gastric epithelioid GISTs that show weaker KIT expression. They also are more often CD34-positive than gastric epithelioid GISTs but almost never express alpha smooth muscle actin that is present in 23% gastric GISTs, at least focally. 24
A molecular genetic clue to SDH-deficient GIST is lack of KIT and PDGFRA mutations (wild type), found here in all analyzed SDH-deficient GISTS, similar to previous observations on a smaller number of cases. 17 Also, these tumors seem to be unrelated to the rare KIT/PDGFRA-wild type GISTs carrying BRAF mutations found in adult patients, more often with intestinal GISTs. 2,16
Clinical behavior of SDH-deficient GISTs largely mirrors pediatric and Carney triad GISTs due to their predominant occurrence at a young age. In our study cohort, 10 of 54 patients with follow-up (19%) developed liver metastases and 7 patients died of disease. Many patients were alive with disease during long-term follow-up. Long latency from primary tumor to metastasis is common, and the longest interval from primary tumor to liver metastasis was 42 years in our study, illustrating the chronic course and need for life-long follow-up. Gastric recurrences, very rare in adult gastric GISTs, are common in SDH-deficient GISTs often ultimately leading to subtotal gastrectomy; they do not seem to have adverse prognostic significance. Neither are lymphovascular invasion, lymph node metastases, or peritoneal micrometastases ominous signs in this group, as many patients with them survived for long times.
SDH-deficient GISTs are unpredictable and metastatic disease may occur in tumors with favorable histologic parameters; two such patients died of disease. In our experience, many patients with metastatic GISTs ≤ 5 cm with mitotic counts ≤ 5/50 HPFs (group 2 GISTs) are SDH-deficient GISTs. On other hand, some patients with SDH-deficient GISTs with mitotic activity in excess of 10/50 HPFs, have uneventful survival again illustrating their unpredictability. The fact that 4 of 7 patients who died of tumor were male in this heavily female dominated group, may suggest that male patients have more aggressive course of disease. The very small number of older adults with SDH-deficient GISTs limits prognostic conclusions, although, with one exception, all patients had a favorable outcome. In many ways: tumor multiplicity, predilection to gastric antrum, potential occurrence of nodal metastases, chronic, somewhat unpredictable course but ultimately low long-term tumor mortality, SDH-deficient GISTs resemble Carney triad-associated and pediatric GISTs in general.5,25,32,38
Carney triad (gastric GIST, pulmonary chondroma, and paraganglioma) is a non-hereditary tumor syndrome including at least of the following: gastric GIST, pulmonary chondroma, and paraganglioma, at varying time intervals. It has a strong predilection to young females.5,38 In this study, we identified only 2 patients with pulmonary chondroma and 2 other patients with paraganglioma. Even if the frequency of Carney triad may be underestimated in our series, the findings suggest that Carney triad is not very common among the SDH-deficient GISTs. Carney triad patients do not have SDH germline mutations, but SDH-deficiency may be mediated by allelic losses in SDH, and losses in chromosome 1p36, the locus of SDHB, may be the alternative mechanism for loss of function of the SDH complex. 21
Carney-Stratakis syndrome (CSS) is a rare hereditary syndrome combining gastric GIST and paraganglioma. In contrast to Carney triad, it has a more equal gender distribution. The pathogenesis is based on a loss-of-function germline mutation in SDH subunits B, C, or D, and functional loss of the other allele based on currently poorly understood mechanisms. 28,29 Lack of familial tumor history and SDHB, C, or D (germline) mutations (analyzed in 10 patients in this study) suggests that SDHB-deficient GISTs are not commonly associated with CSS. However, we were not able to analyze DNA from two male patients with GIST and paraganglioma, good candidates for CSS. However, 54% of patients with paragangliomas, commonly SDH-deficient tumors, were found to have germline SDH mutations in one study. 3 In their rarity of SDH germline mutations, despite SDH-deficient status, GISTs differ from paragangliomas, for which SDH-deficiency generally signals a germline mutation. 26
Very recently, loss-of-function SDHA germline mutations were reported in two gastric KIT/PDGFRA wildtype GISTs 27 indicating that mutations of this subunit should also be screened in SDH-negative GISTs. SDHA mutations have been previously found in one patient with a cathecholamine-secreting adrenal paraganglioma. 4 Previously SDHA loss-of function mutation was reported in Leigh syndrome featuring a severe neurodegenerative disorder. 15
Renal onkocytoma was diagnosed in one patient with an SDH-deficient GIST. Renal cell carcinoma has been reported to be associated with SDHB paraganglioma syndrome, and this tumor is also otherwise known to be associated with pseodohypoxia pathway.36 Esophageal leiomyoma or adrenal adenoma, other tumors seen in Carney Triad, 5 were not encountered in our patients.
The pathogenesis-based definition, SDH-deficient GIST, common to GISTs of Carney triad, Carney-Stratakis syndrome and pediatric GISTs in general, obtained by a single immunostain for SDHB, seems to be more efficient than the identification of a syndrome based on the accompanying tumors, paragangliomas and pulmonary chondromas, or young age only. First, different components of these syndromes can present over a long period of time making life-long follow-up necessary to observe the complete syndrome phenotype. Secondly, based knowledge on the related paraganglioma syndromes also associated with SDH-deficiency, these syndromes may have incomplete to low penetrance, perhaps in only 30%, making it more difficult to observe familial disease without germline mutation analysis. 13 Thirdly, there are pediatric GISTs other than SDH-deficient GISTs; these comprise the very rare non-gastric GISTs at a young age. Similar gene expression profiles reported in CT, CSS, pediatric GISTs, and a small number of adult GISTs also seem to support the unifying concept of SDH-deficient GIST. 1
SDHB-deficient GISTs seem to exclusively occur in the stomach, and none of the GISTs from other locations were SDHB-negative. This included the rare non-gastric GISTs in young populations, NF1-associated GISTs, and sporadic, non-gastric wild-type GISTs, whose pathogenesis remains poorly understood. However, one report described two small intestinal (jejunal) GISTs in paraganglioma patients. 30 Although these cases were negative for SDH subunit mutations, immunohistochemical testing for SDHB was not yet available, leaving it open whether these jejunal GISTs were SDH-deficient.
For treatment, patients with SDH-deficient GISTs should undergo complete tumor resection with attention to secondary lesions and peritoneal and nodal metastases. For adjuvant therapy, they may require different approach than KIT/PDGFRA mutated GISTs that typically respond to imatinib mesylate, the first line KIT tyrosine kinase inhibitor. Instead, second generation tyrosine kinase inhibitors, such as sunitib malate, may be more effective, as noted for pediatric GISTs.1 Lifelong follow-up is necessary for the potential of liver metastases and accompanying tumors, especially paragangliomas, for which also radiologic screening might be indicated. In this retrospective study, only a few patients received imatinib for some periods of time and no one sunitinib, so that data are insufficient for further comments on effectiveness of tyrosine kinase inhibitor treatment, especially in view of generally slow natural course of disease.
In conclusion, we analyzed a series of 66 gastric SDH-deficient GISTs. Most patients were young, with a small population of older adults. Only 4 patients had accompanying tumors defining Carney triad, and none of the patients was found to have germline mutations of SDH subunits defining Carney-Stratakis syndrome. SDH-deficient GISTs are KIT/PDGFRA wild type, typically have plexiform muscularis propria involvement, epithelioid morphology, lymphovascular invasion, and some also have lymph node metastases. Multiple synchronous or asynchronous tumors are common leading to gastric recurrences. Potential, somewhat unpredictable development of liver metastases (in 20% of patients) after long latency and asynchronous paragangliomas necessitate long-term follow-up.
Supplementary Material
Acknowledgments
This study was supported by the NIH/NCI intramural research program and in part by grant NN402 2092 35 from the Ministry of Science and Higher Education, Republic of Poland.
References
- 1.Agaram NP, Laquaglia MP, Ustum B, et al. Molecular characterization of pediatric gastrointestinal tumors. Clin Cancer Res. 2008;14:3204–3215. doi: 10.1158/1078-0432.CCR-07-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agaram NP, Wong GC, Guo T, et al. Novel BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosom Cancer. 2008;47:853–859. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnichon N, Rohmer V, Amar L, et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J Endocr Clin Metab. 2009;94:2817–2827. doi: 10.1210/jc.2008-2504. [DOI] [PubMed] [Google Scholar]
- 4.Burnichon N, Briere JJ, Libe R, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011–3020. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carney JA. Carney triad: a syndrome featuring paraganglionic, adrenocortical, and possibly other endocrine tumors. J Clin Endocr Metab. 2009;94:3656–3662. doi: 10.1210/jc.2009-1156. [DOI] [PubMed] [Google Scholar]
- 6.Cervera AM, Apostolova N, Crespo FL, et al. Cells silenced for SDHB expression display characteristic features of the tumor phenotype. Cancer Res. 2008;68:4058–4067. doi: 10.1158/0008-5472.CAN-07-5580. [DOI] [PubMed] [Google Scholar]
- 7.Dahia PLM, Ross KN, Wright ME, et al. A HIF regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genetics. 2005;1:72–79. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaal J, Stratakis CA, Carney JA, et al. SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol. 2011;24:147–151. doi: 10.1038/modpathol.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill AJ, Benn DE, Chou A, et al. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromocytoma syndromes. Hum Pathol. 2010;34:636–644. doi: 10.1016/j.humpath.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Gill AJ, Chou A, Vilain R, et al. Immunohistochemistry for SDHB divides gastrointestinal stromal tumors (GISTs) into 2 distinct types. Am J Surg Pathol. 2010;34:805–814. doi: 10.1097/PAS.0b013e3181d6150d. [DOI] [PubMed] [Google Scholar]
- 11.Gimenez-Roqueplo AP, Favier J, Rustin P, et al. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am J Hum Genet. 2001;69:1186–1197. doi: 10.1086/324413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 13.Hes FJ, Weiss MM, Woortman SA, et al. Low penetrance of a SDHB mutation in a large Dutch paraganglioma family. BMC Med Genet. 2010;11:92–97. doi: 10.1186/1471-2350-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit gene in gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 15.Horvath R, Abicht A, Holinski-Feder E, et al. Leigh syndrome caused by mutations in the flavoprotein (Fp) subunit of succinate dehydrogenase (SDHA) JNeurol Neurosurg Psychiatry. 2006;77:74–76. doi: 10.1136/jnnp.2005.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hostein I, Faur N, Primois C, et al. BRAF mutation status in gastrointestinal stromal tumors. Am J Clin Pathol. 2010;133:141–148. doi: 10.1309/AJCPPCKGA2QGBJ1R. [DOI] [PubMed] [Google Scholar]
- 17.Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita K, Hirota S, Isozaki K, et al. Absence of c-kit gene mutations in gastrointestinal stromal tumours from neurofibromatosis type 1 patients. J Pathol. 2004;202:80–85. doi: 10.1002/path.1487. [DOI] [PubMed] [Google Scholar]
- 20.Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008;53:245–266. doi: 10.1111/j.1365-2559.2008.02977.x. [DOI] [PubMed] [Google Scholar]
- 21.Matyakhina L, Bei TA, McWhinney SR, et al. Genetics of Carney triad: recurrent losses of chromosome 1 but lack of germline mutations in genes associated with paragangliomas and gastrointestinal stromal tumors. J Clin Endocr Metab. 2007;92:2938–2943. doi: 10.1210/jc.2007-0797. [DOI] [PubMed] [Google Scholar]
- 22.McWhinney SR, Pasini B, Stratakis CA. Familial gastrointestinal stromal tumors and germ-line mutations. N Engl J Med. 2007;357:1054–1056. doi: 10.1056/NEJMc071191. [DOI] [PubMed] [Google Scholar]
- 23.Miettinen M, Paal E, Lasota J, et al. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol. 2002;26:301–311. doi: 10.1097/00000478-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach. A clinicopathologic, immunohistochemical and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 25.Miettinen M, Lasota J, Sobin LH. Gastrointestinal stromal tumors of the stomach in children and young adults. A clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol. 2005;29:1373–1381. doi: 10.1097/01.pas.0000172190.79552.8b. [DOI] [PubMed] [Google Scholar]
- 26.van Nederveen FH, Gaal J, Favier J, et al. An immunohistochemical procedure to detect patients with paraganglioma and pheochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol. 2009;10:764–771. doi: 10.1016/S1470-2045(09)70164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantaleo MA, Astolfi A, Indio V, et al. SDHA loss-of-function mutations in KIT-PDGFRA wild-type gastrointestinal stromal tumors identified by massively parallel sequencing. J Natl Cancer Inst. 2011;103:1–5. doi: 10.1093/jnci/djr130. [DOI] [PubMed] [Google Scholar]
- 28.Pasini B, McWhinney SR, Bei T, et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Human Genet. 2008;16:79–88. doi: 10.1038/sj.ejhg.5201904. [DOI] [PubMed] [Google Scholar]
- 29.Pasini B, Stratakis CA. SDH mutations in tumorigenesis and inherited endocrine tumours: lesson from the phaechromocytoma-paraganglioma syndromes. J Int Med. 2009;266:19–42. doi: 10.1111/j.1365-2796.2009.02111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry CG, Young WF, McWhinney SR, et al. Functioning paraganglioma and gastrointestinal stromal tumor of the jejunum in three women. Syndrome or coincidence. Am J Surg Pathol. 2006;30:42–49. doi: 10.1097/01.pas.0000178087.69394.9f. [DOI] [PubMed] [Google Scholar]
- 31.Pollard PJ, Briere JJ, Alam NA, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1α in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 32.Prakash S, Sarran L, Socci N, et al. Gastrointestinal stromal tumors in children and young adults. A clinicopathologic, molecular, and genomic study of 15 cases and review of the literature. J Pediatr Hematol Oncol. 2005;27:179–187. doi: 10.1097/01.mph.0000157790.81329.47. [DOI] [PubMed] [Google Scholar]
- 33.Rege TA, Wagner AJ, Corless CL, et al. “Pediatric type” gastrointestinal stromal tumors in adults: distinctive histology predicts genotype and clinical behavior. Am J Surg Pathol. 2011;35:494–504. doi: 10.1097/PAS.0b013e31820e5f7d. [DOI] [PubMed] [Google Scholar]
- 34.Rutter R, Winge DR, Shiffman JD. Succinate dehydrogenase – Assembly, regulation and role in human disease. Mitochondrion. 2010;10:393–401. doi: 10.1016/j.mito.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stratakis CA, Carney JA. The triad of paragangliomas, gastric stromal tumours, and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications. J Int Med. 2009;266:43–52. doi: 10.1111/j.1365-2796.2009.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanharanta S, Buchta M, McWhinney SR, et al. Early onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet. 2004;74:153–159. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang JH, Lasota J, Miettinen M. Succinate dehydrogenase subunit B (SDHB) is expressed in neurofibromatosis 1-associated gastrointestinal stromal tumors (GISTs): Implications for the SDHB expression based classification of GISTs. J Cancer. 2011;2:90–93. doi: 10.7150/jca.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Smyrk TC, Young WF, et al. Gastric stromal tumors in Carney triad are different clinically, pathologically, and behaviorally from sporadic gastric gastrointestinal stromal tumors: Findings in 104 cases. Am J Surg Pathol. 2010;34:53–64. doi: 10.1097/PAS.0b013e3181c20f4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.