Abstract
Stimulation of the insulin and insulin-like growth factor I (IGF-I) receptor activates the phosphoinositide-3-kinase/Akt/mTOR pathway causing pleiotropic cellular effects including an mTOR-dependent loss in insulin receptor substrate-1 expression leading to feedback down-regulation of signaling through the pathway. In model systems, tumors exhibiting mutational activation of phosphoinositide-3-kinase/Akt kinase, a common event in cancers, are hypersensitive to mTOR inhibitors, including rapamycin. Despite the activity in model systems, in patients, mTOR inhibitors exhibit more modest antitumor activity. We now show that mTOR inhibition induces insulin receptor substrate-1 expression and abrogates feedback inhibition of the pathway, resulting in Akt activation both in cancer cell lines and in patient tumors treated with the rapamycin derivative, RAD001. IGF-I receptor inhibition prevents rapamycin-induced Akt activation and sensitizes tumor cells to inhibition of mTOR. In contrast, IGF-I reverses the antiproliferative effects of rapamycin in serum-free medium. The data suggest that feedback down-regulation of receptor tyrosine kinase signaling is a frequent event in tumor cells with constitutive mTOR activation. Reversal of this feedback loop by rapamycin may attenuate its therapeutic effects, whereas combination therapy that ablates mTOR function and prevents Akt activation may have improved antitumor activity.
Introduction
Activation of phosphoinositide-3-kinase (PI3K)/Akt/mTOR signaling through mutation of pathway components as well as through activation of upstream signaling molecules occurs in a majority of cancers contributing to deregulation of proliferation, resistance to apoptosis, and changes in metabolism characteristic of transformed cells (1, 2). This pathway is normally regulated by upstream receptor tyrosine kinases, especially the insulin and insulin-like growth factor I (IGF-I) receptors (IGF-IR; ref. 3). Physiologic activation of these receptors also results in feedback down-regulation of the pathway, mediated in part by mTOR/S6K-dependent loss of insulin receptor substrate-1 (IRS-1) expression (4-8). IRS-1 is the major substrate of the IGF-I and insulin receptors, responsible for the propagation of the signal induced by ligand binding (2, 9). We speculated that constitutive activation of the Akt/mTOR pathway in cancer cells would induce upstream feedback inhibition of signaling via IGF-I/insulin and perhaps other transmembrane receptors. Feedback inhibition could have marked biological and therapeutic implications. First, feedback inhibition of upstream signaling pathways could cause hypersensitivity to mTOR inhibitors and inhibition of other elements of the activated signaling pathway (so-called “oncogene addiction”; refs. 10, 11). Second, inhibition of mTOR could cause the release of feedback inhibition, paradoxically activating IGF-I signaling and reducing the antitumor effects of mTOR inhibitors. We show herein that inhibition of mTOR in cancer cell lines and in patient tumors causes activation of Akt kinase which is associated with induction of IRS-1 and is prevented by IGF-IR inhibition. Furthermore, we show that IGF-I antagonizes the antiproliferative effects of rapamycin in serum-free medium and IGF-IR inhibitors sensitize cancer cell lines to rapamycin’s antiproliferative effects.
Materials and Methods
Cell lines, antibodies, and reagents
DU-145, MCF-7, and MDA-MB-468 were purchased from the American Type Culture Collection (Manassas, VA). Anti-IRS-1, anti-IRS-2, and anti-FKHRL1 antibodies were from Upstate Biotechnology (Waltham, MA). pAkt(S473), p-p70/S6K (T389), p70/S6K, p-GSK3α/β, GSK3α, GSK3β, Akt, Akt1, Akt 2, Akt 3, p-FKHR(S256)/p-AFX(S193), FKHR, AFX, and p-FKHR(T24)/p-FKHRL1(T32) antibodies were from Cell Signaling Technology (Beverly, MA). Rapamycin and LY294002 were from Calbiochem (San Diego, CA). NVP-AEW541 was a gift from Novartis Pharma AG (Basel, Switzerland) and A12 was a gift from ImClone Systems, Incorporated (New York, NY).
Western blotting
MDA-MB-468, DU-145, and MCF-7 cells were harvested and lysed in mRIPA or NP40 lysis buffer. Protein concentrations were determined with the bicinchoninic acid method (Pierce, Rockford, IL). Samples were subjected to SDS-PAGE. Proteins were detected using the enhanced chemiluminescence kit (Amersham Biosciences, Piscataway, NJ) and bands quantitated with Science Lab 2003 Image Gauge (Fujifilm, Tokyo, Japan).
In vitro Akt kinase assay
Cells were treated with either 1 nmol/L rapamycin for 1 or 4 hours, DMSO vehicle (time 0), 10 μmol/L LY294002 pretreatment for 1 hour followed by 1 or 4 hours of 1 nmol/L rapamycin exposure, 800 nmol/L NVP-AEW541 pretreatment for 1 hour followed by 1 or 4 hours of 1 nmol/L rapamycin, or 10 nmol/L A12 pretreatment for 1 hour followed by 4 hours of 1 nmol/L rapamycin. Cells were harvested and the Akt kinase assay from Cell Signaling Technology was used. Akt1 was immunoprecipitated from lysates and used in an in vitro kinase assay to catalyze phosphorylation of a GSK-3 fusion protein (1 μg) in the presence of 200 μmol/L ATP. The reaction product was subjected to SDS-PAGE and probed with p-GSK3α/β antibody (S21/9).
Human solid tumor biopsy and immunohistochemistry
Tumor biopsies from patients treated with RAD001 were analyzed. Patients were treated with RAD001 administered p.o. daily (10 mg, four patients) or weekly (50 mg, four patients). Sequential tumor biopsies at baseline time point—prior to start treatment—and 28 days after therapy were done in all patients. All tissue specimens were fixed in 10% buffered neutral formalin for 24 hours at room temperature, then dehydrated and paraffin embedded. Immunostaining was done on 4 μmm tissue sections placed on charged plus glass slides. After deparaffinization in xylene and graded alcohols, heat antigen retrieval was done in citrate buffer (pH 6) for 5 minutes in an autoclave. Following epitope retrieval, endogenous peroxidase was blocked by immersing the sections in 0.03% hydrogen peroxide for 10 minutes. Slides were washed for 5 minutes with TBS. Incubation with polyclonal anti-S473 pAkt antibody was made at room temperature for 2 hours at 1:50 dilution on 0.05 mol/L Tris-HCl buffer [DakoCytomation (Carpinteria, CA) Antibody Diluent]. The peroxidase-labeled polymer conjugated to goat anti-rabbit method was used to detect antigen-antibody reaction (DakoCytomation EnVision+ System) for 30 minutes at room temperature. Sections were then visualized with 3,3′-diaminobenzidine as a chromogen for 5 minutes and counterstained with Mayer’s hematoxylin. All immunohistochemical stainings were done in a Dako Autostainer under the same conditions. The same sections incubated with rabbit nonimmunized serum were used as negative controls; for the positive control, sections of a breast carcinoma human tumor with a known expression of pAkt by immunohistochemistry and Western blot were stained. Tumor sections were studied on a light microscope with an ocular magnification of ×400. To score a tumor cell as positive for pAkt, nuclear and cytoplasmic staining was required. The percentage of stained tumor cells was scored in a whole section and the average percentage and intensity of tumor cell staining was calculated as a histoscore as described previously (12). Tumors with >1% of tumor cells staining were considered positive for such markers. Grading of scoring ranged from a score of 0 to 300. Scoring was blinded to time point data. Statistical analysis for Wilcoxon signed ranks test was done between pretherapy and posttherapy levels of pAkt (SPSS analysis software 10.0).
Cell proliferation studies
100,000 DU-145 or MCF-7 cells, and 50,000 MDA-MB-468 cells were plated in normal growth medium. The cells were grown overnight before treatment with DMSO, 1 nmol/L rapamycin, 1 μmol/L NVP-AEW541, or combined 1 nmol/L rapamycin and 1 μmol/L NVP-AEW541. Cells were trypsinized and counted on a Coulter counter.
Cell cycle analysis
Cells (1 × 106) were plated in 10 cm dishes in normal growth medium and grown overnight before treatment with DMSO, 1 nmol/L rapamycin, or a combination of rapamycin (1 nmol/L) and NVP-AEW541 (1 μmol/L). The nuclei were isolated by the Nusse method (13) and subjected to flow cytometry to determine fraction of cells in sub-G1, G1, S, and G2.
Results
Inhibition of mTOR induces Akt activity in tumor cells
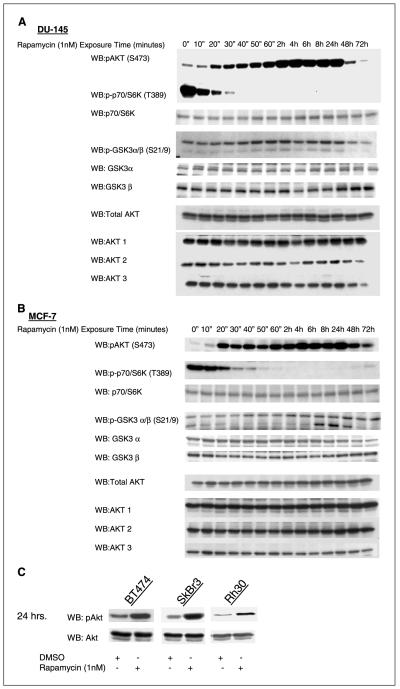
We tested whether inhibition of mTOR caused activation of PI3K/Akt signaling by relieving feedback inhibition of upstream signaling. Prolonged exposure of breast cancer cell lines MCF-7 (PI3K mutant; ref. 14), and MDA-MB-468 (PTEN mutant), or the prostate cancer cell line DU-145 (PTEN wild-type) to the mTOR inhibitor rapamycin caused a marked increase in S473 phosphorylation and Akt kinase activity (Fig. 1A and B). These results are consistent with the recent report by Sun et al. who observed that rapamycin induced pAkt(S473) in several human cancer cell lines including DU-145 and MCF-7 (15). We also observed the induction of pAkt by rapamycin in the breast cancer cell lines, SkBR3 and BT474, cells in which PI3K/Akt is driven by overexpression of human epidermal growth factor receptor 2, and in the rhabdomyosarcoma line Rh30 (Fig. 1C). In DU-145 and MCF-7, induction of S473 phosphorylation occurred within 20 minutes of rapamycin treatment and followed inhibition of mTOR-dependent S6K phosphorylation (Fig. 1A and B). In both cell lines, pAkt was increased 4-fold to 5-fold by 24 hours of rapamycin exposure and remained elevated for at least 48 hours after the addition of the drug. As measured by an in vitro kinase assay, Akt activity in DU-145 increased in parallel with the induction of phosphorylation and was elevated 3-fold compared with controls 4 hours after the addition of the drug (Fig. 1D).
Figure 1.
mTOR inhibition activates Akt in tumor cells. A, 1 nmol/L rapamycin treatment induced S473 Akt and S21/9 GSK3α/β phosphorylation in vitro in a DU-145 prostate cancer cell line. Akt1, Akt2, and Akt3, total Akt, and total GSK3α/β did not change. Phosphorylation of p70/S6K decreased with rapamycin treatment whereas total p70/S6K levels did not change. B, 1 nmol/L rapamycin treatment induced S473 Akt and S21/9 GSK3α/β phosphorylation in vitro in a MCF-7 cancer cell line. Akt1, Akt2, and Akt3, total Akt, and total GSK3α/β did not change. Phosphorylation of p70/S6K decreased with rapamycin treatment whereas total p70/S6K levels did not change. C, 1 nmol/L rapamycin treatment for 24 hours induced S473 Akt in the breast cancer cell lines BT474, SkBr3, and the rhabdomyosarcoma cell line Rh30. Total Akt levels did not change.
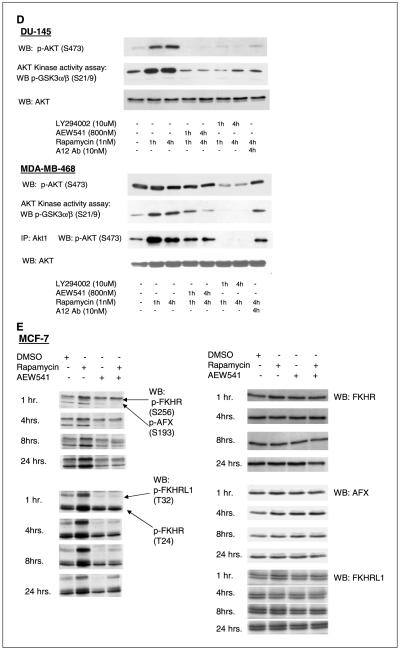
D, mTOR inhibition with 1 nmol/L rapamycin induced Akt Kinase activity in an IGF-IR-dependent manner. An Akt kinase assay in DU-145 cells confirmed that the increased pAkt (S473) resulted in increased Akt activity, which was abrogated by the inhibition of PI3K with the small-molecule LY294002 (10 μmol/L) and by inhibition of IGF-IR with either the small-molecule NVP-AEW541 (800 nmol/L) or the monoclonal antibody A12 (10 nmol/L). A Western blot of pAkt (S473) at the same time points in DU-145 showed the increased Akt phosphorylation which was abrogated by IGF-IR or PI3K inhibition. Although there is no detectable increase in pan-phospho-Akt as measured by Western blot, an Akt kinase assay in MDA-MB-468 cells reveals an induction of Akt activity and p-Akt1 (S473) that is abrogated by LY294002 as well as the IGF-IR inhibitors. E, treatment of MCF-7 cells with rapamycin (1 nmol/L) induced phosphorylation of the FoxO transcription factors, FKHR (S256 and T24), AFX (S193), and FKHRL1 (T32). The rapamycin-induced FoxO phosphorylation was abrogated by IGF-IR inhibition with 800 nmol/L NVP-AEW541. Total FKHR, AFX, and FKHRL1 levels did not change with rapamycin or NVP-AEW-541 treatment.
DU-145 and MCF-7 contain wild-type PTEN and have modest basal levels of p-Akt. In MDA-MB-468, PTEN is mutationally inactivated and p-Akt levels are constitutively elevated. In this cell line, it is difficult to discern a further increase in the S473 phosphorylation of total Akt after rapamycin exposure. However, a 3-fold increase in Akt kinase activity was noted at 1 hour and was accompanied by a 6-fold increase in the phosphorylation of Akt1 (Fig. 1D). Thus, in both PTEN-wt and PTEN-mutated tumor cell lines, inhibition of mTOR function by rapamycin could lead to activation of Akt kinase.
Rapamycin-induced activation of Akt kinase was also associated with increased phosphorylation of endogenous Akt substrates. In MCF-7 cells treated with rapamycin, the phosphorylation of FoxO1a, FoxO3a, and FoxO4 transcription factors (Fig. 1E) and GSK3α/β was markedly increased as compared with control cells (Fig. 1A and B). The increase in GSK3α/β phosphorylation occurred in both DU-145 and MCF-7 cells and began after 40 minutes of rapamycin exposure, remaining elevated for at least 24 hours after initiation of rapamycin treatment. Phosphorylation of FoxO transcription factors was elevated after 1 hour of rapamycin exposure in MCF-7 cells and remained elevated for at least 24 hours. Total FKHR, AFX, and FKHRL1 levels did not change with treatment (Fig. 1E). Thus, rapamycin-induced Akt phosphorylation and kinase activity leads to functional activation of Akt signaling in tumor cells.
Inhibition of mTOR induces S473 Akt phosphorylation in vivo in human tumors
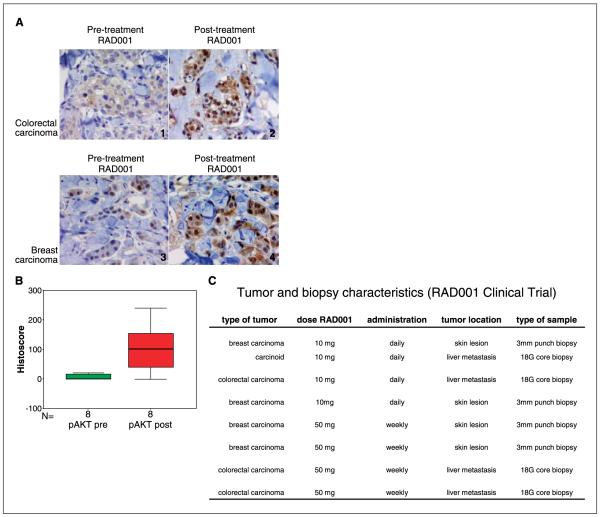
In model systems, tumors with activated Akt secondary to PTEN loss or other causes have been shown to be hypersensitive to rapamycin (16, 17). This data has led to clinical trials of rapamycin derivatives (2, 18). As indicated above, we have shown that mTOR inhibition can activate Akt signaling in tumor cells in tissue culture. To determine if this occurs in patient tumors in vivo as well, we obtained tumor tissue from patients with advanced solid tumors who were being treated on a phase I protocol of the rapamycin derivative RAD001 (Everolimus, Novartis Pharma). The levels of S473 phosphorylated Akt in the tumor biopsies increased after RAD001 treatment (P = 0.018, Wilcoxon signed rank test; Fig. 2A-C). Biopsies of liver metastases or skin lesions were taken from patients with colon or breast carcinoma before and after 4 weeks of RAD001 treatment. The levels of pAkt, as determined by immunohistochemistry and quantitated as a histoscore ranging from 0 to 300, were elevated in biopsies from patients receiving daily RAD001 (n = 4), as well as in biopsies from patients on a weekly dosing schedule (n = 4). Given that Akt activation results in cancer cell survival, proliferation, and growth, the induction of phosphorylated Akt is an unexpected and potentially undesirable consequence of mTOR inhibition.
Figure 2.
mTOR inhibition activates Akt in humans. A, liver metastasis of a colorectal carcinoma from a patient treated with a daily administration of RAD001 for 4 weeks. pAkt was expressed in nuclei and cytoplasm of tumor cells (1) and levels of expression increased after therapy (2). Skin infiltration by ductal carcinoma in a patient treated with a weekly administration of RAD001 for 4 weeks. A similar increment of pAkt was observed before (3) and after therapy (4); (3,3′-diaminobenzidine, pAkt ×400). B, evaluation of levels of pAkt in paired tumor samples from patients treated with daily and weekly administration of RAD001 by immunohistochemistry. A significant increment (P = 0.018) of this protein was observed in eight patients. C, tumor and biopsy characteristics. The characteristics of each tumor biopsy and the dose schedule of the eight patients in the RAD001 clinical trial are described.
Induction of Akt kinase activity is IGF-IR dependent and is associated with up-regulation of IRS-1 protein levels
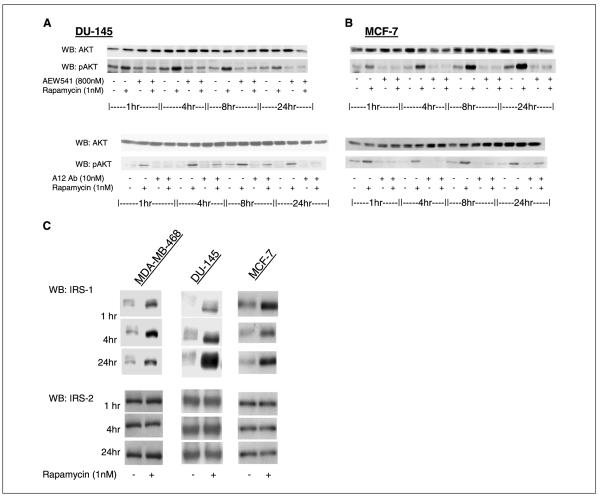
In normal cells, IGF-I and insulin induction of PI3K/Akt/mTOR signaling leads to an mTOR-dependent feedback inhibition of signaling, due in part to mTOR/S6K-dependent loss of IRS-1 expression (4-8). Rapamycin reactivates signaling, reverses insulin resistance, and induces IRS-1 expression in these cells (4). We asked whether induction of Akt signaling by rapamycin in tumor cells is due to loss of negative regulation of IGF-I signaling and whether this induction is IGF-IR-dependent. To determine whether the induction of Akt activity and signaling by rapamycin is dependent on IGF-IR, we employed a small-molecule IGF-IR kinase inhibitor (NVP-AEW541, Novartis Pharma; ref. 19), and a human monoclonal antibody to the IGF-IR (A12, ImClone Systems; ref. 20). NVP-AEW541 shows selectivity for IGF-IR (IC50, 0.086 μmol/L) as compared with the closely related insulin receptor (IC50, 2.3 μmol/L). A12, a human monoclonal antibody to IGF-IR, blocks IGF-I binding to the receptor with an IC50 of 0.6 to 1 nmol/L and does not interfere with insulin binding. One hour of pretreatment with either of the inhibitors abrogated rapamycin-induced pAkt induction (Fig. 3A and B). The induction of Akt kinase activity was also abrogated by IGF-IR blockade and PI3K inhibition with LY294002 (Fig. 1D). In addition, FoxO substrate phosphorylation was prevented by pretreatment with NVP-AEW541 in MCF-7 cells (Fig. 1E).These data suggest that rapamycin-induced Akt activation is due to release of mTOR-dependent negative regulation of IGF-I signaling. Consistent with these data, we found that rapamycin increased IRS-1 protein levels, but not IRS-2 in DU-145, MCF-7, and MDA-MB-468 cells (Fig. 3C). We also found that, inserum-free medium, IGF-I stimulated Akt activation in MCF-7 cells and that cotreatment with rapamycin enhanced this activation >2-fold (Fig. 4A). This further supports the idea that mTOR negatively regulates IGF-I signaling in these cells.
Figure 3.
IGF-I signaling mediates AKT activation induced by mTOR inhibition. A and B, the induction of pAkt (S473) by rapamycin was abrogated by the small-molecule inhibitor of IGF-IR, NVP-AEW541, and by the monoclonal antibody against IGF-IR, A12, in both the DU-145 prostate cancer cell line (A) and the MCF-7 breast cancer cell line (B). Total Akt levels did not change.
C, 1 nmol/L rapamycin treatment up-regulates IRS-1 levels in MCF-7, DU-145, and MDA-MB-468 cell lines by 1 hour, and this induction persists for 24 hours. IRS-2 levels remain unchanged.
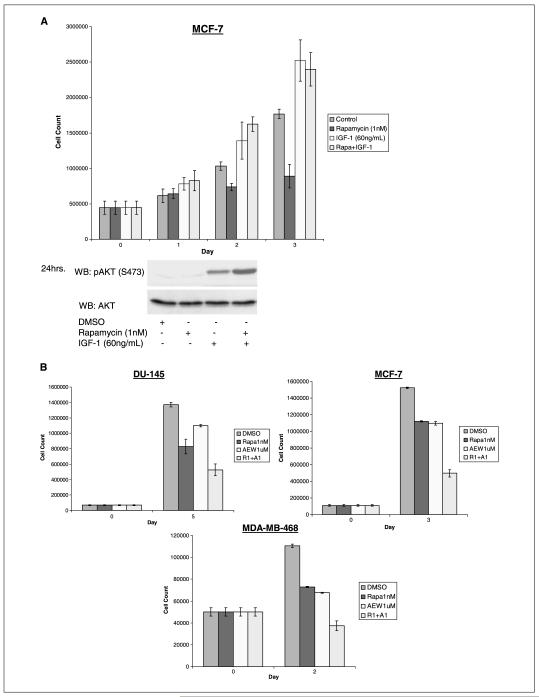
Figure 4.
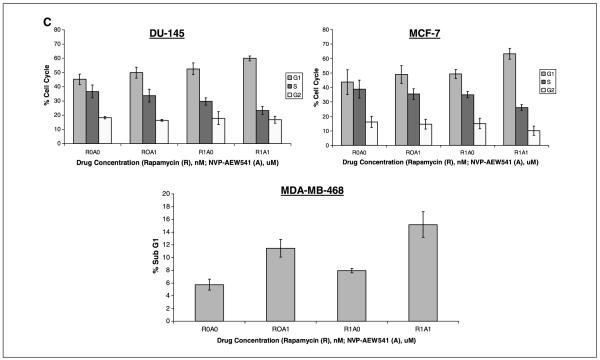
IGF-I prevents and IGF-IR inhibitors enhance the antiproliferative effects of rapamycin. A, IGF-I (60 ng/mL) rescued MCF-7 cells from rapamycin’s antiproliferative effects in serum-free medium and the induction of pAKT was greater in cells cotreated with rapamycin and IGF-I than in cells treated with either single agent. B, the combination of mTOR inhibition with IGF-IR inhibition resulted in enhanced antitumor effects. In vitro, combined mTOR and IGF-IR inhibition with rapamycin (1 nmol/L) and NVP-AEW541 (1 μmol/L) resulted in additive inhibition of proliferation in DU-145 cells, MCF-7 cells, and MDA-MB-468 cells as compared with either single agent. C, in the breast cancer cell line, MCF-7, and the prostate cancer cell line, DU-145, 2 days of combined mTOR and IGF-IR inhibition with rapamycin (1 nmol/L) and NVP-AEW541 (1 μmol/L) resulted in enhanced G1 arrest. In the breast cancer cell line, MDA-MB-468, 2 days of combination treatment resulted in enhanced apoptosis compared with single agent treatment groups.
IGF-I prevents and IGF-IR inhibitors enhance the antiproliferative effects of rapamycin
Rapamycin analogues have shown limited antitumor activity in clinical trials (21). IGF-I has been shown to rescue rhabdomyosarcoma cells from rapamycin-induced apoptosis, suggesting that IGF-I may antagonize the effects of mTOR inhibition (22). If so, our data implies that induction of IGF-I-dependent Akt activation by rapamycin could be responsible for attenuating its anticancer effects. We tested whether IGF-I could interfere with rapamycin-induced inhibition of tumor cell proliferation. MCF-7 cells were serum-starved for 16 hours and treated with rapamycin in the presence or absence of IGF-I (Fig. 4A). Rapamycin treatment dramatically reduced the cell proliferation rate, but cells treated with both rapamycin and IGF-I were not inhibited and proliferated at a rate similar to the cells treated with IGF-I alone. When added to rapamycin-treated cells in serum-free medium, IGF-I acted to completely abrogate the antiproliferative effects of rapamycin. In addition, the induction of pAkt(S473) was >2-fold higher in cells treated with both rapamycin and IGF-I as compared with cells treated with either agent alone.
As IGF-I rescued cells from the antiproliferative effects of rapamycin, we tested whether IGF-IR inhibition enhanced the antiproliferative effect of rapamycin in cells growing in serum. Cells were treated with rapamycin, with or without NVP-AEW541, for 3 days. Simultaneous administration of NVP-AEW541 and rapamycin to DU-145, MCF-7, and MDA-MB-468 (Fig. 4B) cancer cells resulted in additive antiproliferative effects as compared with either agent alone. Cell cycle analysis revealed additive effects on G1 arrest in the MCF-7 and DU-145 cell lines after 2 days of treatment with the combination of IGF-IR and mTOR inhibitors as compared with cells treated with either single agent or vehicle (Fig. 4C). On comparison with control and single agent–treated cells, an enhanced sub-G1 population was observed in MDA-MB-468 cells after 2 days of treatment with combined mTOR and IGF-IR inhibitors (Fig. 4C).
Discussion
Dysregulation of mTOR function via physiologic or mutational activation of upstream pathways is a common event in tumors from many lineages (2, 11). We speculated that tumor cells with activated mTOR display a phenotype functionally equivalent to insulin-resistant diabetes with an exaggerated down-regulation of upstream signaling molecules like IRS-1 (4-8). We report here that, in a variety of tumor cell lines, the mTOR inhibitory drug rapamycin up-regulates IRS-1 protein levels and induces Akt phosphorylation, protein kinase activity, and downstream signaling. In parallel clinical studies with the rapamycin derivative RAD001 (Novartis Pharma), the intensity of immunohistochemical staining of tumor biopsies for p-Akt was significantly elevated after 4 weeks of drug treatment. These data suggest that the induction of Akt activity observed in tumor cells in tissue culture is biologically relevant and may be important clinically.
Rapamycin and rapamycin-like molecules inhibit mTOR efficiently in patients, are useful as immunosuppressants, and suppress S6 kinase activity in normal and tumor cells in vitro and in vivo (18). Pharmacologic inhibition of mTOR has been shown to potently inhibit tumor cells with activation of PI3K/Akt signaling due either to PTEN loss, expression of Akt, or growth factor activation (16). In this regard, rapamycin derivatives are effective in in vivo models, as recently shown in myr-Akt-driven transgenic models of prostatic neoplasia (17). The Akt activation induced by rapamycin in tumor cells, however, is likely to reduce its antitumor effects, by activating pathways that attenuate its effects on proliferation and apoptosis. In tumors in which Akt activation is induced, rapamycin will not effectively inhibit PI3K/Akt kinase signaling except insofar as it is mediated through mTOR. We show here that IGF-I overcomes the rapamycin-induced inhibition of MCF-7 proliferation in serum-free medium. This result is consistent with those of Houghton and coworkers, who showed that induction of apoptosis by rapamycin via ASK-1 activation occurs in serum-free but not serum-containing medium (22). We find that inhibition of induction of Akt activation with agents that block IGF-I signaling enhances cell cycle arrest and apoptosis induction by rapamycin.
Despite the results from model systems, the clinical antitumor activity of mTOR inhibitor analogues has been modest at best. Our demonstration that rapamycin can induce Akt phosphorylation in tumors implies that its potential antitumor activity is attenuated by release of feedback inhibition of growth signaling pathways. The results also suggest a new model for the development of effective combinatorial anticancer therapy. Combined inhibition of constitutively activated oncoproteins and of normal pathways that are down-regulated by oncoprotein-inhibition (and thus up-regulated by oncoprotein-targeted drugs) may be much more effective than either alone. For the specific case discussed in this article, the work provides a rationale for tailored combination therapy with an mTOR inhibitor and an inhibitor of the growth factor receptor, such as IGF-IR, that normally drives PI3K activity in that tumor. Considering our results in which LY294002 abrogates rapamycininduced Akt kinase activity, and the report by Sun et al. detailing the combined efficacy of LY294002 and rapamycin in non-small cell lung cancer cell lines, mTOR inhibitors and PI3K inhibitors might also be a promising combination therapy (15).
We have shown that rapamycin induces Akt activity and that abrogating this induction could enhance the antitumor effects of rapamycin in vitro, but the mechanism of rapamycin-induced Akt activity remains unclear. Our finding that rapamycin treatment induces IRS-1 expression suggests that rapamycin’s inhibition of p70/S6K results in increased IGF-IR/IRS-1/PI3K signaling to Akt. Previous reports have shown that p70/S6K mediates phosphorylation of IRS-1 inhibitory serine sites (S312 and/or S636/639) which lead to IRS-1 degradation (8, 23, 24). Thus, suppression of p70 activity by rapamycin may prevent inhibitory IRS-1 phosphorylation, thereby stabilizing IRS-1. An increase in IRS-1 adapter protein levels may induce Akt activity by augmenting IGF-IR signaling to PI3K/Akt. The inhibition of pAkt induction with LY294002 implies that the phenomenon is PI3K-dependent and supports this mechanism. However, LY294002 inhibits several PI3K-like kinases, including mTOR, and has been reported to inhibit the activity of rictor-mTOR, recently described as the Akt S473 kinase/PDK2 (25-27). It is possible that rapamycin, by some unknown mechanism, induces rictor-mTOR activity, resulting in increased S473 Akt levels. These possibilities are currently under investigation.
Acknowledgments
Grant support: NIH grant PO1-CA094060 and the generous support of the Taub Foundation. K.E. O’Reilly was supported by the Medical Scientist Training Program Grant from the NIH.
References
- 1.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 2.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–48. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 3.Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 2003;13:79–85. doi: 10.1016/s0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 4.Haruta T, Uno T, Kawahara J, et al. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14:783–94. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- 5.Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes. 2001;50:24–31. doi: 10.2337/diabetes.50.1.24. [DOI] [PubMed] [Google Scholar]
- 6.Sun XJ, Goldberg JL, Qiao LY, Mitchell JJ. Insulin-induced insulin receptor substrate-1 degradation is mediated by the proteasome degradation pathway. Diabetes. 1999;48:1359–64. doi: 10.2337/diabetes.48.7.1359. [DOI] [PubMed] [Google Scholar]
- 7.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–6. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–5. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 9.White MF, Yenush L. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Curr Top Microbiol Immunol. 1998;228:179–208. doi: 10.1007/978-3-642-80481-6_8. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein IB. Cancer. Addiction to oncogenes—the Achilles heal of cancer. Science. 2002;297:63–4. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 11.Mills GB, Lu Y, Kohn EC. Linking molecular therapeutics to molecular diagnostics: inhibition of the FRAP/RAFT/TOR component of the PI3K pathway preferentially blocks PTEN mutant cells in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:10031–3. doi: 10.1073/pnas.191379498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulding H, Pinder S, Cannon P, et al. A new immunohistochemical antibody for the assessment of estrogen receptor status on routine formalin-fixed tissue samples. Hum Pathol. 1995;26:291–4. doi: 10.1016/0046-8177(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 13.Giaretti W, Nusse M. Light scatter of isolated cell nuclei as a parameter discriminating the cell-cycle subcompartments. Methods Cell Biol. 1994;41:389–400. doi: 10.1016/s0091-679x(08)61730-6. [DOI] [PubMed] [Google Scholar]
- 14.Bachman KE, Argani P, Samuels Y, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–5. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 15.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 16.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98:10314–9. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 18.Boulay A, Zumstein-Mecker S, Stephan C, et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–61. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Echeverria C, Pearson MA, Marti A, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–9. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 20.Burtrum D, Zhu Z, Lu D, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–21. [PubMed] [Google Scholar]
- 21.Sawyers CL. Will mTOR inhibitors make it as cancer drugs? Cancer Cell. 2003;4:343–8. doi: 10.1016/s1535-6108(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 22.Galvan V, Logvinova A, Sperandio S, Ichijo H, Bredesen DE. Type 1 insulin-like growth factor receptor (IGF-IR) signaling inhibits apoptosis signal-regulating kinase 1 (ASK1) J Biol Chem. 2003;278:13325–32. doi: 10.1074/jbc.M211398200. [DOI] [PubMed] [Google Scholar]
- 23.Harrington LS, Findlay GM, Gray A, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–23. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 26.Hresko RC, Mueckler M. mTOR/RICTOR is the Ser473 kinase for Akt/PKB in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–16. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 27.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]