Abstract
The emergence of air-liquid interface (ALI) culturing of mammalian airway epithelium is a recent innovation for experimental modeling of airway epithelial development, function, and pathogenic mechanisms associated with infectious agent and irritant exposure. This construct provides an experimental platform for in vitro propagation, manipulation, and testing of airway epithelium in a structural and physiologic state that emulates in vivo organization. In this study, we have cultured nasal epithelial biopsies from human subjects with variable histories of tobacco smoke exposure and assessed ciliary beat frequency (CBF) after an extended interval in vitro relative to CBF determined on biopsies from the same subjects immediately upon acquisition. We observed elevated CBF in nasal epithelial biopsies as well as persistence of accelerated CBF in ALI cultures deriving from biopsies of smokers and non-smokers exposed to environmental tobacco smoke compared to CBF in cultures from biopsies of well-documented non-smokers. Moreover, cultures deriving from smokers exhibited reduced ciliation as the cultures matured. These studies document that nasal epithelium cultured in the ALI system retains physiologic and phenotypic characteristics of the epithelial layer in vivo even through rounds of proliferative expansion. These observations suggest that stable epigenetic factors affecting regulation of ciliary function and phenotype commitment may be operative.
Keywords: Cilia, Epithelium, Tobacco smoke, Inhalation toxicology, Cell culture
Introduction
The pseudostratified columnar epithelial layer lining the mammalian conducting airways provides a primary line of defense to the respiratory system from inhaled particulates and irritants. The histologic organization of this layer as well as its ability to upregulate mucus secretion, modify baseline ciliary activity, and maintain a critical volume of airway surface liquid is critical to effecting optimal mucociliary clearance (Davis and Lazarowski 2008). Phenotypic changes in the distribution of epithelial cell types lining the airways associated with infectious agent and irritant exposure may lead to functional deficits in clearance and consequent disease. Such changes in the quantitative distribution of epithelial cell types are well-documented modifications in airway epithelium of individuals exposed to tobacco smoke and in tobacco-related diseases (Saetta et al. 2000; Maestrelli et al. 2001; Yoshida and Tuder 2007). In addition to histologic changes that may predispose exposed individuals to the development of respiratory disease, we recently have shown that nasal epithelial ciliary beat frequency (CBF) is persistently upregulated among individuals exposed to tobacco smoke through active or passive means (Zhou et al. 2009). In the present study, we have appropriated a novel technology, the air-liquid interface (ALI) culture, whereby small fragments of epithelium obtained from human subjects are mitotically expanded and grown to confluence to yield a well-differentiated epithelial layer. In the present study, we have compared CBF and phenotypic characteristics of cultured nasal epithelium obtained from well-documented non-smokers, active smokers, and non-smokers exposed by lifestyle to secondhand tobacco smoke.
Materials and Methods
Human subjects
This study was reviewed and approved by The University of North Carolina at Chapel Hill Office of Human Research Ethics. Twenty subjects (6 male/14 female) participated in the study. There were nine African-American, nine Caucasian, and two Asian subjects. All the subjects, regardless of smoking history, reported subjectively that their overall health was good. There were neither asthmatics nor participants with other previously diagnosed chronic respiratory diseases. Participants self-reported their status as belonging to one of three groups: (1) non-smokers (NS), (2) active smokers (S), or (3) non-smokers but domestically or occupationally exposed to environmental tobacco smoke (NS/ETS). Individuals identifying themselves as smokers subjectively reported recent cigarette use as no more than two packs of cigarettes per day. There were seven non-smokers, seven smokers, and six ETS-exposed individuals in the study groups. The mean age of the subject population was 32.9 yr. The mean age was 31 yr for non-smokers, 33 yr for smokers, and 34.8 yr for NS/ETS-exposed subjects.
Nasal biopsy and determination of CBF ex vivo and in vitro
A non-invasive nasal biopsy to retrieve ciliated epithelium lining the inferior surface of the inferior nasal turbinates was performed on each subject for the determination of CBF. The subjects participating in this study were young to middle-aged adults and reported upon recruitment their subjective sense, regardless of their smoking status, that their overall health was good. Subjects with any documented chronic disease, including asthma, were excluded from the study. Subjects were sampled at random times during a typical day and sampling was not logistically targeted to times when smoking subjects had recently smoked or times when NS/ETS subjects had encountered specific lifestyle ETS exposures. Nasal biopsies were obtained without anesthesia using a sterile, plastic nasal curette (Rhino-Pro™, Arlington Scientific, Arlington, TX). Both nasal turbinates of each subject were sampled by gentle curettage of the inferior turbinate with the curette under direct vision with an otoscope. Immediately following acquisition, small fragments of the mucosa were transferred to a microscope slide and cover slipped. Each specimen was viewed at room temperature (25C) under a Nikon Diaphot inverted microscope equipped with a 40× phase contrast objective. CBF was determined using a high-speed digital camera interfaced to the microscope (Basler AG, Ahrensburg, Germany) and a computer running software designed for making CBF measurements (Sisson et al. 2003; SAVA, Ammons Engineering, Mt. Morris, MI). Mean point CBF was determined at 50 points for each specimen by assessing five random points in each of ten random microscopic fields. The same measurement protocol was used for determination of CBF in the ALI cultures.
Cotinine assays
Each study subject provided a urine specimen at the time of the nasal biopsy to obtain a quantitative measure of tobacco smoke exposure reported as a standardized cotinine/creatinine ratio (CCR) which verified each subject’s interview report of smoking status and validation of their placement within the different experimental groups. These assays not only verified the interview report of smoking status but provided an important perspective of the variability of smoking habits and tobacco smoke exposure levels in the different groups which did not necessarily coincide with subject reporting. Urine specimens were frozen at the time of collection so that analyses could be performed en masse. Cotinine analyses were performed using a commercially available ELISA test (Bio-Quant, San Diego, CA).
Establishment of human airway epithelial air-liquid interface cultures
Nasal epithelial mucosa obtained at biopsy was immediately transferred to a standard tissue culture medium on ice and transported to the laboratory. These fragments are diffuse but coherent and can be easily transferred from the specimen container using a glass pipette. The histologic integrity of the mucosal surface is frequently preserved in these biopsies as the curettage procedure often retrieves layers of cells. Typically, a biopsy retrieving mucosa from both turbinates yields approximately 3 × 105 –1 × 106 cells with 30–50% viability. A micropipette is used to further fragment the biopsy and generate a diffuse cluster of cells in a small volume of the same medium. The biopsy is not subjected to any enzyme or mucolytic substances. The cells are gently centrifuged and the soft pellet is positioned in the center of a 12-well Vitrogen-coated plate using only sufficient medium to retain moisture over the well surface. The plate is then incubated for 24 h at 37C and 5% CO2. The following day, the well is flooded with 1 ml of medium and the pellet seeded again in a new well and incubated. Medium is changed daily in the plates until the cells reach confluence when they are lifted with trypsin/EDTA and seeded in culture flasks. Cells were expanded through two passages in bronchial epithelial growth medium (Cambrex Bioscience Walkersville, Inc., Walkersville, MD) over several days and plated on collagen-coated filter supports with a 0.4 µm pore size (Trans-CLR; Costar, Cambridge, MA, USA) and cultured in a 1:1 mixture of bronchial epithelial cell basic medium and DMEM-H with SingleQuot supplements (Cambrex), bovine pituitary extracts (13 mg/ml), bovine serum albumin (1.5 µg/ml), and nystatin (20 units). When the cultured cells reached confluence, all-trans retinoic acid was added to the medium and ALI culture conditions (removal of the apical medium) were created to promote differentiation (Jaspers et al. 2007). Upon establishment of ALI conditions, cultures typically require approximately 4 weeks to achieve their full level of differentiation. After this point, little further differentiation is evident but the cultures will remain viable for several months with continued regular replenishment of culture medium. The CBF software simultaneously captures the number of motile points within each microscopic field and these data were used as a relative measure of the degree of ciliation in the cultures deriving from each group.
Statistical analyses
Statistical analyses were performed on the data from the 20 subjects who completed the study. The outcome variables were culture CBFs, for which 50 CBF points per individual were obtained in evaluating their response to tobacco smoke exposure and the number of motile points; for the latter outcome, each subject yielded 10 points. In addition to the tabular statistical method (e.g., means shown with one SE. Fig. 1), we detected a potential association between the culture CBFs and the three smoking groups (NS, NS/ETS, and S) using a linear mixed-effects regression model. Linear mixed models are commonly used to analyze clustered data collected on repeated measures from the same individuals as is the case for the data sets in this study. We used the fixed effects to model the smoking group effect and modeled the potential correlation for the 50 CBF points within an individual with the random effects. This approach allows each of the 20 individuals to have their own unobserved yet individual-specific traits (the random effect); and conditional on these individual-specific traits, the 50 CBF points within each individual’s data set are assumed to be independent. Similar statistical approaches were used in the analyses of the number of motile points. All statistical analyses were performed using SAS system software version 9.1.3 (SAS Institute, Cary, NC).
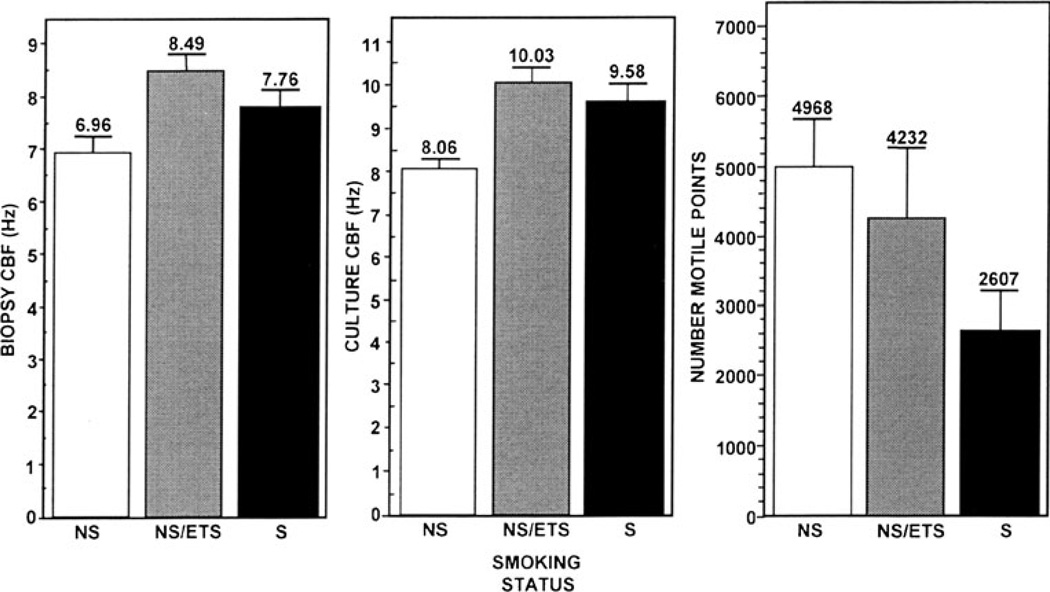
Fig. 1.
Comparative mean CBF of biopsies and subsequent ALI cultures deriving from same biopsies and mean motile points among the three experimental groups. NS non-smokers, NS/ETS non-smokers exposed by domestic or occupational conditions to environmental tobacco smoke, and S active smokers.
Results
Cotinine assays
Cotinine assays exhibited an expected range of CCR among the three experimental groups (Table 1). These quantitative assessments of tobacco smoke exposure confirmed the self-reporting smoking status of all subjects obtained at interview. These analyses served to validate subject exposure in the three exposure groups with very low CCR in the non-smoker group, intermediate levels in the non-smoker/ETS-exposed group, and the highest levels in the smoker group.
Table 1.
Gender and race distribution of subjects and mean cotinine levels in the experimental groups
| Non-smokers (NS) | Non-smokers/ETS-exposed (NS/ETS) | Smokers (S) | |
|---|---|---|---|
| Gender | |||
| Female | 5 | 5 | 4 |
| Male | 2 | 1 | 3 |
| Race | |||
| African-American | 1 | 2 | 6 |
| Asian | 1 | 1 | 0 |
| Caucasian | 5 | 3 | 1 |
| Mean log10 CCR | 0.50±0.30 | 1.46±0.31 | 6.13±0.10 |
CCR cotinine/creatinine ratio
Comparative assessment of epithelial CBF in biopsies and cultures
Epithelial CBF in freshly acquired biopsies from NS/ETS and S subjects was slightly elevated relative to NS subjects. The means and the standard errors for the three outcome variables across the subjects’ measurement values classified by the groups are summarized in Fig. 1. It is clear that the mean of the culture CBFs among the subjects exposed to tobacco smoke, regardless of the route of exposure, was increased relative to that for non-smokers although the mean CBF for the smokers was slightly lower than that of the NS/ETS group. This suggests nevertheless that smoking status is associated with elevated culture CBF values. We employed the linear mixed effects regression models to formally test the overall hypothesis that there is no difference in the CBF level across the three groups. The p value for this test is marginally significant (p=0.09). However, there is a more statistically significant result if we test the S and NS/ETS combined groups against the NS group (p=0.029). Furthermore, individual group comparisons showed that subjects in the S group were more likely to manifest elevation in the culture CBFs over the NS group (p<0.001) while subjects in the NS/ETS group also have higher culture CBFs than those in the NS group (P=0.001).
Tobacco smoke exposure and degree of ciliation
Because the luminal surfaces of large airway mucosa are predominantly populated by ciliated cells and because the mucosal samples lose their anatomic orientation in the biopsy, there was no discernable difference in the experimental groups relative to epithelial cell type distribution. We have found little difficulty in obtaining multiple viable ALI cultures from single individual biopsies regardless of smoking status. However, it is as the cultures deriving from these biopsies approach maturity that difference in cell type distribution becomes more apparent. Moreover, we typically experienced better success in propagating ALI cultures from these generally healthy subjects, regardless of a relevant smoking history, than in similar efforts to culture nasal mucosa from patients with documented chronic disease (unpublished data). Determination of the mean number of motile points among cultured cells as assessed by SAVA showed an obvious decline associated with the exposure level to tobacco (Fig. 1). It is noteworthy that the NS/ETS-exposed group exhibited the greatest variability in mean number of motile points among the three groups. We employed the same random effects regression method above to analyze the relationship between smoking status and the number of motile points as a measure of the degree of ciliation in the cultures. Since the distribution of the motile points was skewed, we applied a log10 scale to the original values of the motile points as a transform to achieve normality for the analyses. While Fig. 1 suggests that the mean motile points in the non-smoker group are the highest and the smoker group the lowest, we did not find any statistical significant difference between the NS and the NS/ETS groups. Similarly, no difference was found between the NS/ETS and the S groups. However, upon combining the groups, we found that the combined NS and NS/ETS group has a significantly higher (+0.36) number of motile points than the S group (p=0.027) although no difference was detected when the NS/ETS and S groups were combined and compared to the NS group.
Discussion
Previous studies from this laboratory (Zhou et al. 2009) have shown that lifestyle exposure to tobacco smoke whether by active smoking or by passive exposure to secondhand smoke elicits upregulation of nasal epithelial ciliary beat frequency. As an extension of these studies, we appropriated ALI airway epithelial cell culture methodology (Jaspers et al. 2007) to investigate the persistence of this pattern in vitro. The establishment of ALI cultures of conducting airway epithelium involves the serial selection of viable epithelial populations taken at biopsy that exhibit good adherence characteristics to a plastic substrate. These populations of cells are propagated using conventional tissue culture techniques in culture flasks where they pass through mitotic cycles to establish a monolayer of largely undifferentiated cells over 2–3 d. These cells are harvested and seeded on commercially available transwells where they are nourished from below with medium supplemented with retinoic acid and positioned with the mucosal surface exposed to air. This configuration leads to the emergence of a well-differentiated epithelial layer of primary cells at approximately 4 wk of further incubation under ALI conditions that emulates morphologic and functional characteristics of the epithelial layer in situ. After 4 weeks, the cultures typically have achieved their maximum level of differentiation but may be maintained for several months following with continued periodic replenishment of the culture medium.
We initially hypothesized that the absence of the chemical stimuli present in tobacco smoke that promote the acceleration of CBF observed in CBF assessment in fresh epithelial biopsies from smokers and individuals exposed to secondhand tobacco smoke would mitigate CBF acceleration in vitro and that the newly differentiated populations of cells deriving from mitotic expansion of the original biopsies would revert to a common baseline CBF having identity with that of non-smokers. To the contrary, we found that the upregulation of CBF in the fresh biopsies from both groups of smoke-exposed individuals persisted even through the rounds of proliferation required to establish new populations of epithelial cells in the cultures. Of particular interest, we noted that CBF was slightly elevated in the cultures deriving from the NS/ETS subjects. Our earlier study of CBF in nasal biopsies of smoke-exposed individuals suggests that even limited and intermittent levels of exposure as would typically occur among non-smokers exposed to tobacco smoke was sufficient to induce a significant elevation in CBF and that active smoking and high levels of exposure are not required to induce this effect. Moreover, we observed in determinations of CBF that cultures derived from smokers and non-smokers exposed to secondhand tobacco smoke exhibited fewer motile points which can be interpreted as a reduced complement of ciliated cells relative to cultures established from biopsies of well-documented non-smokers. Other investigations (Jaspers et al. 2009) in this laboratory also have demonstrated an increased complement of cell phenotypes that localize Muc5B, a biomarker of non-ciliated secretory cell types, in cultures derived from tobacco smoke-exposed subjects.
Our measurements of CBF in cultures from all groups exhibited a modest overall increase in CBF relative to comparable measurements of CBF in fresh biopsies. In contrast to biopsy CBF which was determined at 25°C, culture CBF was determined immediately upon removal from an incubator maintained at 37°C. Although no microscope stage warming device was used, the short time required to capture CBF data likely did not have a detectable effect on CBF in any of the groups. Since this increase was evident and consistent across all groups, we concluded that this differential was accounted for by the difference in temperature at which these determinations were made as biopsy CBF was determined at room temperature immediately after acquisition of the biopsy and culture CBF was determined immediately upon removal of the transwell plates from an incubator maintained at 37°C. The consistency of this difference across the groups is further evidence of a documented generalized effect of temperature to elevate CBF (Schipor et al. 2006).
The stimulatory modification, subsequent to an environmental exposure, of a fundamental physiologic characteristic such as CBF as well as changes in epithelial phenotype distribution through multiple cycles of proliferation is suggestive of the contribution of the exposure to an epigenetic effect. It is plausible that the culturing process promotes population of the cultures by some terminally differentiated cells that already have undergone phenotypic and physiologic modification in vivo and that these characteristics persist in vitro. However, it is also equally plausible that this process selects for populations of progenitor cells that also have undergone epigenetic modification and that their progeny reflect these modifications through subsequent generations as the cultures mature under ALI conditions. Previous studies from this laboratory (Zhou et al. 2009) and others have suggested that CBF may be upregulated in the presence of a variety of stimuli through a nitric oxide-cyclic guanosine monophosphate pathway (Jain et al. 1993; Jain et al. 1995; Li et al. 2000). Using epifluorescence microscopy, we demonstrated the enhanced generation of nitric oxide above background in ALI cultured airway epithelium in the presence of cigarette smoke condensate suggesting that the contents of the tobacco smoke contain components capable of activating nitric oxide synthases. We also have recently demonstrated the elevated excretion, relative to controls from the same isolate, of nitric oxide gas from ALI cultures from non-smoking subjects that have been stimulated with interferon-gamma (unpublished results). Nitric oxide, first recognized as the endothelium-derived relaxing factor in endothelial cells (Furchgott and Zawadzki 1980; Ignarro et al. 1987; Palmer et al. 1987) is now recognized for its physiologic role in other organs including the respiratory epithelium (Moncada et al. 1991; Kobzik et al. 1993). This more extended role for nitric oxide has brought additional investigational attention to nitric oxide as a potential biomarker of pathophysiology in the airways (Tamaoki et al. 1995; Yang et al. 1997). Recent studies have suggested that methylation of nitric oxide synthases may factor into their expression. Of particular interest, Chan et al. (2005) have reported that reduced DNA methylation in the iNOS gene promoter results in increased expression. Other investigators also have shown modified expression and activity of iNOS associated with mechanical injury (Chyu et al. 1999), reactive oxygen species (Zhen et al. 2008), particulate matter (Tarantini et al. 2009), and tobacco smoke (Wright et al. 1999; Anazawa et al. 2004). Moreover, Chan et al. (2004) have demonstrated that in the vascular endothelium DNA methylation regulates expression of eNOS. In related studies in this laboratory (Ilona Jaspers, personal communication), DNA methylation has been shown to be increased in ALI cultures derived from active smokers and individuals exposed to secondhand tobacco smoke. Taken together and in the context of the present study, it is plausible to hypothesize that the basis for the persistence of accelerated CBF in cultured ciliated nasal epithelium from tobacco smoke-exposed human subjects may derive from epigenetic modification of NOS genes. Although nitric oxide regulation appears to have little if any bearing on baseline CBF (Jain et al. 1993), the supranuclear localization of NOS enzymes in ciliated cells (Xue et al. 1996) suggests their role to rapidly upregulate CBF in response to environmental stimuli.
Our investigation of contrasting CBF in biopsies from non-smokers and smoke-exposed subjects indicated that CBF among NS/ETS subjects was comparable to that in smokers and that indeed their ciliary function exhibited greater identity to smokers than non-smokers. Freshly acquired biopsies are not amenable to determination of epithelial phenotype distribution and assessment of how it might vary between non-smokers and smoke-exposed subjects. However, upon growing out these biopsies in ALI culture it became evident that NS/ETS subject biopsies occupied an intermediate niche in terms of epithelial phenotype having fewer motile points than comparable cultures from non-smokers but more motile points than smokers (Fig. 1). We initially hypothesized that the in vitro mitotic expansion and extended propagation of epithelial cells from smoke-exposed subjects in the absence of tobacco smoke constituents that typically act to stimulate CBF would serve to bring all culture CBF to a common baseline regardless of smoking history. Not only was this not the case relative to CBF but similarly, smoke-exposed individuals exhibited a decline in overall ciliation that persisted through in vitro passages.
Conclusions
In summary, our studies have demonstrated the persistence of accelerated ciliary beat frequency in cultured human nasal epithelium obtained from smokers and individuals exposed to secondhand tobacco smoke subsequent to in vitro mitotic expansion. These studies also revealed phenotypic modification in vitro that favors expression of non-ciliated epithelial cell types in specimens deriving from smokers and secondhand smoke-exposed subjects, a pattern consistent with the altered distribution of epithelial cell types associated with chronic bronchitis in smokers (Saetta et al. 2000; Maestrelli et al. 2001). The ALI culture of airway epithelium from patients with documented smoking histories provides an optimal experimental platform to facilitate the design for future experiments involving morphologic and physiologic monitoring and analysis of nitric oxide expression and its role in airway pathophysiology.
Acknowledgments
This study was supported by a Clinical Innovator Award to JLC from the Flight Attendant Medical Research Institute, Grant #R01CA79949 from the National Institutes of Health to HZ, and the US Environmental Protection Agency. Although the research described in this article has been funded wholly or in part by the US Environmental Protection Agency through cooperative agreement CR833463-01 with the Center for Environmental Medicine, Asthma, and Lung Biology at the University of North Carolina at Chapel Hill, it has not been subjected to the agency’s required peer and policy review, and therefore does not necessarily reflect the views of the agency and no official endorsement should be inferred. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Contributor Information
Johnny L. Carson, Email: jcarson@med.unc.edu, Department of Pediatrics, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Center for Environmental Medicine, Asthma, and Lung Biology, The University of North Carolina at Chapel Hill, CB #7310, 104 Mason Farm Road, Chapel Hill, NC 27599-7310, USA.
Tsui-Shan Lu, The Department of Mathematics, National Taiwan Normal University, Taipei, Taiwan, Republic of China.
Luisa Brighton, Center for Environmental Medicine, Asthma, and Lung Biology, The University of North Carolina at Chapel Hill, CB #7310, 104 Mason Farm Road, Chapel Hill, NC 27599-7310, USA.
Milan Hazucha, Center for Environmental Medicine, Asthma, and Lung Biology, The University of North Carolina at Chapel Hill, CB #7310, 104 Mason Farm Road, Chapel Hill, NC 27599-7310, USA.
Ilona Jaspers, Department of Pediatrics, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Center for Environmental Medicine, Asthma, and Lung Biology, The University of North Carolina at Chapel Hill, CB #7310, 104 Mason Farm Road, Chapel Hill, NC 27599-7310, USA.
Haibo Zhou, Department of Biostatistics, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Center for Environmental Medicine, Asthma, and Lung Biology, The University of North Carolina at Chapel Hill, CB #7310, 104 Mason Farm Road, Chapel Hill, NC 27599-7310, USA.
References
- Anazawa T, Dimayuga PC, Li H, Tani S, Bradfield J, Chyu KY, Kaul S, Shah PK, Cercek B. Effect of exposure to cigarette smoke on carotid artery intimal thickening: the role of inducible NO synthase. Arterioscler Thromb Vasc Biol. 2004;24:1652–1658. doi: 10.1161/01.ATV.0000139925.84444.ad. [DOI] [PubMed] [Google Scholar]
- Chan GC, Fish JE, Mawji IA, Leung DD, Rachlis AC, Marsden PA. Epigenetic basis for the transcriptional hyporesponsiveness of the human inducible nitric oxide synthase gene in vascular endothelial cells. J Immunol. 2005;175:3846–3861. doi: 10.4049/jimmunol.175.6.3846. [DOI] [PubMed] [Google Scholar]
- Chan Y, Fish JE, D'Abreo C, Lin S, Robb GB, Teichert A-M, Karantzoulis-Fegaras F, Keightley A, Steer MB, Marsden PA. The cell-specific expression of endothelial nitric-oxide synthase. J Biol Chem. 2004;279:35087–35100. doi: 10.1074/jbc.M405063200. [DOI] [PubMed] [Google Scholar]
- Chyu KY, Dimayuga P, Zhu J, Nilsson J, Kaul S, Shah PK, Cercek B. Decreased neointimal thickening after arterial wall injury in inducible nitric oxide synthase knockout mice. Circ Res. 1999;85:1192–1198. doi: 10.1161/01.res.85.12.1192. [DOI] [PubMed] [Google Scholar]
- Davis CW, Lazarowski E. Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling. Respir Physiol Neurobiol. 2008;163:208–213. doi: 10.1016/j.resp.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain B, Rubinstein I, Robbins RA, Leise KL, Sisson JH. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun. 1993;191:83–88. doi: 10.1006/bbrc.1993.1187. [DOI] [PubMed] [Google Scholar]
- Jain B, Rubinstein I, Robbins RA, Sisson JH. TNF-alpha and IL1-beta upregulate nitric oxide-dependent ciliary motility in bovine airway epithelium. Am J Physiol. 1995;268:L911–L917. doi: 10.1152/ajplung.1995.268.6.L911. [DOI] [PubMed] [Google Scholar]
- Jaspers I, Horvath KM, Zhang W, Brighton LE, Carson JL, Noah TL. Reduced expression of IRF7 in nasal epithelial cells from smokers after infection with influenza. Am J Respir Cell Mol Biol. 2009 Oct 30; doi: 10.1165/rcmb.2009-0254OC. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers I, Zhang W, Brighton LE, Carson JL, Styblo M, Beck MA. Selenium deficiency alters epithelial cell morphology and responses to influenza. Free Radic Biol Med. 2007;42:1826–1837. doi: 10.1016/j.freeradbiomed.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, Stamler JS. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993;9:371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- Li D, Shirakami G, Zhan X, Johns RA. Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. Am J Respir Cell Mol Biol. 2000;23:175–181. doi: 10.1165/ajrcmb.23.2.4022. [DOI] [PubMed] [Google Scholar]
- Maestrelli P, Saetta M, Mapp CE, Fabbri LM. Remodeling in response to infection and injury: airway inflammation and hypersecretion of mucus in smoking subjects with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:s76–s80. doi: 10.1164/ajrccm.164.supplement_2.2106067. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Saetta M, Turato G, Baraldo S, Sanin A, Braccioni F, Mapp CE, Maestrelli P, Cavallesco G, Papi A, Fabbri LM. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med. 2000;161:1016–1021. doi: 10.1164/ajrccm.161.3.9907080. [DOI] [PubMed] [Google Scholar]
- Schipor I, Palmer JN, Cohen AS, Cohen NA. Quantification of ciliary beat frequency in sinonasal epithelial cells using differential interference contrast microscopy and high speed digital imaging. Am J Rhinol. 2006;20:124–127. [PubMed] [Google Scholar]
- Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole field analysis of ciliary beat frequency. J Microsc. 2003;211:103–111. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- Tamaoki J, Chiyotani A, Kondo M, Konno K. Role of NO generation in β-adrenoreceptor-mediated stimulation of rabbit airway ciliary motility. Am J Physiol. 1995;268:C1342–C1347. doi: 10.1152/ajpcell.1995.268.6.C1342. [DOI] [PubMed] [Google Scholar]
- Tarantini L, Bonzini M, Apostoli P, Pegoraro V, Bottati V, Marinelli B, Cantone L, Rizzo G, Hou L, Schwartz J, Bertazzi PA, Baccarelli A. Effects of particulate matter on genomic DNA methylation content and iNOS promoter methylation. Environ Health Perspect. 2009;117:217–222. doi: 10.1289/ehp.11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JL, Dai J, Zay K, Price K, Gilks CB, Churg A. Effects of cigarette smoke on nitric oxide synthase expression in the rat lung. Lab Invest. 1999;79:975–983. [PubMed] [Google Scholar]
- Xue C, Botkin SJ, Johns RA. Localization of endothelial NOS at the basal microtubule membrane in ciliated epithelium of rat lung. J Histochem Cytochem. 1996;44:463–471. doi: 10.1177/44.5.8627003. [DOI] [PubMed] [Google Scholar]
- Yang B, Schlosser RJ, McCaffrey TV. Signal transduction pathways in modulation of ciliary beat frequency by methacholine. Ann Otol Rhinol Laryngol. 1997;106:230–236. doi: 10.1177/000348949710600309. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007;87:1047–1082. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- Zhen J, Lu H, Wang XQ, Vaziri ND, Zhou XJ. Upregulation of endothelial and inducible nitric oxide synthase expression by reactive oxygen species. Am J Hypertens. 2008;21:28–34. doi: 10.1038/ajh.2007.14. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wang X, Brighton L, Hazucha M, Jaspers I, Carson JL. Increased nasal epithelial ciliary beat frequency associated with lifestyle tobacco smoke exposure. Inhal Toxicol. 2009;10:875–881. doi: 10.1080/08958370802555898. [DOI] [PMC free article] [PubMed] [Google Scholar]