Abstract
Background:
Bilberry (Vaccinium myrtillus L.) is one of the richest sources of anthocyanins which are known to have anticancer, wound healing and anti-allergic effects. Here, we examined whether bilberry extract (Bilberon-25) alleviates pruritus in a mouse model of chronic allergic contact dermatitis.
Materials and Methods:
BALB/c mice with chronic allergic contact dermatitis induced by 3 weeks of repeated application of 2,4,6-trinitro-1-chlorobenzene (TNCB) were administered Bilberon-25 orally for 3 weeks after sensitization with TNCB. The effects of Bilberon-25 on pruritus and inflammation were evaluated by measurement of scratching behaviour and ear swelling, respectively.
Results:
Treatment with Bilberon-25 significantly attenuated the TNCB-induced increase in scratching behaviour, but dexamethasone did not. In contrast, ear swelling was ameliorated by dexamethasone treatment, and significantly decreased by Bilberon-25. Repeated application of TNCB induced a shift in the cutaneous cytokine milieu from a T helper cell type (Th)1 to a Th2 profile; Bilberon-25 and dexamethasone alleviated this Th2 predominance of the lesional skin.
Conclusion:
Anthocyanins from bilberry might be beneficial for the treatment of chronic pruritus which can occur in patients with inflammatory skin diseases such as atopic dermatitis.
Keywords: Bilberon-25, bilberry anthocyanins, ear swelling, Interleukin-4 messenger Ribonucleic acid, pruritis
INTRODUCTION
Bilberry (Vaccinium myrtillus L.) is one of the best sources of anthocyanins and provides 15 anthocyanin analogues, each composed of one of five anthocyanidins and one of three glucosides.[1] Many pharmacologic studies have confirmed the efficacy of bilberry and other anthocyanin-containing extracts on the inhibition of cancer cell growth,[2] improvement of eyesight[3] and α-glucosidase inhibition.[4] Furthermore, anthocyanins from bilberry are well known for their potent antioxidative effects,[5–7] and the results of a recent study suggested a certain degree of synergism among the various anthocyanins in terms of antioxidant activity.[8]
In addition, it was recently shown that oral administration of cyanidin-3-o-β-D-glucoside, an anthocyanin found in black rice, as well as its metabolite cyanidin, promotes anti-scratching behavior in pruritogen-induced acute pruritus in mice.[9] As an acute sensation, pruritus fulfils an essential part of the body's innate defense mechanism to remove possibly harmful substances from the skin. If an itch persists, it will lead to chronic pruritus, which is a major diagnostic and therapeutic problem, and can have a profound impact on quality of life. Chronic pruritus can occur in patients suffering from numerous diseases such as inflammatory skin diseases, metabolic disorders and liver and kidney diseases.[10]
The effect of anthocyanins on chronic pruritus, however, remains unclear. Repeated elicitation of contact hypersensitivity with 2, 4, 6-trinitro-1-chlorobenzene (TNCB) not only increases the serum level of Immunoglobulin E (IgE) but also induces a shift in the cutaneous cytokine milieu from a T helper cell type (Th)1 to a Th2 profile,[11] followed by the development of chronic allergic contact dermatitis that is similar clinically, histologically and immunologically to atopic dermatitis. In the present study, we examined whether anthocyanins from bilberry can alleviate chronic pruritus in a mouse model of chronic allergic contact dermatitis induced by long-term repeated application of TNCB.
MATERIALS AND METHODS
Materials
Bilberon-25, a concentrated extract of bilberry, was generously donated by Tokiwa Phytochemical Co. Ltd. (Japan). The bilberry extract contained 15 anthocyanin analogues, each comprising one anthocyanidin (delphinidin, cyanidin, petunidin, peonidin, or malvidin) and one glucoside (glucopyranose, galactopyranose, or arabinopyranose). Naloxone and dexamethasone were obtained from Sigma Chemical (St. Louis, MO), substance P was provided by from Peptide Institute (Osaka, Japan), and TNCB was obtained from Tokyo Chemical (Tokyo, Japan). Bilberon-25 and dexamethasone were dissolved in distilled water. Naloxone and substance P were dissolved in physiologic saline solution and TNCB was dissolved in acetone.
Animals
All experiments and procedures were approved by the Chiba University Institutional Animal Care and Use Committee. Male ICR mice and female BALB/c mice, 6 weeks of age, were obtained from Japan SLC Inc. (Hamamatsu, Japan) and housed under controlled conditions of light (07:00–19:00) and temperature (24°C) with food and water available ad libitum.
Substance P-induced acute pruritus
The hair on the rostral part of the back region of the ICR mice was shaved with a hair clipper 1 day before the start of the experiment. Bilberon-25 (400 or 2,000 mg/kg) and naloxone (1 mg/kg) were administered orally for 5 days and subcutaneously once, respectively, to 6 mice. Substance P (100 nmol; volume, 20 μl) was injected once intradermally into the shaved part of the back 20 minutes after the final treatment with Bilberon-25 (or 15 minutes after treatment with naloxone). Scratching behaviour was evaluated immediately after injection with substance P. Distilled water and physiologic saline solution were applied as vehicle controls for Bilberon-25 and naloxone, respectively.
2,4,6-trinitro-1-chlorobenzene -induced chronic allergic contact dermatitis
The experimental protocols are illustrated in Figure 1. Abdominal hair of the BALB/c mice was shaved with a hair clipper a day before sensitization. On day -7, the mice were sensitized with a single epicutaneous application of 100 μl TNCB (1.1% w/v) solution to the shaved abdomen, and the animals were challenged on day 0 with 10 μl/ear TNCB solution. TNCB was then applied repeatedly to each ear 3 times a week until day 16. Bilberon-25 (400 or 2,000 mg/kg) was administered orally every day from day –7 to 17. Dexamethasone (1.5 mg/kg) was administered orally every day from day –7 and then every other day from day 0 to 17. Each reagent was administered 4 hours before the TNCB solution on each day of application. Scratching behaviour was evaluated 24 hours after application of TNCB on day 14. Acetone and distilled water were applied respectively as vehicle controls for TNCB (Nil) and non-treatment of reagents (Nil and vehicle).
Figure 1.
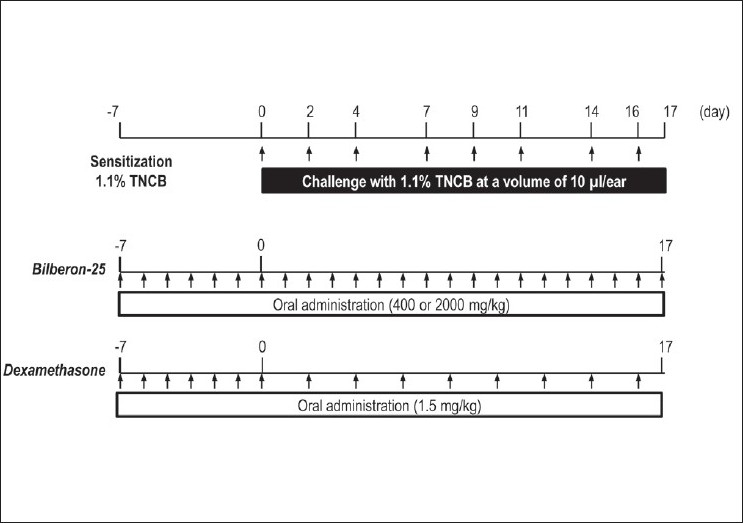
Schedule for the elicitation of chronic allergic contact dermatitis and application of reagents
Measurement of pruritus
Scratching bouts were counted for 30 minutes immediately after substance P application. For TNCB-induced pruritus, scratching behaviour was evaluated for 2 hours after 24 hours of TNCB application. Pruritus was assessed by automated counting of the scratching bouts using MicroAct (Neuroscience Inc., Tokyo, Japan), as reported previously.[12]
Measurement of ear thickness
Right and left ear thickness was measured with a micrometer (Mitsutoyo, Kanagawa, Japan) under light ether anesthesia, 24 hours after each challenge. Ear swelling was calculated by averaging the thickness values for both ears.
Expression of cytokine messenger ribonucleic acid in skin
After evaluation of pruritus on day 17, mice ears were sampled. Each specimen was homogenized, and total RNA was extracted using an RNeasy Mini Kit (QIAGEN, Hilden, Germany). Complementary Deoxyribonucleic acid (cDNA) was prepared from the RNA by reverse transcription using a PrimeScript RT reagent kit (TAKARA Bio INC., Shiga, Japan). Real-time quantitative polymerase chain reaction (PCR) was performed on a Step One TM Real Time PCR System (Applied Biosystems Inc., Carlsbad, CA) using SYBR Premix Ex Taq for mouse β-actin, mouse interleukin (IL)-4, and mouse interferon (IFN)-γ in accordance with the manufacturer's instructions (TAKARA Bio INC.). Results are expressed as mRNA level relative to β-actin mRNA as an internal control.
Statistical analysis
All data are presented as mean ± Standard Error of Mean (SEM). Statistical significance was analyzed using Dunnett's method for multiple comparisons. Statistical differences in two groups were analyzed using the Student's t-test or Welch's test following an F-test. Differences at P<0.05 were considered statistically significant. All statistical analyses were conducted using StatLight software (Yukms Co., Ltd. Tokyo, Japan).
RESULTS
Effects on substance P-induced pruritus
Pruritic responses to substance P were examined in ICR mice. Oral administration of Bilberon-25 showed no reduction in substance P-induced scratching. In contrast, subcutaneous dosing of naloxone, an opioid receptor antagonist, reduced substance P-induced scratching [Figure 2].
Figure 2.
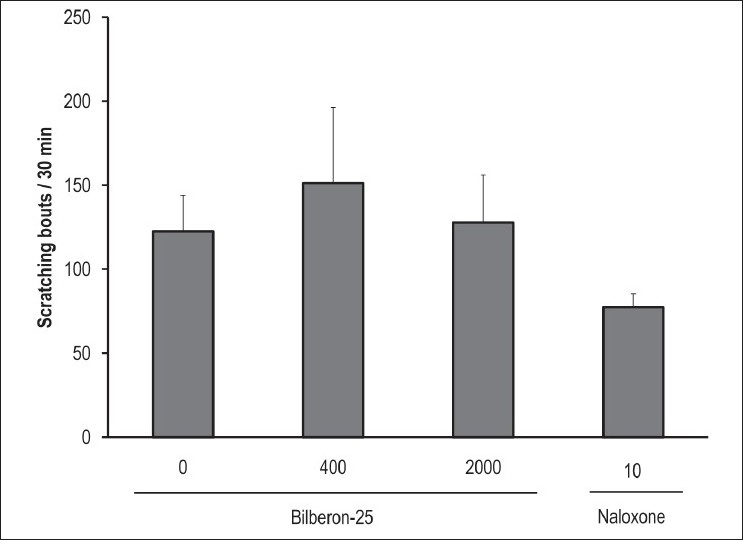
Effect of Bilberon-25 and naloxone on substance P-induced pruritus Bilberon-25 was orally administered for 5 days, and after final administration substance P was injected intradermally. Naloxone was administered once subcutaneously 15 minutes before treatment with substance P. Scratching bouts were counted for 30 minutes using Micro Act. Values represent the mean ± Standard error of mean of 6 mice
Effects on ear swelling as a marker of 2,4,6-trinitro-1-chlorobenzene-induced chronic allergic contact dermatitis
Repeated exposure of mouse ears to TNCB induced progressive and chronic allergic contact dermatitis characterized by skin swelling. Oral administration of Bilberon-25 (400 and 2,000 mg/kg) decreased ear swelling from day 1 until the final day, in a dose-dependent manner. Dexamethasone (1.5 mg/kg) significantly reduced the amount of ear swelling during the experimental period [Figure 3].
Figure 3.
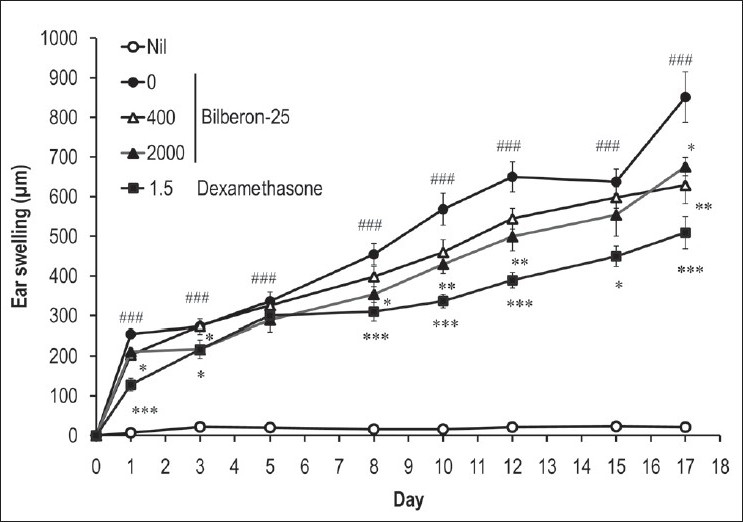
Effect of Bilberon-25 and dexamethasone on ear swelling induced by repeated application of 2,4,6-trinitro-1-chlorobenzene Ear thickness was measured 24 hours after each 2,4,6-trinitro-1-chlorobenzene challenge. Values represent the mean ± Standard error of mean for 4–7 mice. *P<0.05, **P<0.01 and ***P<0.001 vs 0 mg/kg Bilberon-25. ###P<0.001 vs Nil
Effects on 2,4,6-trinitro-1-chlorobenzene -induced chronic allergic contact dermatitis pruritus
Repeated application of TNCB evoked a significant increase in the number of scratching bouts on day 17. Oral administration of Bilberon-25 (400 and 2,000 mg/kg) significantly suppressed this increase; 400 mg/kg was more effective than 2,000 mg/kg in suppression of pruritus. On the other hand, administration of dexamethasone (1.5 mg/kg) showed no suppressive effect on the number of scratching bouts [Figure 4].
Figure 4.
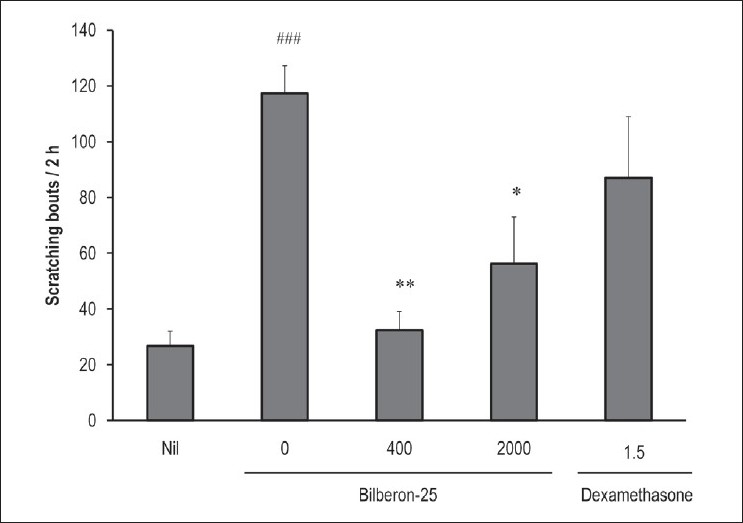
Effect of Bilberon-25 and dexamethasone on scratching behaviour induced by repeated application of 2,4,6-trinitro-1-chlorobenzene. Scratching bouts were counted for 2 hours using MicroAct 24 hours after the application of 2,4,6-trinitro-1-chlorobenzene on day 14. Values represent the mean ± Standard error of mean for 4–7 mice. *P<0.05 and **P<0.01 vs 0 mg/kg Bilberon-25. ###P<0.001 vs Nil
Effects on the expression of messenger Ribonucleic acid for T helper cell type 1 and 2 cytokines in mouse ears
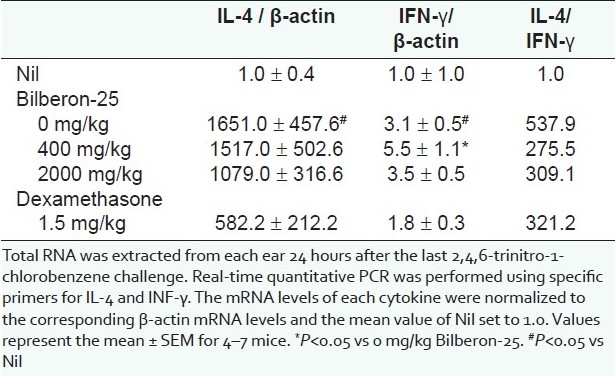
We analyzed the expression of mRNA for IL-4 and IFN-γ using a quantitative polymerase chain reaction (PCR) method to evaluate the relative dominance of Th1 and Th2 cytokines in ear lesions after repeated application of TNCB, and to elucidate the effect of Bilberon-25. The expression of mRNA for IL-4 detected in ears 24 hours after the last challenge was significantly (1,651 times) higher than that in acetone-treated ears (Nil). Oral administration of dexamethasone significantly suppressed this increase, whereas Bilberon-25 (2,000 mg/kg) produced a slight decrease. In contrast, the levels of mRNA for IFN-γ were increased by repeated challenge with TNCB, but the increase was only 3 times greater than that of acetone-treated ears (Nil). Oral administration of Bilberon-25 (400 mg/kg) significantly enhanced this increase, whereas Bilberon-25 (2,000 mg/kg) and dexamethasone had no obvious effect. In addition, the ratio of IL-4/IFN-γ mRNA, which indicates the relative proportions of Th2 and Th1 cytokines, was significantly increased (538 times) by repeated challenge with TNCB. A decrease of 49% and 43% was observed with 400 and 2,000 mg/kg Bilberon-25, respectively, whereas dexamethasone treatment led to a 40% decrease [Table 1].
Table 1.
Effect of Bilberon-25 and dexamethasone on the expression of messenger Ribonucleic acid for Interleukin-4 (IL-4) and Interferon-γ (IFN- γ) in the lesions induced by repeated application of 2,4,6-trinitro-1- chlorobenzene
DISCUSSION
To our knowledge, this is the first study to show the effectiveness of anthocyanins from bilberry on chronic pruritus of experimental chronic allergic dermatitis induced by repeated application of TNCB in mice.
In our study, repeated application of TNCB in mice induced significant ear swelling, which was found to be inhibited by treatment with Bilberon-25. We have previously reported that the citrus fruit natsumikan (Citrus natsudaidai), which is high in antioxidants, demonstrated inhibitory effects on ear swelling in a mouse model of chronic allergic dermatitis induced by TNCB. Furthermore, it is assumed that the inhibitory effect of natsumikan on this form of chronic dermatitis might be mediated, at least in part, by its antioxidative properties.[13] As bilberry anthocyanins are potent antioxidants, we also investigated the antioxidative properties of Bilbellon-25, but found no sign of efficacy (data not shown). These results suggest that the anti-dermatitis effect of Bilberon-25 is not mediated by its antioxidant activity.
Bilberry anthocyanin extract, Bilberon-25, showed no reduction in substance P-induced scratching. Substance P-induced pruritus is resistant to histamine H1 receptor antagonists;[14] given its occurrence in mast cell-deficient mice,[15] it is likely that histamine is not involved in this effect.
In our preliminary study, Bilberon-25 did not show any obvious inhibition of histamine-induced pruritus (data not shown). It appears that IL-31, which is mainly produced by Th2 cells, may be involved in promoting skin disorders. Transgenic mice overexpressing IL-31 developed severe pruritus and skin lesions.[16] In the present study, an obvious increase in IL-4 mRNA and IL-4/IFN-γ ratio, suggesting a response of the Th2 cells, was observed upon application of TNCB to mouse ears, which is consistent with other reports.[11] Oral administration of Bilberon-25 suppressed the increase in IL-4/IFN-γ ratio, and this suppression correlated well with the effect on scratching behavior. Therefore, it is likely that the inhibitory effect of Bilberon-25 on chronic pruritus in our mouse model is mediated, at least in part, by its inhibitory effect on Th2 polarization; however, further studies are required to confirm this.
The findings of a recent study indicate that the anthocyanin delphinidin suppresses expression of the IgE receptor (FceRI) on human mast cells.[17] The content of anthocyanins that carry delphinidin as aglycon is largest (36%) in Bilberon-25.[18] It is therefore considered that this effect of delphinidin might partially be involved in the inhibition of scratching behaviour by Bilberon-25.
Oral administration of Bilberon-25 (400 mg/kg) significantly suppressed the increase in the number of scratching bouts, but with minor effects on ear swelling. In contrast, dexamethasone strongly suppressed ear swelling without any effect on scratching behaviour. Pruritus is a major characteristic of atopic dermatitis, which exacerbates the associated cutaneous inflammation as a result of scratching. However, it remains unclear whether scratching itself precedes the induction of atopic dermatitis, or whether itching and scratching are a consequence of the presence of eczema.[19]
After long-term dexamethasone treatment, mice showed obvious and significant reductions in body weight and immune organ weight (data not shown), indicating possible adverse effects of glucocorticoid treatment. We therefore modified the drug regimen for the dexamethasone group after day 0, from daily treatment to treatment on alternate days. In contrast, Bilberon-25 treatment did not show any such effects.
Atopic dermatitis is one of the most common chronic inflammatory and pruritic skin disorders, marked by alternating periods of relapse and remission.[20,21] All patients with atopic dermatitis suffer from pruritus,[22] and glucocorticoids provide the most widely used therapy. Topical as well as systemic glucocorticoid therapy can induce numerous cutaneous side effects. Therefore, there is a considerable medical need for an effective and safe treatment for this condition. Our results suggest that daily intake of Bilberon-25 may be useful for the treatment of chronic pruritus which can occur in patients with atopic dermatitis.
ACKNOWLEDGEMENT
We thank Tokiwa Phytochemical. Co. Ltd. for providing Bilberon-25.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Ichiyanagi T, Kashiwada Y, Ikeshiro Y, Hatano Y, Shida Y, Horie M, et al. Complete assignment of bilberry (Vaccinium myrtillus L.) anthocyanins separated by capillary zone electrophoresis. Chem Pharm Bull. 2004;52:226–9. doi: 10.1248/cpb.52.226. [DOI] [PubMed] [Google Scholar]
- 2.Zhao C, Giusti MM, Malik M, Moyer MP, Magnuson BA. Effects of commercial anthocyanin-rich extracts on colonic cancer and nontumorigenic colonic cell growth. J Agric Food Chem. 2004;52:6122–8. doi: 10.1021/jf049517a. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto H, Nakamura Y, Tachibanaki S, Kawamura S, Hirayama M. Stimulatory effect of cyanidin 3-glycosides on the regeneration of rhodopsin. J Agric Food Chem. 2003;51:3560–3. doi: 10.1021/jf034132y. [DOI] [PubMed] [Google Scholar]
- 4.Matsui T, Ueda T, Oki T, Sugita K, Terahara N, Matsumoto K. alpha-Glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J Agric Food Chem. 2001;49:1948–51. doi: 10.1021/jf001251u. [DOI] [PubMed] [Google Scholar]
- 5.Ichikawa H, Ichiyanagi T, Xu B, Yoshii Y, Nakajima M, Konishi T. Antioxidant activity of anthocyanin extract from purple black rice. J Med Food. 2001;4:211–8. doi: 10.1089/10966200152744481. [DOI] [PubMed] [Google Scholar]
- 6.Kähkönen MP, Heinonen M. Antioxidant activity of anthocyanins and their aglycons. J Agric Food Chem. 2003;51:628–33. doi: 10.1021/jf025551i. [DOI] [PubMed] [Google Scholar]
- 7.Noda Y, Kaneyuki T, Mori A, Packer L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J Agric Food Chem. 2002;50:166–71. doi: 10.1021/jf0108765. [DOI] [PubMed] [Google Scholar]
- 8.Rahman MM, Ichiyanagi T, Komiyama T, Hatano Y, Konishi T. Superoxide radical- and peroxynitrite-scavenging activity of anthocyanins: Structure-activity relationship and their synergism. Free Radic Res. 2006;40:993–1002. doi: 10.1080/10715760600815322. [DOI] [PubMed] [Google Scholar]
- 9.Han SJ, Ryu SN, Trinh HT, Joh EH, Jang SY, Han MJ, et al. Metabolism of cyanidin-3-O-beta-D-glucoside isolated from black colored rice and its antiscratching behavioral effect in mice. J Food Sci. 2009;74:H253–8. doi: 10.1111/j.1750-3841.2009.01327.x. [DOI] [PubMed] [Google Scholar]
- 10.Metz M, Ständer S. Chronic pruritus--pathogenesis, clinical aspects and treatment. J Eur Acad Dermatol Venereol. 2010;24:1249–60. doi: 10.1111/j.1468-3083.2010.03850.x. [DOI] [PubMed] [Google Scholar]
- 11.Kitagaki H, Ono N, Hayakawa K, Kitazawa T, Watanabe K, Shiohara T. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J Immunol. 1997;159:2484–91. [PubMed] [Google Scholar]
- 12.Inagaki N, Igeta K, Shiraishi N, Kim JF, Nagao M, Nakamura N, et al. Evaluation and characterization of mouse scratching behavior by a new apparatus, Micro Act. Skin Pharmacol Appl Skin Physiol. 2003;16:165–75. doi: 10.1159/000069755. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama N, Yamaura K, Shimada M, Ueno K. Extract from peel of Citrus natsudaidai alleviates experimental chronic allergic dermatitis in mice. Pharmacognosy Res. 2011;3:154–8. doi: 10.4103/0974-8490.84999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, et al. Antipruritic activity of the kappa-opioid receptor agonist, TRK-820. Eur J Pharmacol. 2002;435:259–64. doi: 10.1016/s0014-2999(01)01588-6. [DOI] [PubMed] [Google Scholar]
- 15.Andoh T, Nagasawa T, Satoh M, Kuraishi Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J Pharmacol Exp Ther. 1998;286:1140–5. [PubMed] [Google Scholar]
- 16.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–60. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 17.Tamura S, Yoshihira K, Fujiwara K, Murakami N. New inhibitors for expression of IgE receptor on human mast cell. Bioorg Med Chem Lett. 2010;20:2299–302. doi: 10.1016/j.bmcl.2010.01.160. [DOI] [PubMed] [Google Scholar]
- 18.Ichiyanagi T, Shida Y, Rahman MM, Hatano Y, Konishi T. Bioavailability and tissue distribution of anthocyanins in bilberry (Vaccinium myrtillus L.) extract in rats. J Agric Food Chem. 2006;54:6578–87. doi: 10.1021/jf0602370. [DOI] [PubMed] [Google Scholar]
- 19.Buddenkotte J, Steinhoff M. Pathophysiology and therapy of pruritus in allergic and atopic diseases. Allergy. 2010;65:805–21. doi: 10.1111/j.1398-9995.2010.01995.x. [DOI] [PubMed] [Google Scholar]
- 20.Rudikoff D, Lebwohl M. Atopic dermatitis. Lancet. 1998;351:1715–21. doi: 10.1016/S0140-6736(97)12082-7. [DOI] [PubMed] [Google Scholar]
- 21.Barnetson RS, Rogers M. Childhood atopic eczema. BMJ. 2002;324:1376–9. doi: 10.1136/bmj.324.7350.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisshaar E, Dalgard F. Epidemiology of itch: Adding to the burden of skin morbidity. Acta Derm Venereol. 2009;89:339–50. doi: 10.2340/00015555-0662. [DOI] [PubMed] [Google Scholar]


