Abstract
Background:
Hoslundia opposita Vahl. (Lamiaceae), a common local shrub in Ghana, is traditionally known not only for its pharmacological benefits but also for its insecticidal properties. Its acaricidal property, however, has not been investigated.
Objective:
To test the acaricidal effects of the crude extract and fractions of H. opposita leaves as well as to isolate and characterize the acaricidal principles.
Materials and Methods:
The crude methanolic extract, pet. ether, ethyl acetate and aqueous fractions of the leaves of H. opposita were tested against the larvae of the cattle tick, Amblyomma variegatum, using the Larval Packet Test. A bioassay-guided isolation was carried out to identify the acaricidal principle obtained from the ethyl acetate fraction.
Results:
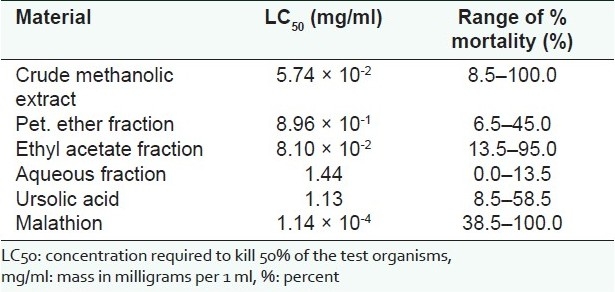
The active principle was characterised as ursolic acid, a triterpene previously isolated from the leaves of the same plant. The extract and fractions were less potent than the control, malathion (LC50 1.14 × 10-4 mg/ml). Among the plant samples however the crude methanolic extract exhibited the highest effect against the larvae (LC50 5.74 × 10-2 mg/ml), followed by the ethyl acetate fraction (LC50 8.10 × 10-2 mg/ml). Ursolic acid, pet. ether and aqueous fractions however showed weak acaricidal effects with LC50 values of 1.13 mg/ml, 8.96 × 10-1 mg/ml and 1.44 mg/ml, respectively.
Conclusion:
Ursolic acid was not as potent as the crude methanolic extract and the ethyl acetate fraction from which it was isolated. The overall acaricidal effect of H. opposita may have been due to synergy with other principles having acaricidal properties.
Keywords: Acaricide, Larval Packet Test, ticks, ursolic acid
INTRODUCTION
Ghana has an estimated cattle population of more than 1.5 million.[1] It is a source of livelihood for many in the rural areas.[2] Considered to be the greatest animal disease problem in Africa, ticks and tick-borne diseases (TDBs) are therefore a great threat and concern to cattle owners in Ghana.[3,4]
A myriad of problems are caused by ticks, notably disease transmission, reduction of animal growth rate, milk yield, livestock productivity and market opportunities.[5] Ticks and TBDs have substantial control costs, requiring considerable expenditures to treat, limit their spread and protect productivity.[3,5]
The use of chemical acaricides in tick control is associated with a number of problems, including environmental pollution, chemical residues in meat, wool and milk products, toxicity to farm animals, high cost of chemicals, pesticide poisoning and the rapid development of tick resistance.[3,6]
The search for safer and effective alternatives has soared and successes have been obtained through natural products, especially those from plant sources.[7,8] They are economically and environmentally friendly and less-toxic to humans and animals.
Hoslundia opposita Vahl. (fam. Lamiaceae) is a common perennial shrub that survives well in both wet and dry areas of Ghana. It has been shown to have several pharmacological benefits, both traditionally and scientifically. Essential oils from the leaves are extensively used as insect repellents. Although H. opposita has been widely studied for many pharmacological activities, its acaricidal potential, however, is yet to be sourced.
This study therefore seeks to investigate the acaricidal potential of H. opposita leaf extract against a common cattle tick in Ghana, Amblyomma variegatum.
MATERIALS AND METHODS
Collection and authentication of plant material
Fresh leaves of H. opposita were collected from a farmland at Juaben, in the Ashanti region of Ghana. It was authenticated at the Department of Pharmacognosy, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah of Science and Technology, Kumasi, Ghana, where a voucher specimen (HO1/2010) has been deposited at the department's Herbarium.
Extraction and isolation of the active acaricidal Principle
Approximately 1.0 kg of the air-dried leaves was cold-macerated with 5.0 L of methanol for 7 days. With the aid of the rotary evaporator, the extract was concentrated under reduced pressure and further dried to give a solid mass of 90.9 g (9.09% w/w).
Subsequently, 50 g of the crude methanolic extract (CME) was partitioned between 5 × 100 ml aliquots of petroleum ether (40–60°) and water [1:1]. The petroleum ether fraction was collected, concentrated into a solid friable mass of 23.7 g and designated F1. Then, 5 × 100 ml aliquots of ethyl acetate were shaken with the aqueous extract. The ethyl acetate fraction was also collected and concentrated into a solid friable mass of 5.5 g and designated F2. The aqueous fraction left (F3) was then concentrated and freeze-dried to yield 10.0 g of amorphous mass.
Five grams of the ethyl acetate fraction (active) was repeatedly column-chromatographed on silica gel 60 F254 (70–230 mesh ASTM), using pet. ether–ethyl acetate step gradient. Guided by their thin layer chromatography (TLC) profiles, fractions collected were bulked into four main fractions labeled A to D. Fraction C (active) was repeatedly column-chromatographed on silica gel 60 F254 (70–230 mesh ASTM), employing gradient elution with pet. ether–ethyl acetate mixture (increasing polarity). Eluates collected were bulked into three main fractions, C1 to C3. Fraction C2 (active) afforded an off-white amorphous powder, X (78 mg), after washing contaminants off with 5% ethyl acetate in pet. ether.
Bioactivity studies
Biological material
Engorged adult female ticks of A. variegatum were obtained from the Kumasi Abattoir and bred for eggs. The 5-day old, unfed larvae were used in this experiment.
Bioactivity studies of extracts and fractions
The crude extract and fractions were screened for acaricidal effect using the Larval Packet Test (LPT). Twenty 5-day old, unfed A. variegatum larvae were brought into contact with drug-impregnated filter papers (Watman No. 1) and observed for mortality after 24 h. A larva was recorded dead if it showed no sign of mobility. Concentrations used were serially diluted and ranged from 5.0 to 5.0 × 10-5 % w/v. A broad-spectrum acaricide, malathion, was used as the positive control, while methanol was used as the negative control. All experiments were run in triplicate.
Acaricidal activity of the isolate
Concentrations ranging from 2.0 to 2.5 × 10-1 % w/v were prepared by serial dilutions. These were tested against the 5-day-old, unfed larvae as described above, using methanol and malathion as negative and positive controls, respectively.
Statistical analysis
Results were expressed as LC50 values compared with the control. One-way ANOVA was used for comparison of the means.
RESULTS AND DISCUSSIONS
A number of ethnomedicinally important plant species abound in Ghana. Essential oils from such plants are commonly used as repellents or pesticides. Traditional livestock farmers employ various herbs to protect their animals and themselves against ticks and TBDs. Although research into local acaricidal plants has been scanty, one study by Annan et al.[9] that investigated the acaricidal effects of a common shrub, Plumbago zeylanica, identified the naphthoquinone, plumbagin, as the main acaricidal priniciple. Their finding therefore suggests the possibility of many more acaricidal principles lying hidden in many local plant species used as acaricides.
Isolate X was isolated as an off-white amorphous powder with an Rf value of 0.38 in pet. ether:ethyl acetate (8:2) and 0.67 in chloroform:methanol (9:1). It recorded a melting point of 270–273°C (uncorrected). ESI-TOF-MS data of X showed a molecular ion peak [M +] at m/z 456.4, which corresponds to the molecular formula C30H48O3.
1H-NMR spectral data showed that X has a vinylic proton triplet at δ5.14 and an alcoholic proton triplet at δ3.09. It also showed an allylic proton multiplet at δ1.87 and several saturated alkane protons with chemical shifts ranging from δ0.63 to δ1.52.
13C-NMR spectrum showed the presence of 30 carbon signals. The DEPT 135 spectrum showed seven methyl groups at δ15.25, δ15.45, δ16.73, δ16.85, δ20.98, δ23.37 and δ27.89. Nine methylene groups were shown at δ18.20, δ23.15, δ24.09, δ26.67, δ29.55, δ30.56, δ32.93, δ36.71 and δ38.57, while seven methine groups were recorded at δ38.80, δ38.98, δ47.45, δ52.72, δ55.15, δ78.70 and δ125.40. Seven quaternary carbons were also shown at δ36.81, δ39.09, δ39.35, δ41.95, δ47.70, δ138.08 and δ180.61. X was shown to possess a carboxylic acid carbon at δ180.61, two olefinic carbons at δ125.40 and δ138.08 and a saturated oxygen-bearing methine at δ78.70. The MS, 1H-NMR and 13C-NMR spectral data compared closely with that of ursolic acid as reported by the literature[10] [Table 1]. The structure of isolate X was therefore assigned as ursolic acid [Figure 1], previously isolated from the leaves of the same species.[11]
Table 1.
1H-NMR and 13C-NMR spectral data for ursolic acid in CDCl3/CD3OD
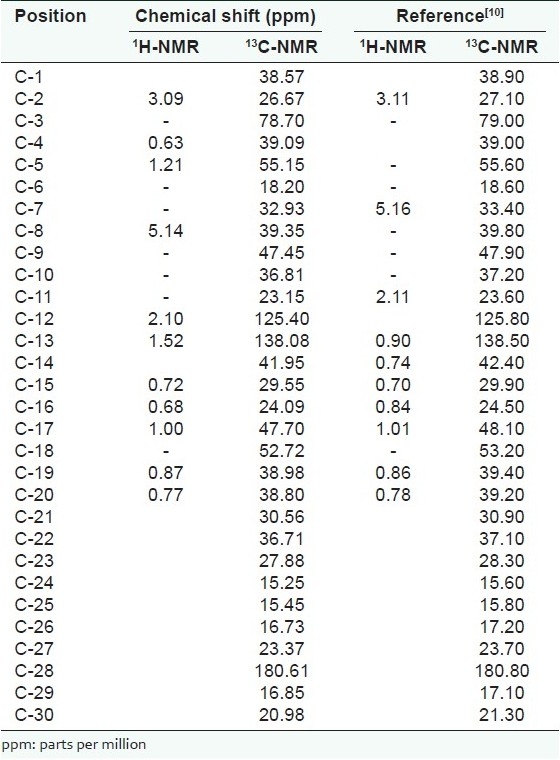
Figure 1.
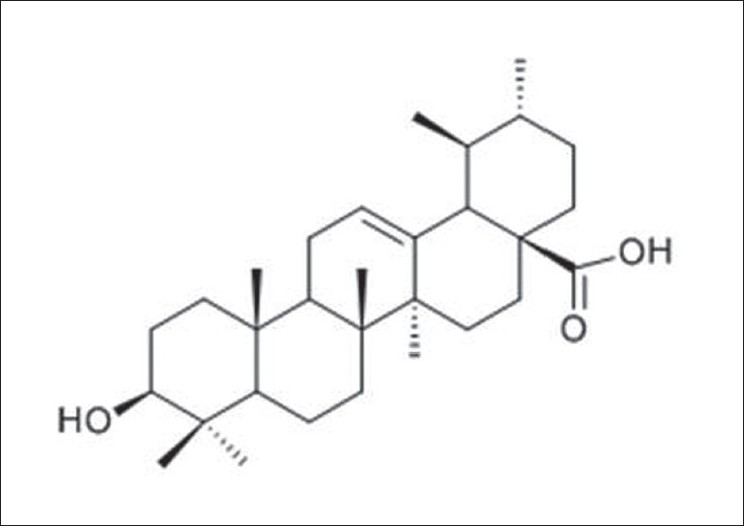
Structure of Ursolic acid
Biological activity
The crude methanolic extract was the most potent among the test samples against A. variegatum larvae (LC50 5.74 × 10-2 mg/ml). The ethyl acetate fraction also showed a relatively high potency (LC50 8.10 × 10-2 mg/ml). The pet. ether and aqueous fractions showed weak acaricidal effects, recording LC50 values of 8.96 × 10-1 mg/ml and 1.44 mg/ml, respectively. Ursolic acid also exhibited a weak acaricidal effect against the larvae of A. variegatum. At 2.0% w/v, it showed 58.5% mortality, with an LC 50 value of 1.13 mg/ml. This was significantly lower (P < 0.001) than the effect exhibited by the control, malathion (LC50 1.14 × 10-4 mg/ml) [Table 2].
Table 2.
Acaridal effects of crude extract and fractions of H. opposita leaves against larvae of A. variegatum compared with control
Malathion is a broad-spectrum acaricide commonly used in Ghana. It is one of several others used by livestock farmers. However, due to the high cost associated with its use,[2] most resource-poor farmers have resorted to cheaper alternative means of arriving at similar outcomes. One such alternative is their traditional knowledge and practices of herbal acaricides. Oils from various parts of plants have been used to repel or kill pests, including seeds, fruit peels and flowers.[12,13]
Ursolic acid is a triterpene, a known component of many essential oils. Literatures supporting the ethnomedicinal use of essential oils in pest control continue to increase. These effects have been attributed to the terpene components of these plants.[13,14]
Acaricidal effects may have been higher in the crude methanolic extract and the ethyl acetate fraction due to the fact that there was a combined effect by other components of the extract and fraction. Previous phytochemical screening of H. opposita showed the presence of other medicinally important terpenes like menthol, thymol, β-caryophyllene, oleanolic acid and germacrene-D. A study has even reported on the presence of benzyl benzoate, a known pesticide[15] identified from samples of essential oils obtained from the plant in West Africa. The combined effects of components such as these may have contributed to the high effects observed. Some structure–activity studies on terpenes suggest that molecules possessing free alcoholic, phenolic or potential olefinic modifications showed the most potent acaricidal activity.[16,17]
CONCLUSION
The results of this study suggest that the crude methanolic extract and ethyl acetate fraction of the leaves of H. opposita have acaricidal activity against the larvae of A. variegatum. Ursolic acid has been found to be a component of the ethyl acetate fraction, which contributes to the acaricidal activity of the plant. The finding therefore corroborates the traditional use of the plant as a repellent; however, its great potential would be limited by using it only as a repellent. The alcoholic extract of the leaves can serve the livestock industry better as a safer and cheaper alternative to chemical acaricides.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Kaljouw M. PhD Thesis. The Netherlands: Utrecht University; 2009. Resistance to acaricides of Boophilus ticks from cattle in Ghana. [Google Scholar]
- 2.Minjauw B, McLeod A. Research report, DFID Animal Health Programme, Centre for Tropical Veterinary Medicine. UK: University of Edinburgh; 2003. Tick-borne diseases and poverty. The impact of ticks and tick-borne diseases on the livelihood of small-scale and marginal livestock owners in India and eastern and southern Africa. [Google Scholar]
- 3.Kaaya GP. Prospects for Innovative Tick Control Methods in Africa. Insect Sci Appl. 2003;23:59–67. [Google Scholar]
- 4.Bell-Sakyi L, Koney EB, Dogbey O, Walker AR. Incidence and prevalence of tick-borne haemoparasites in domestic ruminants in Ghana. Vet Parasitol. 2004;124:25–42. doi: 10.1016/j.vetpar.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 5.McDermott J, Richard D, Randolph T. Incidence of animal diseases and their current and future impacts on crop-livestock systems in West Africa. In: Williams TO, Tarawali S, Hiernaux P, Fernandez-Rivera S, editors. Sustainable crop-livestock production for improved livelihoods and natural resource management in West Africa. Proceedings of an international conference; 2001 Nov. 19-22. Wageningen, The Netherlands: ILRI, Nairobi (Kenya) and CTA; 2004. [Google Scholar]
- 6.Miller GT. 6th ed. California: Thompson Learning Inc, Pacific Grove; 2004. Sustaining the Earth. [Google Scholar]
- 7.De Boer H, Vongsombath C, Pålsson K, Björk L, Jaenson TG. Botanical repellents and pesticides traditionally used against hematophagous invertebrates in lao people's democratic republic: A comparative study of plants used in 66 villages. J Med Entomol. 2010;47:400–14. doi: 10.1603/me09273. [DOI] [PubMed] [Google Scholar]
- 8.Rasikari HL, Leach DN, Waterman PG, Spooner-Hart RN, Basta AH, Banbury LK, et al. Acaricidal and Cytotoxic Activities of Extracts from Selected Genera of Australian Lamiaceae. J Econ Entomol. 2005;98:1259–66. doi: 10.1603/0022-0493-98.4.1259. [DOI] [PubMed] [Google Scholar]
- 9.Annan K, Dickson R, Mensah A, Fleischer TC. Acaricidal Effect of Plumbago zeylanica L. against Amblyomma variegatum. Pharmacogn J. 2009;1:190–4. [Google Scholar]
- 10.Nthambeleni R. MSc. Thesis. Pietermaritzburg, SA: University of KwaZulu-Natal; 2008. Isolation and characterisation of cytotoxic compounds from Anthospermum hispidulum and Eriocephalus tenuifolius. [Google Scholar]
- 11.Ngadjui BT, Tsopmo A, Ayafor JF, Connolly JD, Tamboue H. Hosloppin, a New Pyrone-substituted flavonoid from Hoslundia opposita. J Nat Prod. 1995;58:109–11. [Google Scholar]
- 12.Dolan MC, Dietrich G, Panella NA, Montenieri JA, Karchesy JJ. Biocidal Activity of three wood essential oils against Ixodes scapularis (Acari: Ixodidae), Xenopsylla cheopis (Siphonaptera: Pulicidae), and Aedes aegypti (Diptera: Culicidae) J Econ Entomol. 2007;100:622–5. doi: 10.1603/0022-0493(2007)100[622:baotwe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Choi BR, Lee SG, Kim SI, Ahn YJ. Toxicity of plant essential oils to acaricide-susceptible and -resistant Tetranychus urticae (Acari: Tetranychidae) and Neoseiulus californicus (Acari: Phytoseiidae) J Econ Entomol. 2010;103:1293–8. doi: 10.1603/ec09222. [DOI] [PubMed] [Google Scholar]
- 14.Cseke LJ, Kirakosyan A, Kaufman SL, Duke JA, Brielman HL. 2nd ed. Boca Raton: Taylor and Francis Group; 2006. Natural Products from Plants. [Google Scholar]
- 15.Tonzibo ZF, Ahibo CA, Chalachat JC, N’guessan YT. Chemical composition of essential oils of Hoslundia opposita Vahl. from Ivory Coast. Flavour Fragr J. 2006;21:789–91. [Google Scholar]
- 16.Perrucci S, Macchioni G, Cioni PL, Flamini G, Morelli I. Structure/activity relationship of some natural monoterpenes as acaricides against Psoroptes cuniculi. J Nat Prod. 1995;58:1261–4. doi: 10.1021/np50122a018. [DOI] [PubMed] [Google Scholar]
- 17.Cantrell CL, Klun JA, Pridgeon J, Becnel J, Duke S. Structure/Activity relationship studies on the arthropod repellent callicarpenal. USDA-ARS. 2009. [Last accessed on 2010 Oct 13]. Available from: http://www.ars.usda.gov .


