Abstract
Background:
Chlorogenic acid (CGA) or 5-caffeoylquinic acid, was found to be the dominant phenolic compound in leaves of Etlingera elatior (Zingiberaceae). The CGA content of E. elatior leaves was significantly higher than flowers of Lonicera japonica (honeysuckle), the commercial source. In this study, a protocol to produce a standardised herbal CGA extract from leaves of E. elatior using column chromatography was developed.
Materials and Methods:
Freeze-dried leaves of E. elatior were extracted with 30% ethanol, and sequentially fractionated using Diaion HP-20 and Sephadex LH-20.
Results:
The CGA fractions, which yielded extracts of 10% and 40% w/w purity, possessed antioxidant, tyrosinase inhibition, and antibacterial properties. The entire fractionation process took only 6.5 hours, using gravity flow. From 50 g of leaves, the final yield of CGA extract was 0.2 g (0.4%). The CGA content of the standardised herbal extract from leaves of E. elatior (40%) is 1.6 times that of commercial extracts from honeysuckle flowers (25%).
Conclusion:
With high CGA content, the standardised herbal extract has a great potential to be developed into functional food and other health products. Leaves of E. elatior, which currently have no economic value, could serve as an alternative source of CGA. Leaves are large, available in abundance, and harvesting is non-destructive to the plants.
Keywords: Chlorogenic acid, column chromatography, fractionation, standardised extract
INTRODUCTION
Etlingera species (Zingiberaceae) are tall ginger plants of disturbed tropical forests. Inflorescences are borne on stalks protruding from the ground or are found at the soil level. The varying shades of pink and red bracts and flowers, make them very attractive plants. In Peninsular Malaysia, a total of 15 Etlingera species has been recorded.[1,2] Plants of Etlingera have various traditional and commercial uses as food, condiment, medicine, and ornamentals. The hearts of young shoots, inflorescences, and fruits of the torch ginger Etlingera elatior (Jack) R.M. Smith or kantan are consumed by indigenous communities as condiment, eaten raw or cooked.[3] In Southeast Asia, inflorescences of E. elatior are widely cultivated as spices for food flavouring and as ornamentals. Fruits are used traditionally to treat earache, while leaves are applied for cleaning wounds.[4] Leaves, mixed with other aromatic herbs in water, are used by post-partum women for bathing to remove body odour.
In our previous study, antioxidant properties in terms of total phenolic content (TPC) and ascorbic acid equivalent antioxidant capacity (AEAC) of leaves of 26 ginger species belonging to eight genera were screened.[5] Results showed that leaves of Etlingera had the strongest TPC and AEAC followed by Alpinia. Among the five Etlingera species assessed, leaves of E. elatior had the highest values.[6] Prompted by their outstanding antioxidant properties, leaves of E. elatior were analysed for phenolic constituents. Three caffeoylquinic acids (CQA) i.e. 3-CQA or neochlorogenic acid, 5-CQA or chlorogenic acid and methyl 5-CQA were reported for the first time in Zingiberaceae.[7,8] Three flavonoids, namely, isoquercitrin, quercitrin, and (+)-catechin were also isolated. Chlorogenic acid (CGA) is the dominant phenolic compound in leaves of E. elatior. CGA content of leaves of E. elatior (294 ± 53 mg CGA/100 g) was found to be significantly higher than flowers of Lonicera japonica Thunb. or Japanese honeysuckle (173 ± 13 mg CGA/100 g), the commercial source.
CGA (5-CQA) is an ester of caffeic and quinic acids that is commonly found in coffee, and in fruits such as prunes and plums.[9–11] CGA is one of the abundant polyphenols in the human diet, and is the only CQA that is commercially available.[9,12] It is a natural antioxidant with commercial applications in medicine, food, and cosmetics. CGA is an antioxidant having two phenolic groups, which are able to scavenge free radicals via proton transfer.[13] It is also a bioactive compound with anti-inflammatory, antitumor, antimutagenic, anticarcinogenic, antidiabetic, analgesic, and antipyretic properties.[14–16] CGA protects against degenerative and age-related diseases in animals, and contributes to the prevention of cardiovascular diseases in humans.[17,18] Consumption of CGA enriched instant coffee induced reduction in glucose absorption, weight, and fat in the body.[19] In this study, a protocol to produce a standardised herbal extract of CGA from leaves of E. elatior is reported. Fractions were analysed for CQA content, CGA content, total phenolic content, radical scavenging activity, antibacterial activity, and tyrosinase inhibition ability.
MATERIALS AND METHODS
Plant material
Leaves of E. elatior were collected from Janda Baik in Pahang. The species is widely cultivated and can be easily identified, as its leaves emit a characteristic pleasant sour scent when crushed. Voucher specimen of E. elatior (EC14) was deposited at the herbarium of Forest Research Institute, Malaysia.
Leaf extraction
Leaves of E. elatior (50 g, in triplicate) were freeze dried overnight at 0.125 mbar and -50°C, and ground in a blender. Ground leaves were extracted four times with 500 ml of 30% ethanol for one hour each time in orbital shaker. Crude extract was filtered under suction and the solvent removed with a rotary evaporator (Eyela) at 50°C. For each batch, residues were weighed (4 g) and stored at -20°C for further use.
Fractionation with Diaion HP-20
The 30% ethanol crude leaf extract (in triplicate) was subjected to column chromatography. The extract (4 g) was dissolved in 10 ml of 20% ethanol and chromatographed over a 40 g Diaion HP-20 (Supelco) column. Fractions were eluted using water:ethanol (H2O:EtOH) : 0–35% step-gradient with an increment of 5% ethanol every 100 ml. The column was flushed with 200 ml of 100% ethanol after elution of each extract. Eluents from 0–5%, 10–35%, and 100% ethanol were pooled into fractions 1, 2, and 3, respectively. Fractions were dried in a rotary evaporator at 50°C prior to analysis. CGA was eluted in fraction 2.
Fractionation with Sephadex LH-20
Attempts were made to further refine fraction 2 (10–35% ethanol) that had the highest CGA content. The fraction (0.9 g) was re-dissolved in 5 ml of 20% ethanol and chromatographed over a 10 g Sephadex LH-20 (Sigma) column. The column was eluted with 100 ml of water (fraction 2.1) followed by 200 ml of 20% ethanol (fraction 2.2) and 200 ml of ethanol (fraction 2.3). Fractions were dried in a rotary evaporator at 50°C prior to analysis. CGA was eluted in fraction 2.2.
Caffeoylquinic acid content
Caffeoylquinic acid (CQA) content was quantified using the molybdate assay.[20] Molybdate reagent was prepared by dissolving 16.5 g sodium molybdate, 8.0 g dipotassium hydrogen phosphate, and 7.9 g potassium dihydrogen phosphate in 1 L deionised water. Plant samples (0.3 ml) were mixed with the reagent (2.7 ml) and incubated at room temperature for 10 minutes. Absorbance was measured at 370 nm. CQA content was expressed as mg chlorogenic acid equivalent (CGAE) per gram of extract. The calibration equation for CQA was y = 8.6966x (R2 = 0.9979) where y represents absorbance while x is concentration of chlorogenic acid in mg/ml.
Chlorogenic acid content
Chlorogenic acid (CGA) content was quantified using an Agilent Technologies 1200 Series reversed-phase high-performance liquid chromatography (RP-HPLC) with Thermo Scientific BDS Hypersil Phenyl Column (4.6 × 100 mm).[7] A 15-minute linear gradient from 5–100% methanol (MeOH), was used to elute samples at 1 ml/minute. Mobile phases were acidified with 0.1% trifluoroacetic acid for better resolution. A 20 μl loop was used for injection and elution was monitored at 280 nm. Identity of CGA was determined by matching UV spectrum and retention time with the standard (Acros Organic). The amount of CGA present was quantified using peak areas. The calibration equation of peak area (mAU*s) against concentration of CGA (mg/ml) was y = 7286.7 × (R2 = 0.9998). CGA content was expressed as mg CGA/g of extract.
Total phenolic content
Total phenolic content (TPC) was analysed using the Folin-Ciocalteu (FC) assay.[21] Fractions (300 μl in triplicate) were introduced into test tubes followed by 1.5 ml of FC reagent (Fluka) at 10 times dilution and 1.2 ml of sodium carbonate (Fluka) at 7.5% w/v. The tubes were allowed to stand for 30 minutes in the dark before absorbance at 765 nm was measured. TPC was expressed as mg gallic acid equivalent (GAE) per g of extract. The calibration equation for GA (Fluka) was y = 0.0111x – 0.0148 (R2 = 0.9998) where y is absorbance and x is mg/ml of GA.
Radical scavenging activity
Radical scavenging activity (RSA) was assessed using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay.[22] Different dilutions of fractions (1 ml; triplicate) were added to 2 ml of DPPH (Sigma) (5.9 mg/100 ml methanol). Absorbance was measured at 517 nm after 30 minutes. Calculated as half maximal inhibitory concentration (IC50), RSA was expressed as ascorbic acid equivalent antioxidant capacity (AEAC) in mg ascorbic acid (AA) per gram = IC50 AA/IC50 extract × 103. The IC50 of ascorbic acid (Merck) used for calculation of AEAC was 0.00387 mg/ml.
Antibacterial activity
Antibacterial activity was screened using the disc-diffusion method.[23] Agar cultures of Gram-positive bacteria of Bacillus cereus, Micrococcus luteus, and Staphylococcus aureus were prepared. Suspensions of bacteria (100 μl) were spread evenly onto 20 ml Mueller-Hinton agar preset in 90 mm Petri dishes. Paper discs (6 mm diameter) impregnated with 1 mg of plant extract dissolved in 100 μl solvent were transferred onto the inoculated agar. Streptomycin susceptibility discs (10 μg) and methanol impregnated discs were used as positive and negative controls, respectively. After incubation overnight at 37°C, inhibition zones were measured and recorded as mean diameter (mm). Results were expressed as minimum inhibitory dose (MID), that is, the minimum dose required to show a zone of inhibition.[24]
Tyrosinase inhibition ability
Tyrosinase inhibition ability (TIA) was assessed using the dopachrome method with 3,4-dihydroxy-L-phenylalanine (L-DOPA) (Sigma) as substrate.[25] Assays were conducted in a 96-well microtiter plate and a plate reader was used to measure absorbance (A) at 475 nm with 700 nm as reference. Samples were dissolved in 50% dimethyl sulphoxide (DMSO). Each well contained 40 μl of sample with 80 μl of phosphate buffer (0.1M, pH 6.8), 40 μl of tyrosinase (Sigma) (31 units/ml), and 40 μl of L-DOPA (2.5 mM). Each sample was accompanied by a blank that has all the components except L-DOPA. This gave a final sample concentration of 0.5 mg/ml. Results were compared with 50% DMSO as control. TIA (%) was calculated as (Acontrol – Asample)/Acontrol × 100.
RESULTS AND DISCUSSION
Chlorogenic acid and caffeoylquinic acid contents
Contents of CGA and CQA of the crude leaf extract of E. elatior were 28 ± 2 mg CGA/g and 53 ± 2 mg CGAE/g, respectively [Table 1]. Yields of CGA and CQA were 234 ± 25 and 437 ± 25 mg/100 g, respectively. An earlier study has reported that E. elatior leaves extracted with methanol followed by successive extraction with water had CGA and CQA contents of 294 ± 53 mg CGA/100 g and 320 ± 62 mg CGAE/100 g, respectively.[7] Initial isolation with Diaion HP-20 yielded fractions 1, 2, and 3. Most of the CGA and CQA were eluted in fraction 2 (10–35% ethanol) with CGA content increasing from 28 ± 2 to 96 ± 4 mg CGA/g (10% w/w purity) after fractionation. CQA content increased from 53 ± 2 to 169 ± 7 mg CGAE/g. This represented a significant increase of 3.4 times in CGA content and 3.2 times in CQA content compared to the crude extract. Fractionation with Diaion HP-20 reduced the yield of CGA and CQA by 28.6 and 32.5%, respectively. Fractions 1 and 3 had very low CGA content (7.5 ± 0.5 and 2.2 ± 1.1 mg CGA/g) and CQA content (73 ± 1.8 and 85 ± 17 mg CGAE/g), respectively. This implies that most of the CGA was eluted in fraction 2 with very little lost to the other fractions. Diaion HP-20 was chosen as the column-packing material because it is capable of elution at extremely high flow rates. Gravity elution in a 20 × 230 mm column was 75 ml/minute. Furthermore, Diaion HP-20 has good selectivity for aromatic hydrophobic compounds.
Table 1.
Content and yield of chlorogenic acid and caffeoylquinic acid after fractionation with Diaion HP-20 and Sephadex LH-20
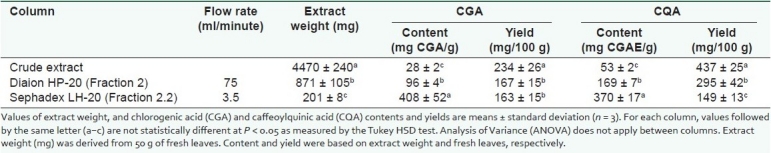
Further isolation of fraction 2 using Sephadex LH-20 yielded fractions 2.1, 2.2, and 2.3. Most of the CGA and CQA were eluted in fraction 2.2 (20% ethanol) with CGA content increasing from 96 ± 4 to 408 ± 52 mg CGA/g (40% w/w purity) after fractionation. CQA content increased from 169 ± 7 to 370 ± 17 mg CGAE/g. This represented a significant increase of 4.3 times in CGA content and 2.2 times in CQA content compared to fraction 2. Fractionation with Sephadex LH-20 resulted in full recovery of the yield of CGA but a reduction of 49.5% in the yield of CQA, as eluents were optimised for the isolation of CGA. Fractions 2.1 and 2.3 had very low CGA content (4.5 ± 0.8 and 5.5 ± 1.2 mg CGA/g) and CQA content (109 ± 6.7 and 71 ± 17 mg CGAE/g), respectively. This implied that most of the CGA was eluted in fraction 2.2 with very little loss to the other fractions. Sephadex LH-20 had a much slower gravity elution than Diaion HP-20. Flow rate in a 30 × 60 mm column was only 3.5 ml/minute. However, Sephadex LH-20 was able to refine CGA with a simple 3-step elution that involved the usage of minimal amounts of ethanol. It has excellent selectivity based on size exclusion and hydrophobic adsorption.
HPLC chromatograms at 280 nm showing CGA peaks of crude extract of leaves of E. elatior, and of standardised extract sequentially fractionated using Diaion HP-20 and Sephadex LH-20 are shown in Figure 1. The chromatogram of the crude extract showed the presence of compounds other than CGA. Subsequently, their presence was progressively reduced through fractionation. Purity of the CGA standardised extract after Sephadex LH-20 fractionation was demonstrated by the presence of a single CGA peak, while peaks corresponding to other compounds were progressively reduced from crude to Sephadex LH-20. HPLC is commonly used to analyse the chemical constituents of standardised extracts of single herbs or polyherbal mixtures.[26,27]
Figure 1.
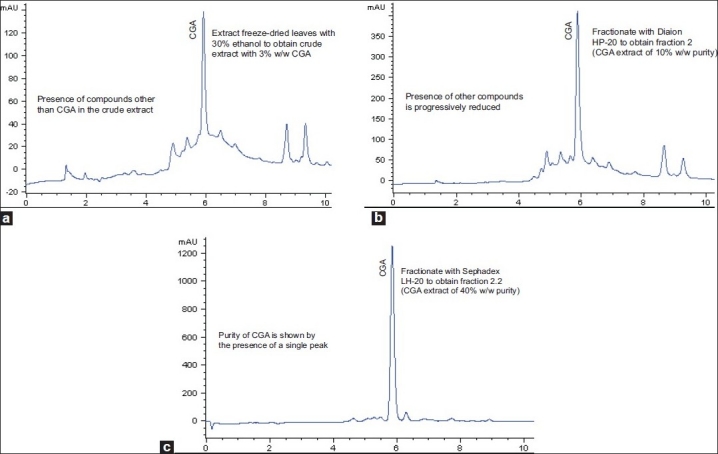
High-performance liquid chromatography chromatograms at 280 nm of Etlingera elatior leaves showing chlorogenic acid peaks at 5.74 minutes retention time of crude extract (a), Diaion HP-20 fractionated extract (b), and Sephadex LH-20 fractionated extract (c)
Bioactivities
TPC, AEAC, and TIA of the crude extract were 444 ± 20 mg GAE/g, 463 ± 20 mg AA/g, and 27%, respectively [Table 2]. Antibacterial activity of the crude extract was good with MID of 0.125 mg/disc against S. aureus, M. luteus, and B. cereus. Fraction 1 had the lowest TPC (283 ± 21 mg GAE/g) and AEAC (359 ± 31 mg AA/g), respectively. Antibacterial activity was moderate with MID of 0.25 mg/disc against S. aureus, M. luteus and B. cereus. TIA of fraction 1 (34%) was comparable to fraction 3 (39%). When compared to the crude extract, fraction 2 had the most outstanding TPC, AEAC, and antibacterial activity. TPC was 706 ± 12 mg GAE/g, AEAC was 993 ± 26 mg AA/g, and MID was 0.06 mg/disc against S. aureus and B. cereus, and 0.13 mg/disc against M. luteus. Fraction 3 showed the highest TIA of 39%. TPC and AEAC values were moderate, and MID values were 0.50 mg/disc against all three Gram-positive bacteria.
Table 2.
Properties of fractions from Diaion HP-20 and Sephadex LH-20 based on total phenolic content, ascorbic acid equivalent antioxidant capacity, tyrosinase inhibition ability, and antibacterial activity
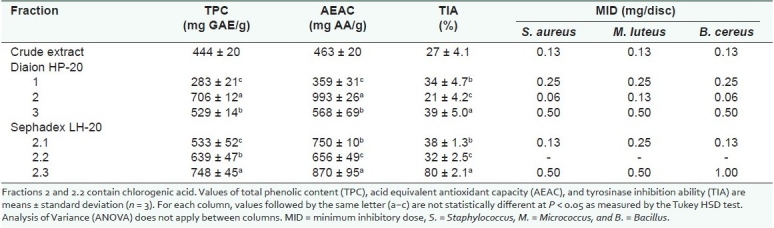
Further fractionation of fraction 2 using Sephadex LH-20 yielded fractions 2.1, 2.2, and 2.3. This resulted in a reduction of antioxidant properties of all fractions except fraction 2.3. However, there was a general decline in antibacterial activity and an increase in TIA. In particular, fraction 2.3 showed enhanced TIA from 39 to 80%, representing a two-fold increase.
Standardised herbal extract
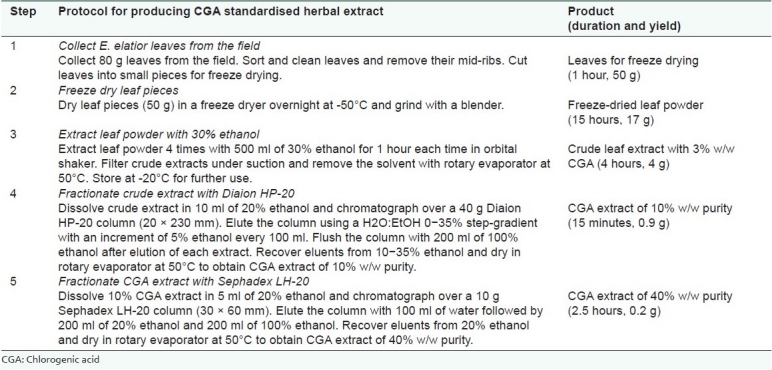
The protocol for producing a standardised herbal extract of CGA from leaves of E. elatior using column chromatography is shown in Table 3. The protocol has been briefly described in two review papers on antioxidant properties of ginger leaves, and on phytochemistry and pharmacological activities of E. elatior.[28,29] Freeze drying of leaves followed by extraction with 30% ethanol, and sequential fractionation using Diaion HP-20 and Sephadex LH-20 yielded an extract with 40% w/w purity. Aqueous ethanol solvents were used for both extraction and isolation. Following collection and freeze drying of leaves, the entire fractionation process took only 6.5 hours, using only gravity flow. From 50 g of leaves, the final yield of CGA extract was 0.2 g (0.4%).
Table 3.
Protocol for producing chlorogenic acid standardised herbal extract of 40% w/w purity from leaves of Etlingera elatior using column chromatography
Diaion HP-20 and Sephadex LH-20 have been used for successive fractionation of polyphenols including caffeic acid derivatives from plant extracts.[30] A similar method for isolating CGA has been reported for flowers of L. japonica (honeysuckle) using HPD-850 chromatography.[16] Following elution with 70% ethanol, the CGA content of the honeysuckle extract increased 4.5 fold. In this study, 10-35% ethanol was used and isolation with Diaion HP-20 yielded comparable results with CGA content increasing by 3.4 fold. It should be noted that both HPD-850 and Diaion HP-20 are macroporous styrene divinylbenzene resins of similar particle size. However, further isolation with Sephadex LH-20 in this study increased the CGA content by 14.6 fold.
A standardised extract of mangiferin from leaves of Mangifera indica as an ideal antioxidant has been reported.[31] As no isolation was involved, the mangiferin content of the extract was only 73 ± 0.17 mg/g dry weight (7.3% w/w). This value is very low compared to the CGA extract of 40% w/w purity. In terms of antioxidant properties, the mangiferin extract had TPC of 590 ± 48 mg GAE/g and IC50 of 0.17 ± 0.02 mg/ml (AEAC of 23 ± 2.7 mg AA/g). TPC value was therefore comparable to that of the CGA extract but AEAC value was about 28 times lower.
A standardised herbal extract contains a specified amount of active compound(s). Various brands of CGA standardised extracts from flowers of honeysuckle are commercially available.[32] With specifications of 25% CGA as the active ingredient, the extracts are sold as natural supplements with antioxidant, antimicrobial and other medicinal properties. In this study, CGA content of the standardised herbal extract produced from E. elatior leaves (40%) is 1.6 times than that of commercial extracts from honeysuckle flowers (25%).
Commercial potential
Leaves of E. elatior, which currently have no economic value, could serve as alternative sources of CGA. The species is widely cultivated in Southeast Asia for its inflorescence and as spice. Leaves are large, available in abundance and harvesting is non-destructive to the plants. This will ensure adequate and constant supply of raw materials essential for product development.
CONCLUSION
The CGA content of E. elatior leaves was found to be significantly higher than flowers of honeysuckle, the commercial source. A protocol to produce a standardised CGA extract from leaves of E. elatior using column chromatography was developed. CGA content of the standardised herbal extract of 40% w/w purity is 1.6 times than that of commercial extracts from honeysuckle flowers (25% w/w purity). With high CGA content, the standardised herbal extract from leaves of E. elatior can be developed into functional food, and other health products.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Lim CK. Taxonomic notes on Etlingera Giseke (Zingiberaceae) in Peninsular Malaysia: The “Nicolaia” taxa. Folia Malay. 2000;1:1–12. [Google Scholar]
- 2.Lim CK. Taxonomic notes on Etlingera Giseke (Zingiberaceae) in Peninsular Malaysia: The “Achasma” taxa and supplementary notes on the “Nicolaia” taxa. Folia Malay. 2001;2:141–78. [Google Scholar]
- 3.Noweg T, Abdullah AR, Nidang D. Forest plants as vegetables for communities bordering the Crocker Range National Park. ASEAN Rev Biodiv Environ Conser. 2003 Jan-Mar;:1–18. [Google Scholar]
- 4.Ibrahim H, Setyowati FM. Etlingera. In: De Guzman CC, Siemonsma JS, editors. Plant Resources of South-east Asia. Vol. 13. Leiden, Netherlands: Backhuys Publisher; 1999. pp. 123–6. [Google Scholar]
- 5.Chan EW, Lim YY, Wong LF, Lianto FS, Wong SK, Lim KK, et al. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008;109:477–83. [Google Scholar]
- 6.Chan EW, Lim YY, Omar M. Antioxidant and antibacterial activity of leaves of Etlingera species (Zingiberaceae) in Peninsular Malaysia. Food Chem. 2007;104:1586–93. [Google Scholar]
- 7.Chan EW, Lim YY, Ling SK, Tan SP, Lim KK, Khoo MG. Caffeoylquinic acids from leaves of Etlingera species (Zingiberaceae) LWT - Food Sci Technol. 2009;42:1026–30. [Google Scholar]
- 8.Chan EW. Bioactivities and chemical constituents of leaves of some Etlingera species (Zingiberaceae) in Peninsular Malaysia. Selangor, Malaysia. PhD thesis, Monash University Sunway Campus, Malaysia. [cited in 2009]. Available from: http://arrow.monash.edu.au/hdl/1959.1/149589 .
- 9.Clifford MN. Chlorogenic acids and other cinnamates - nature, occurrence, and dietary burden. J Sci Food Agric. 1999;79:362–72. [Google Scholar]
- 10.Nakatani N, Kayano S, Kikuzaki H, Sumino K, Katagiri K, Mitani T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.) J Agric Food Chem. 2000;48:5512–6. doi: 10.1021/jf000422s. [DOI] [PubMed] [Google Scholar]
- 11.Fang N, Yu S, Prior RL. LC/MS/MS characterization of phenolic constituents in dried plums. J Agric Food Chem. 2002;50:3579–85. doi: 10.1021/jf0201327. [DOI] [PubMed] [Google Scholar]
- 12.Clifford MN. Chlorogenic acids and other cinnamates - nature, occurrence, dietary burden, absorption and metabolism. J Sci Food Agric. 2000;80:1033–43. [Google Scholar]
- 13.Bonita JS, Mandarano M, Shuta D, Vinson J. Coffee and cardiovascular disease: In vitro, cellular, animal, and human studies. Pharm Res. 2007;55:187–98. doi: 10.1016/j.phrs.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 14.dos Santos MD, Almeida MC, Lopes NP, de Souza GEP. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol Pharm Bull. 2006;29:2236–40. doi: 10.1248/bpb.29.2236. [DOI] [PubMed] [Google Scholar]
- 15.Xiang Z, Ning Z. Scavenging and antioxidant properties of compound derived from chlorogenic acid in South-China honeysuckle. LWT - Food Sci Technol. 2008;41:1189–203. [Google Scholar]
- 16.Zhang B, Yang R, Zhao Y, Liu CZ. Separation of chlorogenic acid from honeysuckle crude extracts by macroporous resins. J Chromatogr B. 2008;867:253–8. doi: 10.1016/j.jchromb.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Niggeweg R, Michael AJ, Martin C. Engineering plants with increased level of the antioxidant chlorogenic acid. Nat Biotechnol. 2004;22:746–54. doi: 10.1038/nbt966. [DOI] [PubMed] [Google Scholar]
- 18.Olthof MR, Hollman PC, Katan MB. Chlorogenic acid and caffeic acid are absorbed in humans. J Nutr. 2001;131:66–71. doi: 10.1093/jn/131.1.66. [DOI] [PubMed] [Google Scholar]
- 19.Thom E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J Int Med Res. 2007;35:900–8. doi: 10.1177/147323000703500620. [DOI] [PubMed] [Google Scholar]
- 20.Clifford MN, Wright J. The measurement of feruloylquinic acids and caffeoylquinic acids in coffee beans.Development of the technique and its preliminary application to green coffee beans. J Sci Food Agric. 1976;27:73–84. doi: 10.1002/jsfa.2740270112. [DOI] [PubMed] [Google Scholar]
- 21.Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–62. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 22.Miliauskas G, Venskutonis PR, van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–7. [Google Scholar]
- 23.Chung PY, Chung LY, Ngeow YF, Goh SH, Imiyabir Z. Antimicrobial activities of Malaysian plant species. Pharm Biol. 2004;42:292–300. [Google Scholar]
- 24.Mackeen MM, Ali AM, El-Sharkawy SH, Manap MY, Salleh KM, Lajis NH, et al. Antimicrobial and cytotoxic properties of some Malaysian traditional vegetables (ulam) Pharm Biol. 1997;35:174–8. [Google Scholar]
- 25.Masuda T, Yamashita D, Takeda Y, Yonemori S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci Biotechnol Biochem. 2005;69:197–201. doi: 10.1271/bbb.69.197. [DOI] [PubMed] [Google Scholar]
- 26.Arawwawala LA, Hewageegana HS, Arambewela LR, Ariyawansa HS. Standardization of spray-dried powder of Piper betle hot water extract. Phcog Mag. 2011;7:157–60. doi: 10.4103/0973-1296.80678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samarakoon SR, Thabrew I, Galhena PB, De Silva D, Tennekoon KH. A comparison of the cytotoxic potential of standardized aqueous and ethanolic extracts of a polyherbal mixture comprised of Nigella sativa (seeds), Hemidesmus indicus (roots) and Smilax glabra (rhizome) Phcog Res. 2010;2:335–42. doi: 10.4103/0974-8490.75451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan EWC, Lim YY, Wong SK. Antioxidant properties of ginger leaves: An overview. Free Rad Antiox. 2011;1:6–16. [Google Scholar]
- 29.Chan EWC, Lim YY, Wong SK. Phytochemistry and pharmacological activities of Etlingera elatior: A review. Phcog J. 2011;3:6–10. [Google Scholar]
- 30.Nuntanakorn P, Jiang B, Yang H, Cervantes-cervantes M, Kronenberg F, Kennelly EJ. Analysis of polyphenolic compounds and radical scavenging activity of four American Actaea species. Phytochem Anal. 2007;18:219–28. doi: 10.1002/pca.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling LT, Yap SA, Radhakrishnan AK, Subramaniam T, Cheng HM, Palanisamy UD. Standardised Mangifera indica extract is an ideal antioxidant. Food Chem. 2009;113:1154–9. [Google Scholar]
- 32.Organic Herb. Honeysuckle flower extract. [cited in 2010]. Available from: http://www.organic-herb.com/Product/OHI-000166.html .


