Abstract
Background:
Citrus natsudaidai (natsumikan) is a typical citrus fruit containing several antioxidative nutrients which are found in higher concentration in the peel than in the pulp of the fruit. In this study, we examined whether extract from immature natsumikan peel prevents development of chronic allergic dermatitis in mice.
Materials and Methods:
Chronic allergic dermatitis was induced by repeated application of 2, 4, 6-trinitro-1-chlorobenzene in BALB/c mice and natsumikan was administrated orally for 30 days. Ear swelling and dermatitis score were measured after each challenge. The level of derivative-reactive oxygen metabolites (d-ROM) in serum was measured on day 30.
Results:
Treatment of natsumikan significantly attenuated the increase in ear swelling and improved dermatitis scores. In addition, increases in serum d-ROM were attenuated by a treatment of natsumikan. Although the routine treatment with dexamethasone resulted in a clear and significant reduction in body weight, natsumikan treatment did not have such effects.
Conclusion:
Immature natsumikan peel is beneficial for the treatment of chronic allergic dermatitis.
Keywords: 2, 4, 6-trinitro-1-chlorobenzene; Citrus natsudaidai; experimental model; oxidative stress
INTRODUCTION
Citrus natsudaidai (natsumikan) have been famous citrus fruits and recognized for their medical properties in many countries. Because natsumikan have a large amount of antioxidative nutrients such as Vitamin C and flavonoids, regular consumption of citrus fruit has been considered good for maintaining health. Additionally, constituents of some citrus plants, such as Aurantii Fructus Immaturus (Kijitsu) prepared from dried immature fruits of Citrus aurantium var. daidai and C. natsudaidai, have been used to develop useful crude drugs. Usually, kijitsu is used as an aromatic bitter stomachic and expectorant agent; Chun and Sankawa reported kijitsu extract to have an inhibitory effect on passive cutaneous anaphylaxis in rats, and suggested that kijitsu may possess antiallergic properties.[1] Natsumikan has many bioactive components such as hesperidin, neohesperidin, naringin, nobiletin, tangeretin, and aurapten, each having been well studied for its pharmacological anti-inflammatory,[2,3] anticarcinogenic,[4] antioxidant,[5] and other properties.
Chronic allergic dermatitis is a common skin disease induced by repeated exposure to an allergen. Atopic dermatitis is a typical chronic allergy skin disease with symptoms such as itching, redness, swelling, and small skin blisters. It is usually treated with steroids as a form of topical therapy, but long-term application results in major side effects such as cushingoid features, cataracts, and hyperglycemia. Thus, a safe and effective treatment is required.
At present, allergic dermatitis is easily simulated in experimental animal models by repeated application of haptens such as 2, 4-Dinitrofluorobenzene,[6] ovalbumin,[7] and 2, 4, 6-trinitro-1-chlorobenzene (TNCB)[8,9] which induce allergic inflammation in the skin. A mouse model reflects some aspects of human diseases including atopic dermatitis[10] or delayed-type hypersensitivity.[11] The antiallergic effect of some citrus spices was studied in vivo. It was reported that extract of unripe Citrus unshiu inhibits ear swelling during both the effector and induction phases in picryl chloride-induced dermatitis model mice.[12] It was also demonstrated that extract of unripe Citrus hassaku and its flavanone glycosides, naringin, and neohesperidin showed antiallergic properties in type I and type IV allergy models in mice.[13] These results suggest that citrus plant extract is beneficial for treatment of allergic dermatitis, but little is known about antiallergic activities of natsumikan. Therefore, the purpose of this study is to examine the effect of peel extracted from immature natsumikan on chronic allergic dermatitis induced by long-term repeated application of TNCB in an experimental mouse model.
MATERIALS AND METHODS
Plant material
Immature C. natsudaidai (natsumikan) were collected in Minamiboso city, Chiba, Japan, in August 2009, and stored at 4°C prior to extraction from their peel. The natsumikan peel contained the following active components per 100 g: hesperidin, 11 mg; neohesperidin, 370 mg; naringin, 780 mg; nobiletin, 1.6 mg; tangeretin, 3.2 mg; and aurapten, 26 mg.
Extraction procedure
Natsumikan peel (1 215.2 g) was cut into small pieces with scissors and air dried at 50°C for 24 hours in a mechanical air-dryer. Extract was obtained by placing the dried peel (412.4 g) in a 5-fold volume of methanol (Wako, Osaka, Japan) at 40°C for 2 hours. The resulting extract was filtered and concentrated under reduced pressure with the methanol evaporated, then lyophilized with freeze-drying machine (Taitec, Saitama, Japan) to give extract of dried natsumikan peel (14.2 g).
Animals
Female, 5-week-old BALB/c mice were obtained from Japan SLC Inc. (Shizuoka, Japan) and were aged 6 weeks at the time of the experiment. The mice were housed under controlled light (7:00-19:00) and temperature (24°C) with food and water available ad libitum. All experiments and procedures were approved by the Chiba University Institutional Animal Care and Use Committee.
Reagents and drugs
TNCB was obtained from Tokyo Chemical Industry (Tokyo, Japan). TNCB was dissolved in acetone/olive oil (3 : 1) to 0.8% (w/v) and used for sensitization and challenge. Dexamethasone (Sigma, St. Louis, MO, USA) was used as a positive control in this study.
Sensitization and challenge procedure
The hair of abdominal regions of mice was shaved with a hair clipper 2 days before sensitization. On day 1, these mice were sensitized with 50 μl of 0.8% TNCB solution to the shaved abdomen, and on day 8, they were challenged with 10 μl of 0.8% TNCB solution applied to the both sides of right ear. After the first challenge, TNCB solution was repeatedly applied to the same area of the right ear every 3 days till day 29.
Treatment
Natsumikan (100, 300, or 1 000 mg/kg) or distilled water as vehicle was administrated orally every day from day 1 to 30. Dexamethasone (3 mg/kg) was administrated orally every day from day 1 to 20, and then every other day from day 21 to 30. All compounds were given as suspensions in distilled water, administrated in a volume of 0.1 ml per 10 g body weight; each compound was administered on the day of each application of TNCB solution, 30 minutes beforehand.
Ear thickness
Right and left ear thickness was measured with a micrometer (Mitutoyo, Kanagawa, Japan) under light ether anesthesia, 24 hours after each challenge. Ear swelling was calculated by subtracting the left ear thickness value from the right ear thickness value.
Dermatitis score
A dermatitis score for the right ear was obtained using the method described by Sunada et al.[14] The severity of dermatitis was assessed 48 hours after each challenge on days 22, 25, and 28, and scored for three symptoms-erythema/hemorrhage, excoriation/erosion, and scaling/dryness, according to the following scale: 0 - no symptoms; 1 - mild; 2 - moderate; 3 - severe. The dermatitis score was defined as the total ranging from 0 to 9.
Oxidative stress
Blood samples were collected by orbital puncture, 24 hours after final application of TNCB solution on day 30 under ether anesthesia. Serums were prepared by centrifugation at 1 000 g for 20 minutes at 4°C, and then stored at -30°C. To measure the levels of reactive oxygen metabolites (ROM) in serum, a derivative-reactive oxygen metabolites (d-ROM) test was performed using an FRAS4 (Diacron, Grosseto, Italy), according to the analysis manual. The results are expressed in conventional units (Carratelli Units, U.CARR; 1 U.CARR corresponds to 0.8 mg/l H2O2 ).
Statistical analysis
All data are presented in the form mean ± SEM. Statistical significance was analyzed by Dunnett's method of multiple comparisons. Values of P < 0.05 were considered statistically significant. All statistical analysis was conducted using StatLight software (Yukms, Tokyo, Japan).
RESULTS
Effect of natsumikan on body weight
Oral administration of natsumikan did not cause a change in body weight compared with vehicle group. On the other hand, dexamethasone resulted in a significant reduction in body weight of the mice from day 9 throughout experimental period [Figure 1].
Figure 1.
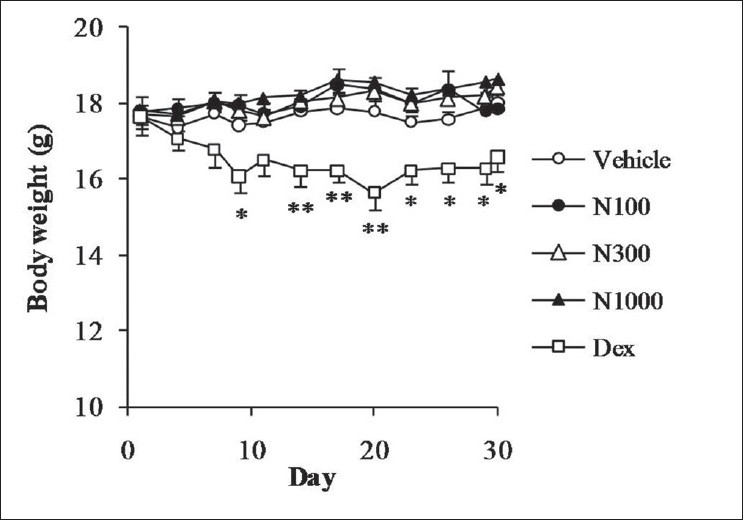
Effect of natsumikan extract and dexamethasone administration on body weight in mice repeatedly exposed to TNCB. Each symbol represents the mean ± SEM of 7 to 8 mice. *, **: Significantly different from the vehicle group at P < 0.05 and P < 0.01, respectively (Dunnett's test). N100, N300, and N1000 = Natsumikan at a dose of 100, 300, and 1 000 mg/kg; Dex = Dexamethasone
Inhibition of ear swelling in a model of allergic dermatitis
As shown in Figure 2, ear swelling was significantly increased in the mouse model of chronic allergic dermatitis, and natsumikan, especially at a dose of 300 mg/kg on day 21 to 30, and 1 000 mg/kg on days 21 and 24, significantly attenuated the increase in ear swelling compared with vehicle group. Dexamethasone significantly reduced the amount of ear swelling during the experimental period.
Figure 2.
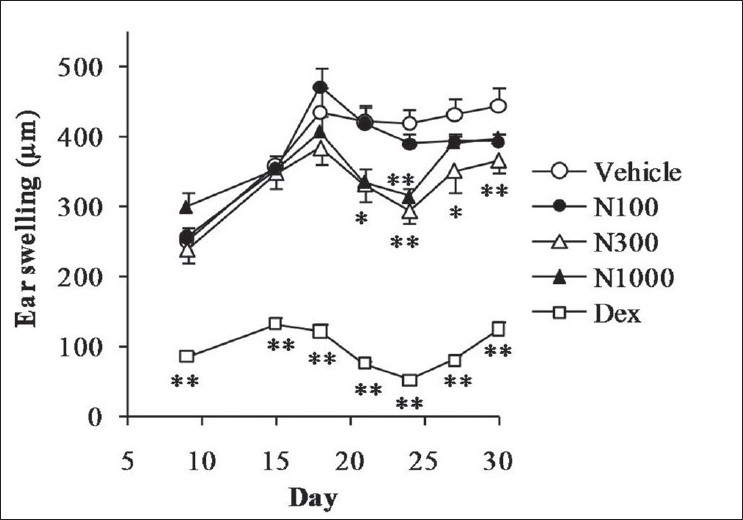
Effect of natsumikan extract and dexamethasone administration on ear swelling in mice repeatedly exposed to TNCB. Each symbol represents the mean ± SEM of 7 to 8 mice. *, **: Significantly different from the vehicle group at P < 0.05 and P < 0.01, respectively (Dunnett's test). N100, N300, and N1000 = Natsumikan at doses of 100, 300, and 1 000 mg/kg; Dex = Dexamethasone
Natsumikan improved the dermatitis score in a model of allergic dermatitis
As shown in Figure 3, repeated application of TNCB resulted in a high dermatitis score in the vehicle group, natsumikan improving the score in dose-dependent manner. Significant decrease in score was seen at day 25 with a dose of 1 000 mg/kg natsumikan. Dexamethasone also showed significant reduction in symptoms of dermatitis throughout the experimental period.
Figure 3.
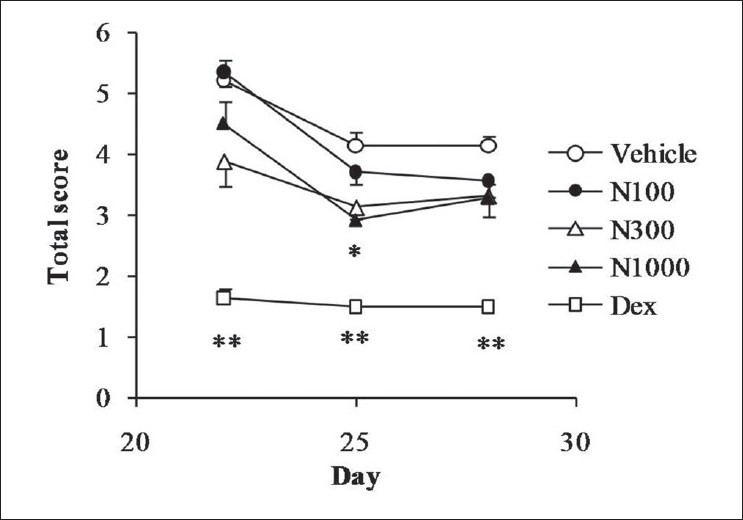
Effect of natsumikan extract and dexamethasone administration on dermatitis score in mice repeatedly exposed to TNCB. Each symbol represents the mean ± SEM of 7 to 8 mice. *, **: Significantly different from the vehicle group at P < 0.05 and P < 0.01, respectively (Dunnett's test). N100, N300, and N1000: Natsumikan at doses of 100, 300, and 1 000 mg/kg; Dex: Dexamethasone
Effect of natsumikan on serum oxidative stress in allergic dermatitis
Serum oxidative stress manifested higher in the vehicle group than the Nil group; natsumikan at a dose of 300 mg/kg showed a slight decrease [Figure 4]. In contrast, dexamethasone did not show obvious reduction in serum oxidative stress.
Figure 4.
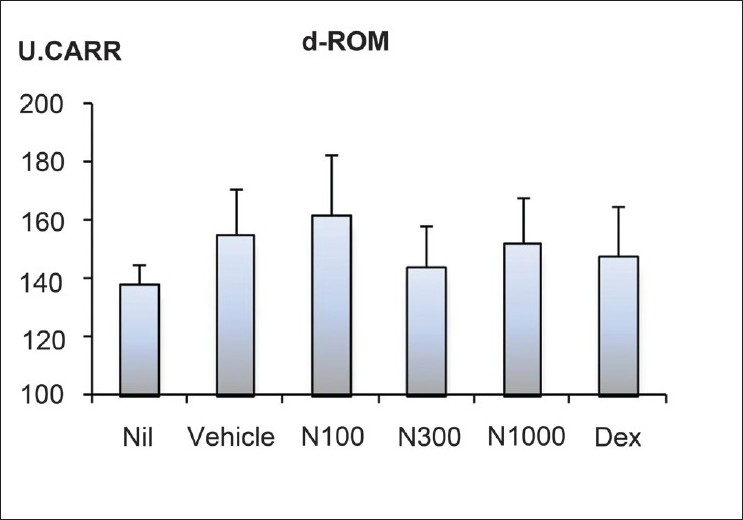
Effect of natsumikan extract and dexamethasone administration on degree of oxidative stress in mice repeatedly exposed to TNCB. All values were expressed as mean ± SEM of 7 to 8 mice (Vehicle, N100, N300, N1000 and Dex) and of three mice (Nil). Nil: Nontreatment; N100, N300, and N1000: Natsumikan at doses of 100, 300, and 1 000 mg/kg; Dex: Dexamethasone
DISCUSSION
This is the first study to show the effectiveness of natsumikan for allergic dermatitis, in which we have developed a chronic allergic dermatitis mouse model by applying TNCB every three days for 3 weeks and examining the antiallergic activity of extract from immature natsumikan peel.
Citrus peel has been regarded as a byproduct of the citrus fruit industry, but it has been reported to contain several active components at far higher concentration than the pulp. Natsumikan peel contains higher concentration of neohesperidin and naringin than other citrus species.[15] Furthermore, Kubo et al. reported the antiallergic effect of C. unshiu to be decreased with its ripening.[16] Therefore, we focused on the peel extract from unripened natsumikan.
Previous studies have demonstrated the antiallergic effects of citrus species. For example, C. unshiu powder attenuated production of pollen-induced inflammatory cytokines by peripheral blood mononuclear cells in seasonal allergic rhinitis patients,[2] and showed inhibitory effect on ear swelling in a mouse model of picryl chloride-induced contact dermatitis.[12] In addition, it was reported that neohesperidin and naringin, both typical active citrus components, inhibit ear swelling in a type IV allergic model mouse.[13] In our study, repeated application of TNCB in mice induced significant ear swelling and serious allergic dermatitis symptoms, which was found to be inhibited by treatment with natsumikan. These results suggest that natsumikan has a beneficial effect on allergic dermatitis which may be mediated by the active components as discussed previously. Furthermore, serum d-ROM value, which indicates oxidative stress, was increased by repeated application of TNCB but reduced by natsumikan, especially at a dose of 300 mg/kg. Thus, it can be hypothesized that oxidative stress is associated with the pathology of allergic dermatitis. It was reported that pathology of atopic dermatitis, a typical allergic dermatitis, was involved in oxidative stress.[17] In addition, the inflammatory response is known to involve the production of reactive oxygen species at sites of inflammation.[18] As described above, there is high concentration of neohesperidin in natsumikan peel fraction and it was reported that neohesperidin and its aglycone, hesperetin, are major secondary metabolites in citrus species, both possessing antioxidative properties.[19,20] Furthermore, some plant resources which possess antioxidant activity demonstrate a potential for an effective management of atopic disease.[21,22] Therefore, it is assumed that the inhibitory effect of natsumikan on chronic allergic dermatitis in our mouse model may be mediated, at least in part, by its antioxidative properties, but further studies are required to confirm this.
The mice after long-term dexamethasone treatment showed obvious and significant reduction in body weight and immune organ weight (data not shown), these results indicating possible adverse effects from steroid treatment. We hence modified the drug regimen for the dexamethasone group from day 20, from daily treatment to treatment on alternate days. By contrast, natsumikan treatment did not show such effects.
In conclusion, extract of immature natsumikan peel might be an effective natural product to treat chronic allergic dermatitis with no apparent side effects. Our results suggest that natsumikan can be a valuable treatment for several allergic diseases.
ACKNOWLEDGEMENT
We thank the Minamiboso Yoi-shoku Entrepreneurs Association for providing the natsumikan used in this study. This work was supported by a grant-in-aid from Chiba University, Japan.
Footnotes
Source of Support: Grant-in-aid from Chiba University, Japan,
Conflict of Interest: None declared.
REFERENCES
- 1.Chun YT, Sankawa U. Screening of antiallergic effect in traditional medicinal drugs and active constituents of Aurantii Fructus Immaturus. [Last accessed on 2010 Nov 30];Shoyakugaku Zasshi. 1998 43:314–23. Available from: http://www.meteo-intergate.com/journal/jsearch.php?jo=cg5yaksjandye=1989andvo=43andissue=4 . [Google Scholar]
- 2.Tanabe S, Kinuta Y, Yasumatsu H, Takayanagi M, Kobayashi S, Takido N, et al. Effects of Citrus unshiu powder on the cytokine balance in peripheral blood mononuclear cells of patients with seasonal allergic rhinitis to pollen. Biosci Biotechnol Biochem. 2007;71:2852–5. doi: 10.1271/bbb.70397. [DOI] [PubMed] [Google Scholar]
- 3.Lee NK, Choi SH, Park SH, Park EK, Kim DH. Antiallergic activity of hesperidin is activated by intestinal microflora. Pharmacology. 2004;71:174–80. doi: 10.1159/000078083. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka T, Kawabata K, Kakumoto M, Makita H, Hara A, Mori H, et al. Citrus auraptene inhibits chemically induced colonic aberrant crypt foci in male F344 rats. Carcinogenesis. 1997;18:2155–61. doi: 10.1093/carcin/18.11.2155. [DOI] [PubMed] [Google Scholar]
- 5.Sugiura M, Ohshima M, Ogawa K, Yano M. Chronic administration of Satsuma mandarin fruit (Citrus unshiu Marc.) improves oxidative stress in streptozotocin-induced diabetic rat liver. Biol Pharm Bull. 2006;29:588–91. doi: 10.1248/bpb.29.588. [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Oh SG, Seo SW, Ahn HJ, Geum D, Cho JJ, et al. Oral administration of Astragalus membranaceus inhibits the development of DNFB-induced dermatitis in NC/Nga mice. Biol Pharm Bull. 2007;30:1468–71. doi: 10.1248/bpb.30.1468. [DOI] [PubMed] [Google Scholar]
- 7.Dahten A, Koch C, Ernst D, Schnöller C, Hartmann S, Worm M. Systemic PPARgamma ligation inhibits allergic immune response in the skin. J Invest Dermatol. 2008;128:2211–8. doi: 10.1038/jid.2008.84. [DOI] [PubMed] [Google Scholar]
- 8.Harada D, Takada C, Tsukumo Y, Takaba K, Manabe H. Analyses of a mouse model of the dermatitis caused by 2,4,6-trinitro-1-chlorobenzene (TNCB)-repeated application. J Dermatol Sci. 2005;37:159–67. doi: 10.1016/j.jdermsci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Seike M, Furuya K, Omura M, Hamada-Watanabe K, Matsushita A, Ohtsu H. Histamine H4 receptor antagonist ameliorates chronic allergic contact dermatitis induced by repeated challenge. Allergy. 2010;65:319–26. doi: 10.1111/j.1398-9995.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- 10.Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J Invest Dermatol. 2009;129:31–40. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunikata T, Tatefuji T, Aga H, Iwaki K, Ikeda M, Kurimoto M. Indirubin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur J Pharmacol. 2000;410:93–100. doi: 10.1016/s0014-2999(00)00879-7. [DOI] [PubMed] [Google Scholar]
- 12.Fujita T, Shiura T, Masuda M, Tokunaga M, Kawase A, Iwaki M, et al. Anti-allergic effect of a combination of Citrus unshiu unripe fruits extract and prednisolone on picryl chloride-induced contact dermatitis in mice. J Nat Med. 2008;62:202–6. doi: 10.1007/s11418-007-0208-x. [DOI] [PubMed] [Google Scholar]
- 13.Itoh K, Masuda M, Naruto S, Murata K, Matsuda H. Antiallergic activity of unripe Citrus hassaku fruits extract and its flavanone glycosides on chemical substance-induced dermatitis in mice. J Nat Med. 2009;63:443–50. doi: 10.1007/s11418-009-0349-1. [DOI] [PubMed] [Google Scholar]
- 14.Sunada Y, Nakamura S, Kamei C. Effect of Lactobacillus acidophilus strain L-55 on the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol. 2008;8:1761–6. doi: 10.1016/j.intimp.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H. Flavonoid composition of fruit tissues of citrus species. Biosci Biotechnol Biochem. 2006;70:178–92. doi: 10.1271/bbb.70.178. [DOI] [PubMed] [Google Scholar]
- 16.Kubo M, Yano M, Matsuda H. Pharmacological study on citrus fruits. I. Anti-allergic effect of fruit of Citrus unshiu Markovich (1) Yakugaku Zasshi. 1989;109:835–42. doi: 10.1248/yakushi1947.109.11_835. [DOI] [PubMed] [Google Scholar]
- 17.Tsukahara H, Shibata R, Ohshima Y, Todoroki Y, Sato S, Ohta N, et al. Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci. 2003;72:2509–16. doi: 10.1016/s0024-3205(03)00145-0. [DOI] [PubMed] [Google Scholar]
- 18.Geronikaki AA, Gavalas AM. Antioxidants and inflammatory disease: Synthetic and natural antioxidants with anti-inflammatory activity. Comb Chem High Throughput Screen. 2006;9:425–42. doi: 10.2174/138620706777698481. [DOI] [PubMed] [Google Scholar]
- 19.Sawa T, Nakao M, Akaike T, Ono K, Maeda H. Alkylperoxyl radical-scavenging activity of various flavonoids and other phenolic compounds: Implications for the anti-tumor-promoter effect of vegetables. J Agric Food Chem. 1999;47:397–402. doi: 10.1021/jf980765e. [DOI] [PubMed] [Google Scholar]
- 20.Kim JY, Jung KJ, Choi JS, Chung HY. Hesperetin a potent antioxidant against peroxynitrite. Free Radic Res. 2004;38:761–9. doi: 10.1080/10715760410001713844. [DOI] [PubMed] [Google Scholar]
- 21.Sini H, Devi KS. Antioxidant Activities of the Chloroform Extract of Solanum trilobatum. Pharm Biol. 2004;42:462–6. [Google Scholar]
- 22.Ranjith MS, Ranjitsingh AJ, Shankar SG, Vijayalaksmi GS, Deepa K, Babu K, et al. Solanum trilobatum in the management of atopy: Through inhibition of mast cell degranulation and moderation of release of interleukins. Pharmacogn Res. 2010;2:10–4. doi: 10.4103/0974-8490.60581. [DOI] [PMC free article] [PubMed] [Google Scholar]


