Abstract
We aimed to compare the positivity of the QuantiFERON TB gold in-tube (QFT-IT antigens) specific Interferon gamma (IFN-γ/QFT-IT) and IFN-γ nducible protein-10 (IP-10/QFT-IT) assays with tuberculin skin test (TST) among human immunodeficiency virus (HIV) infected individuals in a TB endemic setting. A total of 180 HIV infected subjects, with no evidence of active TB were recruited. IFN-γ nd IP-10 levels specific to QFT-IT antigens were measured in plasma from QFT-IT tubes. The overall positivity of TST at 5mm cut-off point (19%) was significantly lower when compared to IFN-γ/QFT-IT (38%) and IP-10/QFT-IT (45%) assays. The positivity of IP-10/QFT-IT was significantly higher than IFN-γ/QFT-IT (p=0.038). Indeterminate results for IFN-γ/QFT-IT and IP-10/QFT-IT were more frequent in subjects with CD4 count <100 cells/µl, than those with >100 cells/µl. IFN-γ/QFT-IT (9%) yielded significantly higher number of indeterminate results than IP-10/QFT-IT (5%). The frequency of these responses is higher than the proportion of individuals with positive TST results. However, 6 IFN-γ/QFT-IT or IP-10/QFT-IT negative subjects were positive for TST at 5mm cut-off point. Prospective and prognostic studies are required to clarify the significance of these data.
Keywords: Tuberculosis, human immunodeficiency virus, diagnosis, QuantiFERON-TB Gold in-tube, interferon gamma inducible protein-10, tuberculin skin test
Introduction
Individuals with human immunodeficiency virus (HIV) infection are at increased risk of rapid progression of a recently acquired tuberculous infection, as well as of re-activation of latent tuberculosis infection (LTBI). Studies from many parts of the world have shown higher incidence of tuberculosis (TB) among HIV infected, ranging from 5 to 10% per year of observation (Markowitz et al., 1997), which is in sharp contrast to the lifetime risk of 10% among people without HIV. The detection and treatment of LTBI with isoniazid has proven efficacy in reducing incidence of active disease (Grant et al., 2005, Pape et al., 1993), but as a policy it is poorly implemented. Lack of accurate diagnostic tool for LTBI is one among the barriers.
For many years, LTBI has been identified using the tuberculin skin test (TST). Despite its widespread use, TST unfortunately suffers from major limitations due to cross-reactions with a wide range of environmental mycobacteria and Bacillus Calmette-Guérin (BCG) vaccination (Andersen and Brennan, 1994), and its sensitivity has been shown to be substantially reduced in HIV infected individuals (Converse et al., 1997).
The introduction of interferon gamma (IFN-γ) based rapid blood tests, called interferon gamma release assays (IGRA) for the diagnosis of LTBI seems to be a significant upgrade of the century-old TST. Unlike TST, IGRA is less influenced by environmental mycobacteria and prior BCG vaccination (Pai et al., 2008). IGRA is commercially available in two formats: the T-SPOT.TB assay (Oxford Immunotec, Oxford, England) is an ELISPOT assay that uses peripheral blood mononuclear cells and QuantiFERON-TB Gold in-tube (QFT-IT; Cellestis Ltd., Victoria, Australia) is an ELISA assay utilizing whole blood. A number of studies reported that IGRA using TB specific antigens were more specific than the TST for the diagnosis of LTBI in endemic settings (Adetifa et al., 2007, Hill et al., 2005, Whalen et al., 2006, Pai et al., 2009). There is, however, little evidence on the value of these assays among immunocompromised individuals such as those with HIV infection.
Earlier, the screening of potential markers for TB diagnosis has identified the interferon gamma inducible protein-10 (IP-10) as a potential diagnostic marker for TB infection (Ruhwald et al., 2008, Lighter et al., 2009, Dheda et al., 2009, Whittaker et al., 2008, Kabeer et al., 2010, Goletti et al., 2010a, Goletti et al., 2010b, Syed Ahamed Kabeer et al., 2010). IP-10 is mainly secreted by macrophages and monocytes upon stimulation with antigen specific T cells (Moser and Loetscher 2001, Ragno et al., 2001, Dhillon et al., 2007). Our earlier study conducted in HIV-TB patients also showed the higher secretion of IP-10 in response to TB antigens of the QFT-IT (QFT-IT antigens) (Kabeer et al., 2010).
In this study, we compared the positivity of QFT-IT antigens specific IFN-γ and IP-10 assays with TST in HIV infected individuals in a setting of high prevalence to TB and HIV, In India, the prevalence of HIV infection was 747/100,000 among adults and the prevalence of TB was 310/100,000 in 2005 (WHO, 2008).
Materials and methods
This study was approved by Institutional Ethical Committee of Tuberculosis Research Centre, Chennai. A written and informed consent was obtained from all the study participants before drawing blood.
Study subjects recruitment
The recruitment of study subjects was done at Government Hospital of Thoracic Medicine, Chennai during April 2007 and March 2009. The subjects identified as HIV infected and willing to participate in this study were assessed. Individuals with previous history of TB, silicosis, end stage renal disease, leukemia / lymphoma, who had TST in the past 16 months, who received chemoprophylaxis, those under anti retroviral therapy or any other immunosuppressive therapy were excluded from the study.
Eligible subjects were confidentially interviewed and subjected to clinical, radiological and microbiological investigations to rule out the presence of active TB. Only the subjects with no evidence of active TB were included for this study. Blood was drawn from all the subjects for total blood count, HIV serology (further to confirm the HIV positivity) and QFT-IT. TST was carried out, after drawing the blood samples.
HIV testing
The HIV status was confirmed by 2 rapid tests (Retroquic Comb Aids-RS, Span Diagnostics, Surat, India and HIV TRI-DOT, J. Mitra & Co, New Delhi, India). When a serum was positive for both tests, it was considered as HIV positive. If a serum was positive for only one EIA (which was rare), Western Blot was done as confirmatory test.
CD4 count
The CD4 cell count was estimated in blood samples of HIV positive individuals by flow cytometry (Syed Ahamed Kabeer et al., 2009) using a combination of anti CD3-FITC, anti CD4-PE and anti CD8-APC (BD Biosciences, CA, USA). The acquisition and analysis were carried out by and Flowjo Software (Tree star, Inc., CA, USA) respectively.
QFT-IT antigens specific IFN-γ assay
QFT-IT antigens specific IFN-γ release assay was performed using commercial QFT-IT (Cellestis Ltd., Victoria, Australia) kits and the results were analyzed using the software supplied by the manufacturer and interpreted as per kit guidelines.
QFT-IT antigens specific IP-10 assay
QFT-IT antigens specific IP-10 levels were measured in the same supernatants as for IFN-γ, from QFT-IT tubes using BD Opt-EIA kits (BD Biosciences, CA, USA) as per manufacture’s instruction (Kabeer et al., 2010, Syed Ahamed Kabeer et al., 2010). The cut-off value 300pg/ml for QFT-IT antigens was determined based on our earlier studies (Kabeer et al., 2010, Syed Ahamed Kabeer et al., 2010). The receiver operating characteristic curve analysis performed with HIV negative healthy subjects who were negative for IFNγ/QFT-IT and TST and pulmonary TB patients showed 300pg/ml as optimal cut-off point for QFT-IT antigens specific IP-10 response.
The subjects with net IP-10 ≥300pg/ml for QFT-IT antigens (QFT-IT antigen – QFT-IT NIL), irrespective of QFT-IT mitogen response were considered as positive; <300pg/ml for QFT-IT antigens and ≥200pg/ml for QFT-IT mitogen were considered as negative; others (<300pg/ml for QFT-IT antigen and <200pg/ml for QFT-IT mitogen) were considered as indeterminate.
Tuberculin Skin Test
The 2 TU (tuberculin unit) of purified protein derivative (PPD) RT23 (Statens Serum Institute, Copenhagen, Denmark) was injected intradermally by Mantoux method and the induration was measured between 48–72 hours after PPD injection by trained professionals.
Statistical analysis
Data were analyzed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, California, USA). In the previous studies, when calculating sensitivity of IFN-γ/QFT-IT, indeterminate results were excluded from the analysis. However, this approach artificially inflates the test sensitivity. The recent studies recommend considering the indeterminate results as negatives for sensitivity calculation, since the indeterminate results do not have any clinical significance (Raby et al., 2008). Thus, to provide the clear picture of IFN-γ/QFT-IT, we did not exclude indeterminate results and combined with negative results for agreement analysis. Positivity was therefore defined as number of positive results over total number tested. Kruskal-Wallis test was carried out to calculate the differences of IFN-γ/QFT-IT and IP-10/QFT-IT levels between the groups. The comparison between the tests was done by using McNemar Chi-square tests, by treating the data as paired. We dropped the 10 individuals who did not have IP-10/QFT-IT data, when comparing the IP-10/QFT-IT positivity with TST and IFN-γ/QFT-IT. The strength of agreement between the tests was measured by using kappa statistics.
Results
A total of 180 HIV infected subjects were recruited. The demographic profile of the study subjects is given in Table 1. The median age of the study subjects was 34 years (IQR: 27.8, 39.3 years) and 62% of them were males.
Table 1.
Demographic and baseline characteristics of study subjects
| Category | Subcategory | N (%) or Median (Range; IQR) |
|---|---|---|
| Sex, Number (%) | Male | 112 (62) |
| Female | 68 (38) | |
| Age, Median in years (Range; IQR) | 34 (18–61; 28–39) | |
| HIV strain, Number (%) | HIV-I | 154 (85) |
| HIV-II | 3 (2) | |
| HIV-I&II | 23 (12) | |
| CD4 cell count (cells/µl)* | <100 | 43 (28) |
| 101–200 | 33 (22) | |
| 201–350 | 33 (22) | |
| >350 | 43 (28) |
IQR-Inter quartile range
N – Number of subjects
% - Percentage
HIV – Human immunodeficiency virus
Data available for 152 subjects
IP-10/QFT-IT and IFN-γ/QFT-IT levels
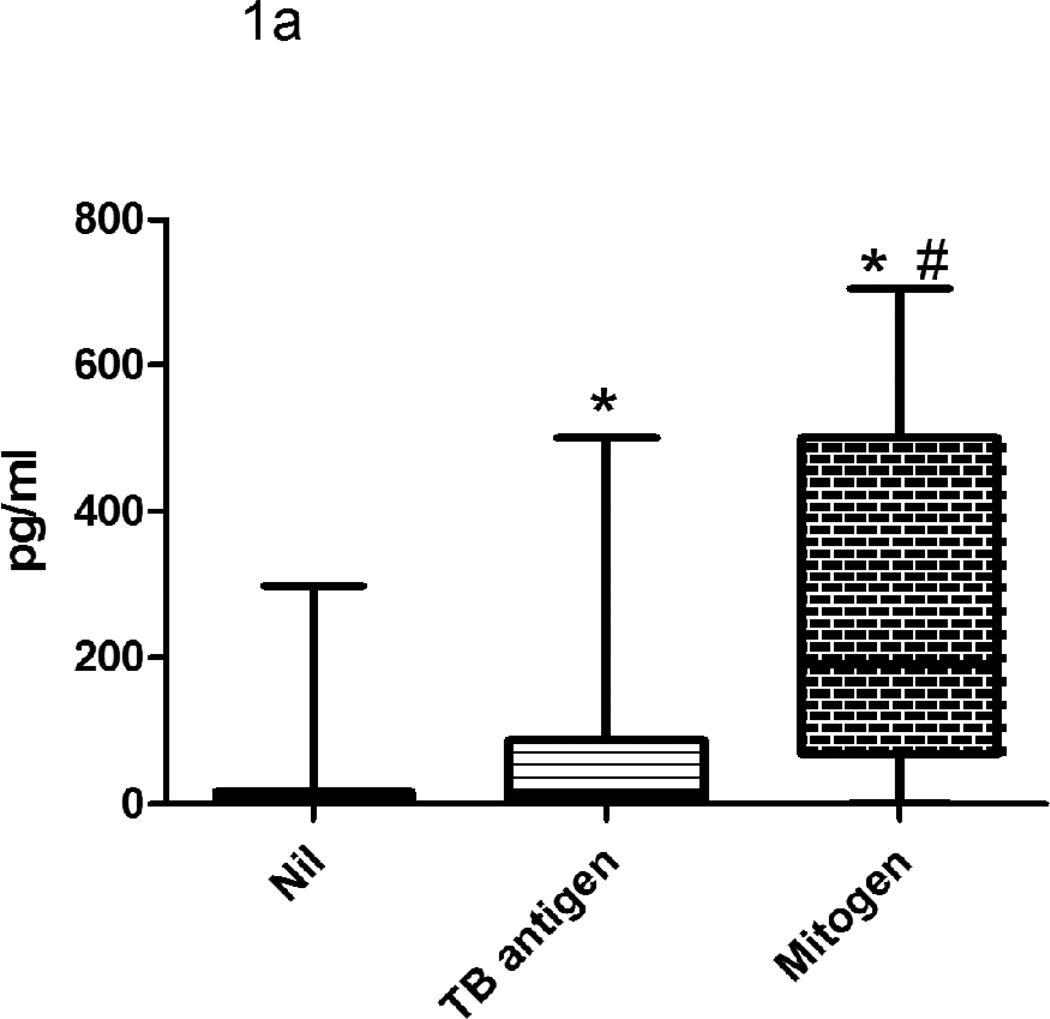
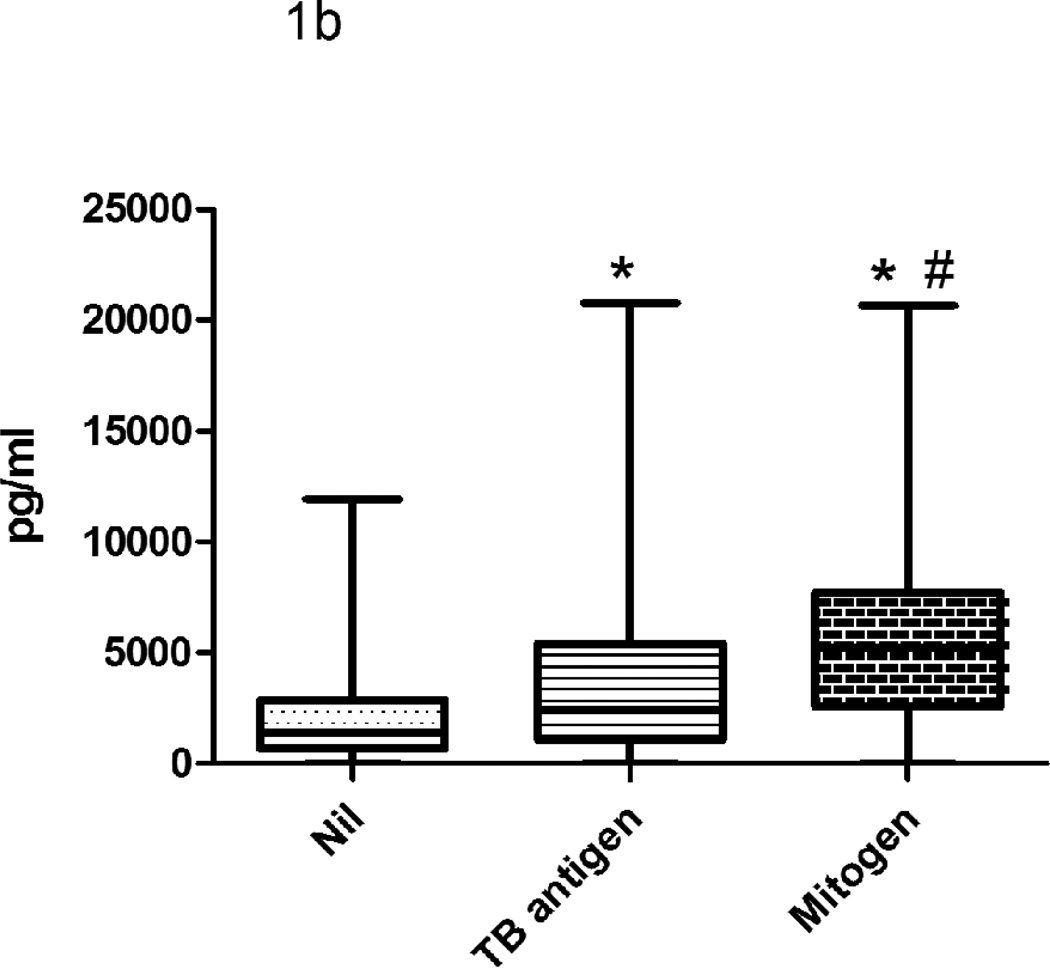
The NIL QFT-IT tube plasma concentration had medians of 1387 pg/ml (range 0–11934 pg/ml) for IP-10 and 7.5 pg/ml (range 0–298 pg/ml) for IFN-γ (Fig 1). QFT-IT TB antigens samples had medians of 2448 pg/ml (0–20786 pg/ml) for IP-10 and 15.8 pg/ml (range 0–500 pg/ml) for IFN-γ. QFT-IT Mitogen samples had medians of 5199 pg/ml (range 0–20648 pg/ml) for IP-10 and 191.3 pg/ml (range 0–705 pg/ml) for IFN-γ. The levels of IFN-γ and IP-10 were significantly higher in QFT-IT TB antigens and QFT-IT mitogen stimulated samples when compared to QFT-IT NIL samples.
Figure 1. The levels of IFN-γ (a) and IP-10 (b) secretion in QFT-IT NIL, QFT-IT TB antigen stimulated and QFT-IT mitogen stimulated samples.
The levels of IFN-γ and IP-10 were significantly higher in QFT-IT TB antigens stimulated, as well as QFT-IT mitogen stimulated samples when compared to QFT-IT NIL samples. Box and Whisker plots show range, inter-quartile range and median. The difference between the groups was calculated by Kruskal-Wallis test. *significant difference p<0.001 compared to unstimulated sample, # significant difference p<0.001 compared to QFT-IT TB specific antigens stimulated sample. QFT-IT – QuantiFERON-TB Gold in-tube, TB- Tuberculosis
Positivities of IFN-γ/QFT-IT, IP-10/QFT-IT and TST
Of 180 HIV infected subjects tested, IFN-γ/QFT-IT was positive in 68 (38%; 95% confidence interval: 31–45), negative in 95 (53%; 95%CI: 46–60) and indeterminate in 17 (9%; 95%CI: 5–13) subjects (Table 2). Due to the plasma sample insufficiency, data of IP-10/QFT-IT were missing for 10 samples. Thus, IP-10/QFT-IT results were available for 170 subjects. There was no difference in the demographic profile and baseline characteristics between subjects with and without IP-10/QFT-IT results. The positivity of IFN-γ/QFT-IT (38.2% and 30.8% respectively) and IP-10/QFT-IT (44.4% and 33.3% respectively) did not differ significantly respectively (p=0.684).
Table 2.
Performance of QFT-IT and TST
| Tests | Number of subjects, % (95% CI) | |||
|---|---|---|---|---|
| Positive | Negative | Indeterminate | Net positive | |
| QFT-IT | 38 (31–45) | 53 (46–60) | 9 (5–13) | 42 (34–50) |
| IP-10 | 45 (38–52) | 50 (42–58) | 5 (2–8) | 47 (39–55) |
| TST (5mm) | 19 (13–25) | 81 (75–87) | NA | NA |
| TST (10mm) | 15 (10–20) | 85 (80–90) | NA | NA |
Net positivity was calculated by excluding the indeterminate results from the analysis.
QFT-IT - QuantiFERON TB-Gold in-tube
IP-10 – Interferon gamma inducible protein-10
TST- Tuberculin Skin test
CI – Confidence interval
With the cut-off value 300 pg/ml for QFT-IT TB antigens, IP-10/QFT-IT was positive in 76 (45%; 95%CI: 38–52) subjects, negative in 85 (51%; 95%CI: 42–58) subjects and indeterminate in 9 (5%; 95%CI: 2–8) subjects. All the indeterminate results were due to poor response to mitogen. When the cut-off value 450 pg/ml was used, 6 subjects became negative. Thus, IP-10/QFT-IT was positive in 70 (41%) negative in 91 (54%) and indeterminate in 9 (5%) subjects.
When the results were considered as binary values (negative results included indeterminate results), positivity of IP-10/QFT-IT was significantly higher than IFN-γ/QFT-IT (Mc Nemar’s test; p=0.038). However, this significance was not observed when indeterminate results were excluded from the analysis (p=0.153). IFN-γ/QFT-IT yielded significantly higher number of indeterminate results than IP-10/QFT-IT (p=0.013). TST was positive in 34 (19%; 95% CI: 13–25) and 27 (15%; 95%CI: 10–20) subjects out of 180 subjects at 5mm and 10mm cut-off points respectively and the difference reached statistical significance (p=0.0412). The positivity of TST was significantly lower than IFN-γ/QFT-IT and IP-10/QFT-IT at both (5mm and 10mm) cut-of points (p<0.001). However, 6 IFN-γ/QFT-IT or IP-10/QFT-IT negative subjects were positive for TST at 5mm cut-off point.
The results for IP-10/QFT-IT and IFN-γ/QFT-IT were available for 170 subjects. Of them either IFN-γ/QFT-IT or IP-10/QFT-IT was positive in 82 (48%, 95% CI: 40%–56%), negative in 79 (46%; 95%CI: 39%–53%) and indeterminate in 9 (5%; 95% CI:2%–8%) subjects. The Positivity of combination of IFN-γ/QFT-IT and IP-10/QFT-IT was significantly higher than IFN-γ/QFT-IT but not than IP-10/QFT-IT.
Agreement between the tests
The agreements between the three tests are given in Table 3. To measure the agreement between the tests, the results of IFN-γ/QFT-IT and IP-10/QFT-IT were transformed into binary values by combining negative and indeterminate values. There was a good agreement between IP-10/QFT-IT and IFN-γ/QFT-IT observed (k=0.66). The agreement of TST at 5mm cut-off point with IFN-γ/QFT-IT was 74% (k=0.38), whereas with IP-10/QFT-IT, it was slightly lower (k=0.29). The difference in the cut-off point for TST did not influence the agreement between the tests.
Table 3.
| a. Agreement between TST and IFN-γ | ||
|---|---|---|
| Test Results | 5mm | 10mm |
| Positive TST/Positive QFT-IT | 27 | 25 |
| Negative TST/Negative QFT-IT | 106 | 110 |
| Positive TST/Negative QFT-IT | 6 | 2 |
| Negative TST/Positive QFT-IT | 41 | 43 |
| Agreement % | 73.9% | 75% |
| k (95% CI) | 0.38 (0.23–0.53) | 0.40 (0.24–0.55) |
| b. Agreement between TST and IP-10 | ||
|---|---|---|
| Test Results | 5mm | 10mm |
| Positive TST/Positive IP-10 | 25 | 22 |
| Negative TST/Negative IP-10 | 89 | 91 |
| Positive TST/Negative IP-10 | 5 | 3 |
| Negative TST/Positive IP-10 | 51 | 54 |
| Agreement % | 67.1 | 66.5 |
| k (95% CI) | 0.29 (0.14–0.44) | 0.28 (0.12–0.43) |
| c. Agreement between IFN-γ and IP-10 | ||||
|---|---|---|---|---|
| Tests | IP-10 | |||
| QFT-IT | Positive | Negative | Total | |
| Positive | 56 | 8 | 64 | |
| Negative | 20 | 86 | 106 | |
| Total | 76 | 94 | 170 | |
| Agreement % | 83.5 | |||
| K (95% CI) | 0.66 (0.55–0.78) | |||
QFT-IT - QuantiFERON TB-Gold in-tube
IP-10 – Interferon gamma inducible protein-10
TST- Tuberculin Skin test
CI – Confidence interval
k – kappa
% - Percentage
Influence of immunosuppression
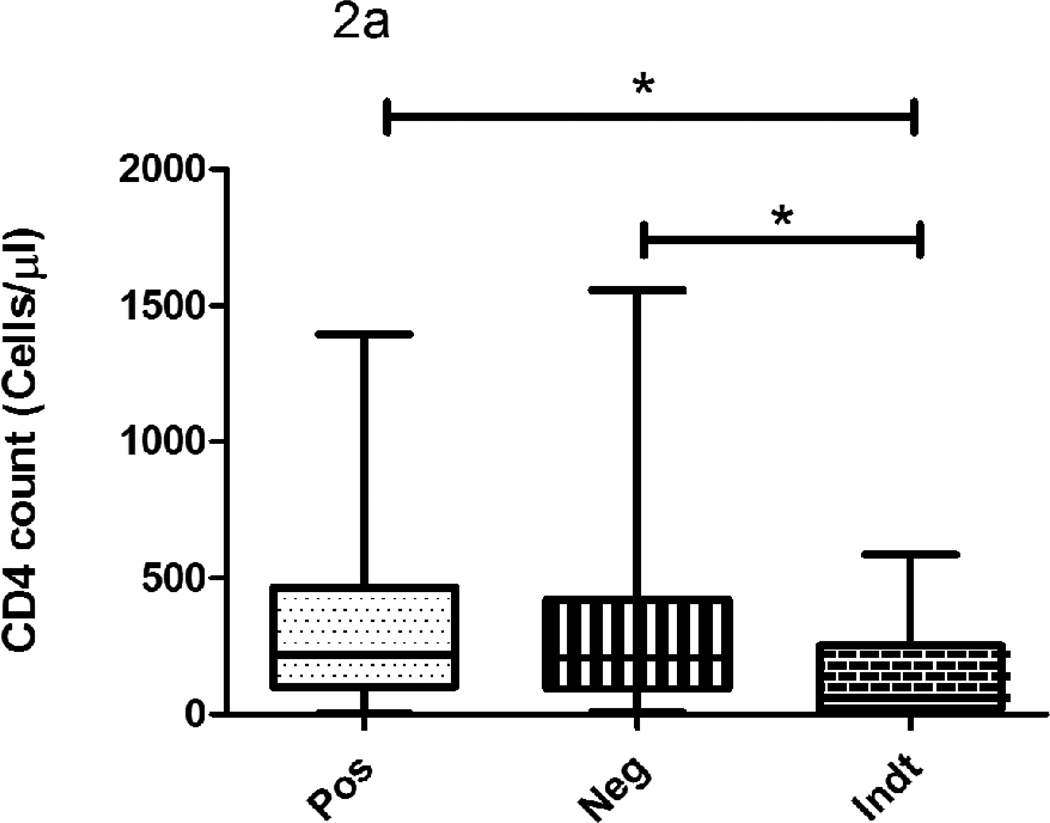
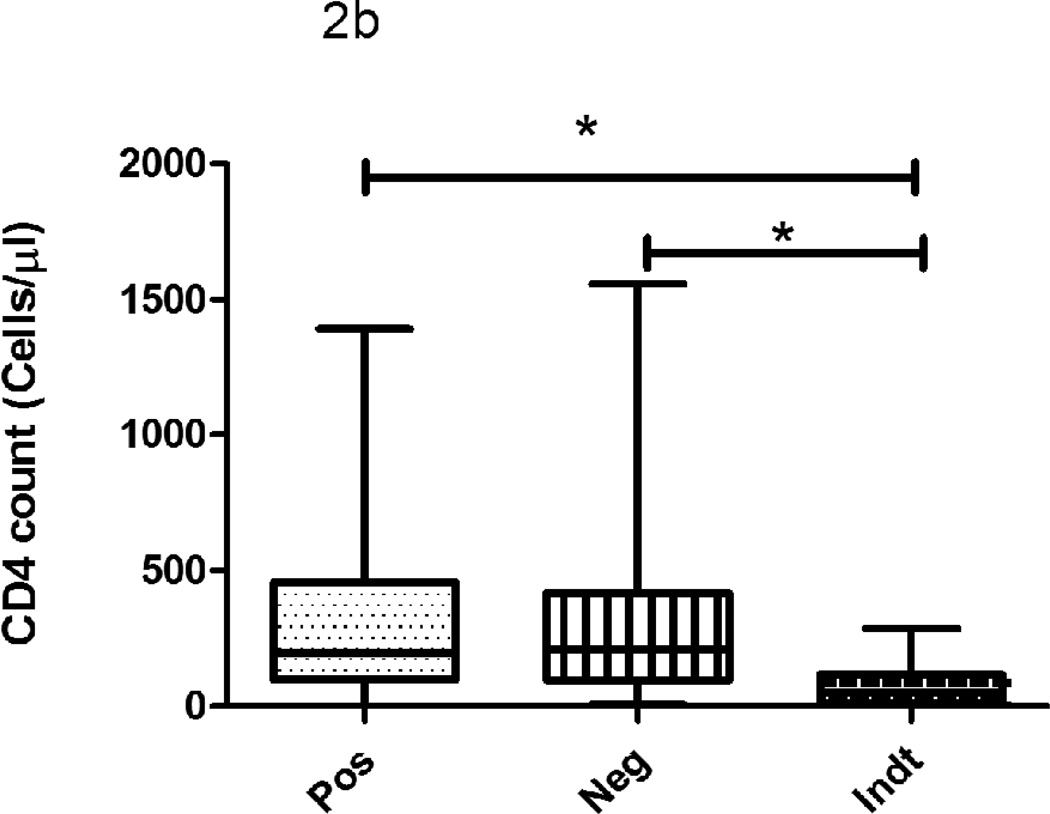
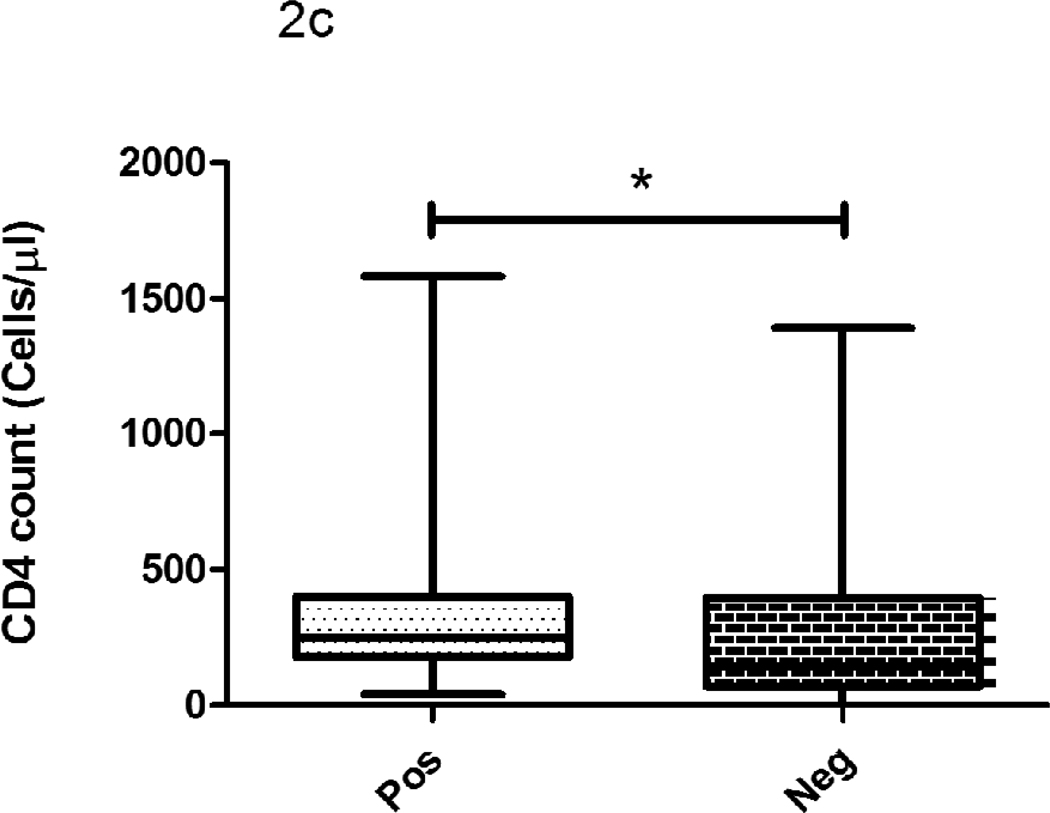
The median CD4 count did not differ significantly between IFN-γ/QFT-IT positive and negative subjects but they were significantly lower in indeterminate subjects when compared to IFN-γ/QFT-IT positives or negatives (Fig 2). The proportion of IFN-γ/QFT-IT indeterminate results was significantly higher in subjects with <100 cells/µl (14 out of 17 subjects; 82%), when compared to subjects with >100 cells/µl (3 out 17 subjects; 18%). Except one subject out of nine, other 8 IP-10/QFT-IT indeterminate subjects (89%) had CD4 cell count <100 cells/µl. However, there was no significant difference observed on CD4 count between IFN-γ/QFT-IT and IP-10/QFT-IT indeterminate results. When comparing the TST positive and negative subjects, the cell counts were significantly lower in TST negative than positive subjects.
Figure 2. The influence of CD4 count on IFN-γ/QFT-IT (a), IP-10/QFT-IT (b) assays and TST (c) outcomes.
The decline of CD4 count was associated with IFN-γ/QFT-IT or IP-10/QFT-IT indeterminate only but not with IFN-γ/QFT-IT or IP-10/QFT-IT negative. The CD4 count was significantly low in TST negatives than TST positives. Box and Whisker plots show range, inter-quartile range and median. QFT-IT–Quantiferon TB Gold in-tube, IP-10 – Interferon gamma inducible protein-10, TST – Tuberculin skin test, *significant difference p<0.05 by Kruskal-Wallis test.
Discussion
The World Health Organization (WHO) currently recommends treatment of LTBI in developing countries for HIV infected patients (WHO, 1998). National guidelines for Thailand—one of the few TB endemic countries with an established LTBI treatment program for HIV infected individuals also recommend the treatment (Hiransuthikul et al., 2005). In all these policies, TST is mentioned as test for LTBI diagnosis. But the high number of false negativity by TST due to the HIV associated anergy (Karam et al., 2008) urges the need for alternative tests for LTBI to make those polices to work better. Of note TST does not have indeterminate results, unlike IFN-γ/QFT-IT. This study compared the IFN-γ/QFT-IT and IP-10/QFT-IT based in-house ELISA method with TST and reported that positivity of IFN-γ/QFT-IT and IP-10/QFT-IT was higher than TST. When comparing IFN-γ/QFT-IT and IP-10/QFT-IT, the latter yielded less indeterminate results than the former. To our knowledge, this is the first study comparing the positivity of IFN-γ/QFT-IT and IP-10/QFT-IT with TST in HIV positive cohort from a setting which has high prevalence to both TB and HIV.
Studies which evaluated IGRA in HIV negative cohorts from endemic countries indicate that IGRA correlates well along with gradient of exposure to M. tuberculosis and performs as good as TST (Dheda et al., 2009). But significantly lower positivity observed for TST, in comparison with IFN-γ/QFT-IT and IP-10/QFT-IT, in this study indicates TST might have failed to detect some of the LTBI cases among HIV infected subjects. However, further Isoniazid preventive trails are needed to confirm which test really gives relevant information.
An earlier study that assessed the performance of TST using 1 TU PPD (Statens Serum Institut, Denmark) in our settings among HIV infected individuals, also reported the depressed positivity even in subjects with >500 cell/µl and it went worse, when CD4 cell count decreased further (Swaminathan et al., 2008). In this study, we report, similar to findings in other endemic countries, the positivity of TST even at 2 TU strength is low among HIV infected individuals in our setting. Of note, in our earlier study, TST at 2 TU strength was positive only in 31% of HIV-TB patients (Syed Ahamed Kabeer et al., 2009).
Indeed, TST has several practical disadvantages like including the necessity for a second visit to the health clinic to assess the result (many subjects fail to return), inter-operator variation as a result of subjective assessment of the test result, a booster phenomenon in repeated testing can result in a false positive result (Huebner et al., 1993, ATS and CDC, 2000). In addition, indeterminate results cannot be interpreted using TST. Although IFN-γ/QFT-IT or IP-10/QFT-IT based in vitro tests are relatively costlier and require appropriately equipped laboratory, they are rapid and overcome all other practical disadvantages of TST (Pai et. al., 2006).
However, five TST positive subjects at 5mm cut-off point were negative for both IFN-γ/QFT-IT and IP-10/QFT-IT and another 2 TST positive subjects were negative for one of the two tests. The antigens used in IFN-γ/QFT-IT are present only M. tuberculosis complex and very few atypical mycobacteria. Earlier studies also suggested that IFN-γ/QFT-IT was less affected by prior BCG vaccination and exposure to environmental mycobacteria (Pai et al., 2008). Since the IP-10/QFT-IT was measured in the same IFN-γ/QFT-IT supernatants, it is unlikely that IP-10/QFT-IT as well as IFN-γ/QFT-IT, yielded false positive results due to the exposure to environmental mycobacteria. Therefore, we hypothesis that the positivity observed only for TST in five subjects might reflect false positivity of TST, associated with non tuberculosis mycobacterial infection. Of note, the effect on TST of BCG received in infancy is minimal, especially ≥10 years after vaccination (Farhat et al., 2006). BCG is given once at birth in our setting and our earlier studies showed that the proportion of TST positive subjects was similar in subjects with and without BCG scar (Kabeer et al., 2010).
But the 2 TST positive subjects, who was positive either for IFN-γ/QFT-IT or IP-10/QFT-IT indicated the IFN-γ/QFT-IT and IP-10/QFT-IT possibly failed to pick few true LTBI cases. It is noteworthy to mention that there was a significant disagreement observed between IFN-γ/QFT-IT and TST in earlier studies in HIV seronegative TB contacts (Pai et al., 2005). Further studies are needed on this issue to recommend replacement of TST by IFN-γ/QFT-IT or IP-10/QFT-IT assays.
Elevated level of IP-10 was shown in serum and plural fluid from the TB patients (Dheda et al., 2009, Okamoto et al., 2005, Azzuri et al., 2005, Juffermans et al., 1999). The secretion of IP-10 was also shown to be induced by M. tuberculosis specific antigens and hence suggested as an alternative test for LTBI diagnosis (Ruhwald et al., 2008, Lighter et al., 2009, Dheda et al., 2009, Whittaker et al., 2008, Kabeer et al., 2010, Goletti et al., 2010a, Goletti et al., 2010b, Syed Ahamed Kabeer et al., 2010). More recently, we showed a higher level of IP-10 secretion in response to M. tuberculosis specific antigens in cultures of whole blood from HIV positive patients with TB (Kabeer et al., 2010). At 300 pg/ml cut-off point, as used in this study, we found 86% of positivity (when excluding the indeterminate results it was 96%) among sputum smear positive HIV-TB patients. Interestingly, none of the TST and IFN-γ/QFT-IT negative healthy control subjects were positive for IP-10/QFT-IT. Hence, 44% of positivity for IP-10/QFT-IT in HIV infected individuals, while IFN-γ/QFT-IT was positive in 39% of subjects from the same cohort, would reflect the true M. tuberculosis infection rather any non-specific response. Overall, our study results support the notion that IP-10/QFT-IT can be used as potential alternative marker for LTBI and here we suggest it can also be used among HIV infected individuals.
As IFN-γ/QFT-IT and IP-10/QFT-IT assays measure the host immune response, there is a concern, similar to TST, the performance of tests could be impaired when CD4 cell count declines (Balcells et al., 2008). While some of the studies reported that IFN-γ/QFT-IT yielded only indeterminate, but not false negative results in advanced immunosuppression in respect to CD4 count (Syed Ahamed Kabeer et al., 2009, Rangaka et al., 2007, Lagrange et al., 2008, Santin et al., 2001), other studies showed the association of IFN-γ/QFT-IT false negativity with CD4 count (Ferrara et al., 2005, Brock et al., 2006). In our study results, there was no significant difference on CD3, CD4 and CD8 cell counts between IFN-γ/IP-10 positive and negative results. However, both IFN-γ/QFT-IT and IP-10/QFT-IT gave indeterminate results and most of them had CD4 cell count <100 cells/µl. The occurrence of indeterminate results is often pointed out in earlier studies as drawback of IGRA in TB diagnosis especially among individuals with strong immunosuppression. Ferrea et al reported that IFN-γ/QFT-IT showed 20% indeterminate results and it was associated with the severity of immunosuppression (Ferrara et al., 2005). In a study assessing response to M. tuberculosis specific antigens among HIV positive subjects in Denmark using the IFN-γ/QFT-IT, the proportion of indeterminate test results due to low IFN-γ production following mitogen stimulation was significantly higher in patients with low CD4 count (<100 cells/µl) compared to those with high CD4 cell count (>100 cells/µl) (Brock et al., 2006). Our earlier observation in HIV-TB patients also suggested IFN-γ/QFT-IT gave indeterminate results when CD4 cell count declined, particularly below 200 cells/µl. As shown in earlier studies, CD4 cell count was significantly lower in subjects with TST negative results, than in subjects TST positive results.
When comparing the positivity of IFN-γ/QFT-IT and IP-10/QFT-IT, the latter appeared to be higher than the former. While IFN-γ/QFT-IT failed to detect 16 of 75 IP-10/QFT-IT positive subjects and IP-10 failed to detect only 8 of 67 IFN-γ/QFT-IT positive subjects. In the 17 IFN-γ/QFT-IT indeterminate subjects, 8 subjects became determinate results (either positive or negative) for IP-10. The higher sensitivity of IP-10/QFT-IT at 300 pg/ml than IFN-γ/QFT-IT was shown in HIV-TB patients in our earlier findings (Kabeer et al., 2010). The possible reasons for higher positivity might be (i) IP-10 is the amplified signal of IFN-γ, thus low quantity of IFN-γ itself is enough to stimulate large quantity of IP-10 (Moser and Loetscher 2001, Ragno et al., 2001) (ii) IP-10 is also induced by IL-23 and IL-17 producing cells, independent of IFN-γ production (Khader et al., 2007).
To conclude, positivity of IFN-γ/QFT-IT or IP-10/QFT-IT based in vitro assays was higher than TST. They are less influenced by HIV infection, when compared to TST. However, further studies are needed to evaluate the cut-off points used for IP-10 in this study, to identify a more potential assay between IFN-γ/QFT-IT and IP-10/QFT-IT. Further studies should also evaluate the discordance between TST and whole blood assays and which one really provides the evidence of LTBI.
Acknowledgements
The authors wish to thank all the study subjects, who participated in this study.
The work contributed by Mr. Saravanakannan, Counselor, Mrs. Jennath, Health visitor and Mrs. Wincy Saravanan, Laboratory technician is gratefully acknowledged. The authors thank the project consultant Dr. Lee W Riley, Division of Infectious Diseases, School of Public Health, University of California, Berkeley, CA, USA for fruitful discussions. The statistical assistance provided by Dr. Venkatesan Perumal, Head, Department of Statistics, Tuberculosis Research Centre is kindly acknowledged.
Basirudeen S is the recipient of Senior Research Fellowship from Indian Council of Medical Research (ICMR), New Delhi, India.
Alamelu Raja - Conceived and designed the experiments: Principle investigator in the NIH R03 Grant: Corrected the manuscript.
Basirudeen Syed Ahamed Kabeer - Performed the experiments; Analyzed the data; Wrote the paper.
Dr. Rajasekaran - Responsible for patient recruitment
Financial Support:
This project was financially supported by NIH grant [AI064055]. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
None of the authors have any conflict of interest, including specific financial interests or relationships or affiliations to the subject matter or materials discussed in the manuscript.
Contributor Information
S Basirudeen, Email: basir2020@gmail.com.
S Rajasekaran, Email: rajtbaids@gmail.com.
References
- 1.Adetifa IM, Lugos MD, Hammond A, Jeffries D, Donkor S, Adegbola RA, et al. Comparison of two interferon gamma release assays in the diagnosis of Mycobacterium tuberculosis infection and disease in The Gambia. BMC Infect Dis. 2007;7:122. doi: 10.1186/1471-2334-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen AB, Brennan P. Proteins and antigens of Mycobacterium tuberculosis. In: Bloom BR, editor. Tuberculosis: pathogenesis, protection, and control. Washington DC: American Society for Microbiology; 1994. pp. 307–332. [Google Scholar]
- 3.Azzurri A, Sow OY, Amedei A, Bah B, Diallo S, Peri G, et al. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:1–8. doi: 10.1016/j.micinf.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Balcells ME, Perez CM, Chanqueo L, Lasso M, Villanueva M, Espinoza M, et al. A comparative study of two different methods for the detection of latent tuberculosis in HIV-positive individuals in Chile. Int J Infect Dis. 2008;12:645–652. doi: 10.1016/j.ijid.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Brock I, Ruhwald M, Lundgren B, Westh H, Mathiesen LR, Ravn P. Latent tuberculosis in HIV positive, diagnosed by the M. tuberculosis specific interferon-gamma test. Respir Res. 2006;7:56. doi: 10.1186/1465-9921-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Converse PJ, Jones SL, Astemborski J, Vlahov D, Graham NM. Comparison of a tuberculin interferon-gamma assay with the tuberculin skin test in high-risk adults: effect of human immunodeficiency virus infection. J Infect Dis. 1997;176:144–150. doi: 10.1086/514016. [DOI] [PubMed] [Google Scholar]
- 7.Dheda K, van Zyl Smit R, Badri M, Pai M. T-cell interferon-gamma release assays for the rapid immunodiagnosis of tuberculosis: clinical utility in high-burden vs. low-burden settings. Curr Opin Pulm Med. 2009;15:188–200. doi: 10.1097/MCP.0b013e32832a0adc. [DOI] [PubMed] [Google Scholar]
- 8.Dheda K, Van-Zyl Smit RN, Sechi LA, Badri M, Meldau R, Symons G, et al. Clinical diagnostic utility of IP-10 and LAM antigen levels for the diagnosis of tuberculous pleural effusions in a high burden setting. PLoS One. 2009;4:e4689. doi: 10.1371/journal.pone.0004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhillon NK, Peng F, Ransohoff RM, Buch S. PDGF synergistically enhances IFN-gamma-induced expression of CXCL10 in blood-derived macrophages: implications for HIV dementia. J Immunol. 2007;179:2722–2730. doi: 10.4049/jimmunol.179.5.2722. [DOI] [PubMed] [Google Scholar]
- 10.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 11.Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192–1204. [PubMed] [Google Scholar]
- 12.Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, Roversi P, et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2005;172:631–635. doi: 10.1164/rccm.200502-196OC. [DOI] [PubMed] [Google Scholar]
- 13.Goletti D, Raja A, Ahamed Kabeer BS, Rodrigues C, Sodha A, Butera O, et al. IFN-gamma, but not IP-10, MCP-2 or IL-2 response to RD1 selected peptides associates to active tuberculosis. J Infect. 2010a;61:133–143. doi: 10.1016/j.jinf.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Goletti D, Raja A, Syed Ahamed Kabeer B, Rodrigues C, Sodha A, Carrara S, et al. Is IP-10 an accurate marker for detecting M. tuberculosis-specific response in HIV-infected persons? PLoS One. 2010b;5:e12577. doi: 10.1371/journal.pone.0012577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant AD, Charalambous S, Fielding KL, Day JH, Corbett EL, Chaisson RE, et al. Effect of routine isoniazid preventive therapy on tuberculosis incidence among HIV-infected men in South Africa: a novel randomized incremental recruitment study. JAMA. 2005;293:2719–2725. doi: 10.1001/jama.293.22.2719. [DOI] [PubMed] [Google Scholar]
- 16.Hill PC, Fox A, Jeffries DJ, Jackson-Sillah D, Lugos MD, Owiafe PK, et al. Quantitative T cell assay reflects infectious load of Mycobacterium tuberculosis in an endemic case contact model. Clin Infect Dis. 2005;40:273–278. doi: 10.1086/427030. [DOI] [PubMed] [Google Scholar]
- 17.Hiransuthikul N, Nelson KE, Hiransuthikul P, Vorayingyong A, Paewplot R. INH preventive therapy among adult HIV-infected patients in Thailand. Int J Tuberc Lung Dis. 2005;9:270–275. [PubMed] [Google Scholar]
- 18.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993;17:968–975. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 19.Juffermans NP, Verbon A, van Deventer SJ, van Deutekom H, Belisle JT, Ellis ME, et al. Elevated chemokine concentrations in sera of human immunodeficiency virus (HIV)-seropositive and HIV-seronegative patients with tuberculosis: a possible role for mycobacterial lipoarabinomannan. Infect Immun. 1999;67:4295–4297. doi: 10.1128/iai.67.8.4295-4297.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabeer BS, Sikhamani R, Raja A. Comparison of interferon gamma and interferon gamma-inducible protein-10 secretion in HIV-tuberculosis patients. AIDS. 16(24):323–325. doi: 10.1097/QAD.0b013e328334895e. [DOI] [PubMed] [Google Scholar]
- 21.Kabeer BSA, Perumal V, Paramasivan P, Raja A. Yield of QuantiFERON-TB gold in tube assay and tuberculin skin test in healthy subjects from a tuberculosis endemic population. J Pediatrics Infect Dis. 2010;5:1–5. [Google Scholar]
- 22.Karam F, Mbow F, Fletcher H, Senghor CS, Coulibaly KD, LeFevre AM, et al. Sensitivity of IFN-gamma release assay to detect latent tuberculosis infection is retained in HIV-infected patients but dependent on HIV/AIDS progression. PLoS One. 2008;3:e1441. doi: 10.1371/journal.pone.0001441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 24.Lagrange PH, Herrmann JL. Diagnosing Latent Tuberculosis Infection in the HIV Era. Open Respir Med J. 2008;2:52–59. doi: 10.2174/1874306400802010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lighter J, Rigaud M, Eduardo R, Peng CH, Pollack H. Latent tuberculosis diagnosis in children by using the QuantiFERON-TB Gold In-Tube test. Pediatrics. 2009;123:30–37. doi: 10.1542/peds.2007-3618. [DOI] [PubMed] [Google Scholar]
- 26.Markowitz N, Hansen NI, Hopewell PC, Glassroth J, Kvale PA, Mangura BT, et al. Incidence of tuberculosis in the United States among HIV-infected persons. The Pulmonary Complications of HIV Infection Study Group. Ann Intern Med. 1997;15:123–132. doi: 10.7326/0003-4819-126-2-199701150-00005. [DOI] [PubMed] [Google Scholar]
- 27.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto M, Kawabe T, Iwasaki Y, Hara T, Hashimoto N, Imaizumi K, et al. Evaluation of interferon-gamma, interferon-gamma-inducing cytokines, and interferon-gamma-inducible chemokines in tuberculous pleural effusions. J Lab Clin Med. 2005;145:88–93. doi: 10.1016/j.lab.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Pai M, Gokhale K, Joshi R, Dogra S, Kalantri S, Mendiratta DK, et al. Mycobacterium tuberculosis infection in health care workers in rural India: comparison of a whole-blood interferon gamma assay with tuberculin skin testing. JAMA. 2005;293:2746–2755. doi: 10.1001/jama.293.22.2746. [DOI] [PubMed] [Google Scholar]
- 30.Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: part I. Latent tuberculosis. Expert Rev Mol Diagn. 2006;6:413–422. doi: 10.1586/14737159.6.3.413. [DOI] [PubMed] [Google Scholar]
- 31.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pai M, Joshi R, Dogra S, Zwerling AA, Gajalakshmi D, Goswami K, et al. T-cell assay conversions and reversions among household contacts of tuberculosis patients in rural India. Int J Tuberc Lung Dis. 2009;13:84–92. [PMC free article] [PubMed] [Google Scholar]
- 33.Pape JW, Jean SS, Ho JL, Hafner A, Johnson WD., Jr Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–272. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- 34.Raby E, Moyo M, Devendra A, Banda J, De Haas P, Ayles H, et al. The effects of HIV on the sensitivity of a whole blood IFN-gamma release assay in Zambian adults with active tuberculosis. PLoS One. 2008;3:e2489. doi: 10.1371/journal.pone.0002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ragno S, Romano M, Howell S, Pappin DJ, Jenner PJ, Colston MJ. Changes in gene expression in macrophages infected with Mycobacterium tuberculosis: a combined transcriptomic and proteomic approach. Immunology. 2001;104:99–108. doi: 10.1046/j.0019-2805.2001.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rangaka MX, Wilkinson KA, Seldon R, Van Cutsem G, Meintjes GA, Morroni C, et al. Effect of HIV-1 infection on T-Cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007;175:514–520. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- 37.Ruhwald M, Bodmer T, Maier C, Jepsen M, Haaland MB, Eugen-Olsen J, et al. Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur Respir J. 2008;32:1607–1615. doi: 10.1183/09031936.00055508. [DOI] [PubMed] [Google Scholar]
- 38.Santin M, Casas S, Saumoy M, Andreu A, Moure R, Alcaide F, Ferrer E, Podzamczer D. Detection of latent tuberculosis by the tuberculin skin test and a whole-blood interferon-γ release assay, and the development of active tuberculosis in HIV-seropositive persons. Diagn Microbiol Infect Dis. 2011;69:59–65. doi: 10.1016/j.diagmicrobio.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Swaminathan S, Subbaraman R, Venkatesan P, Subramanyam S, Kumar SR, Mayer KH, et al. Tuberculin skin test results in HIV-infected patients in India: implications for latent tuberculosis treatment. Int J Tuberc Lung Dis. 2008;12:168–173. [PubMed] [Google Scholar]
- 40.Syed Ahamed Kabeer B, Sikhamani R, Swaminathan S, Perumal V, Paramasivam P, Raja A. Role of interferon gamma release assay in active TB diagnosis among HIV infected individuals. PLoS One. 2009;4:e5718. doi: 10.1371/journal.pone.0005718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Syed Ahamed Kabeer B, Raman B, Thomas A, Perumal V, Raja A. Role of QuantiFERON-TB gold, interferon gamma inducible protein-10 and tuberculin skin test in active tuberculosis diagnosis. PLoS One. 2010;5:e9051. doi: 10.1371/journal.pone.0009051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whalen CC, Chiunda A, Zalwango S, Nshuti L, Jones-Lopez E, Okwera A, et al. Immune correlates of acute Mycobacterium tuberculosis infection in household contacts in Kampala, Uganda. Am J Trop Med Hyg. 2006;75:55–61. [PMC free article] [PubMed] [Google Scholar]
- 43.Whittaker E, Gordon A, Kampmann B. Is IP-10 a better biomarker for active and latent tuberculosis in children than IFNgamma? PLoS One. 2008;3:e3901. doi: 10.1371/journal.pone.0003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO statistical information system. 2008 Available online at www.who.int/whosis/en.
- 45.WHO Global Tuberculosis Programme, UNAIDS. Geneva, Switzerland: WHO; 1998. Policy statement on preventative therapy against tuberculosis in people living with HIV. WHO/TB/98.255 and UNAIDS/98.34. [Google Scholar]