Abstract
Background:
Doxorubicin (DOX) is the most active cytotoxic agents having efficacy in malignancies either alone or combined with other cytocidal agents. The clinical usefulness of the anthracycline drug has been precluded by cardiac toxicity. Many therapeutic interventions have been attempted to improve the therapeutic benefits of the drug. This study is based on the possible protective effects of combination of p-coumaric acid (PC) and naringenin (NR) on DOX induced cardiac toxicity in male Swiss albino rats.
Methods:
Total nine groups of Swiss albino rats were used, Group I (vehicle control) receive saline solution daily and Group II (disease control) receive saline solution daily up to 29th day and at 30th day a single dose of DOX (15 mg/kg i.p.) is given. PC alone (100 mg/kg/day p.o.) and (200 mg/kg/day p.o.) also NR alone (15 mg/kg/day) orally administer for 30 days. Similarly a standard drug Vit. E (100 mg/kg/day) administers alone for 30 days. Group PC/DOX and PC and NR/DOX receive PC (200 mg/kg/day) and combine PC (200 mg/kg/day).
Results:
Doxorubicin induced marked biochemical alterations characteristic of cardiac toxicity including increase in MDA level and decrease SOD, CAT & GSH level but prior administration of combination of PC & NR ahead of doxorubicin challenge ameliorated all these biochemical markers.
Conclusion:
The study proves the beneficial effects of combination of PC and NR in protecting animal against DOX induced cardiotoxicity.
Keywords: Antioxidents, cardiotoxicity, doxorubicine, naringenin, p-coumaric acid
INTRODUCTION
Doxorubicin (DOX) is one of the most active anthracycline antibiotics that has been used for long time in the therapy of array of human malignancies such as, hematopoietic, lymphoblastic,[1] and solid tumors[2] either alone or combination with other cytocidal agents.[3] The clinical usefulness of DOX, however, has been hampered by its detrimental cardiac toxicity,[4] so the cardiac protection during the use of DOX in the treatment of cancer can be achieved by limiting its cumulative dose.
Several in vivo–in vitro studies have demonstrate that reactive oxygen metabolites including free radical species, superoxide anions (O-●), hydrogen peroxide (H2O2), and hydroxyl radicals (●OH) are the important mediators of tissue injury.[5] The involvement of oxygen radical injury of membrane lipid has been reported as the main causative factor for DOX-induced cardiotoxicity.[6–9] DOX form semiquinone free radicals by reducing one electron,[6–9] this free radical donates its electrons and forms superoxide anions.[10] The dismutation of superoxide yields hydrogen peroxide (H2O2).[11] Under biological conditions semiquinone reductively cleaves hydrogen peroxide to produce hydroxyl radicals which is the most reactive and destructive species. This leads to lipid peroxidation causing irreversible damage of the membrane structure and function.[12]
Metabolic machineries of heart tissue are very active and antioxidant resources are very low in this organ compared with other organs in the body, made heart quite vulnerable to free radical damage by DOX[13] which ultimately leads to cardiotoxicity.
In this study, we estimate the effect of combination of two drugs, i.e. p-coumaric acid (PC) and naringenin (NR) against cardiotoxicity caused due to the DOX use in the treatment of cancer.
PC is the phenolic acid widely distributed in plants and forms the part of human diet.[14] Sources of phenolic acid are peanut, tea, coffee, wine, chocolate, beer, etc.[15] The antioxidative mechanism of phenolic acid includes binding of metal ions, scavenging of reactive oxygen species (ROS), reactive nitrogen species (RNS), or other precursors and upregulation of endogenous antioxidant enzyme or the repair of oxidative damage to biomolecules.[16] Recently interests in food phenolics have increased due to their role of antioxidants and scavengers of free radicals and their implication in the prevention of many pathological diseases such as cardiovascular[16,17] and certain types of cancer.[18]
Also the other drug used in the study, i.e. NR is flavonoids from the class of benzogamma pyron having high pharmacological potency. Sources of NR are grape fruit, orange, and lemon. Due to their free radical scavenging and ion chelating properties[13] flavonoids can be consider as a possible potential protector against DOX-induced cardiotoxicity.
In this study, the cardioprotective effect of combination of both drugs in male Swiss albino rats challenge with a single cumulative dose of DOX. Also with this, the effect of this combination on antioxidant enzymes such as SOD (superoxide dismutase), CAT (catalase), GSH (glutathione), MDA (malondialdehyde) is also determined.
MATERIALS AND METHODS
Drugs
Doxorubicin (Dabur research laboratory, Ghaziabad), p-coumaric acid (PC Chem, Mumbai), Naringenin (Sigma chemicals), and Vitamin E (E Merk, Mumbai) were obtained.
Chemicals, reagent, solvents
Disodium hydrogen phosphate (Na2HPO3), 5, 5-dithiobis-(2-nitrobenzoic acid) (DTNB), EDTA, pyrogallol, tri-chloroacetic acid, Tris-HCl buffer, and thio-barbituric acid were used.
Equipments
Centrifugation machine (REMI, Mumbai), Tissue homogenizer, and UV spectrophotometer (Double Beam) (Teknik, an ISO 9001–2000 company) were also used.
Animal selection
Fifty-four Swiss albino rats, weighing 180–200 g, were obtained from our animal breeding facility of Sudhakarrao Naik Institute of Pharmacy, Pusad. Rats were maintained in our facility under standard laboratory conditions (the photoperiod was 12 h artificial light and 12 h darkness, at 20–23°C with humidity 65–67%) and all the pharmacological experimental protocols were approved by the Institutional Animal Ethics Committee (Reg no: SNIOP/264/03c/ CPCSEA, Feb 2009).
Experimental design
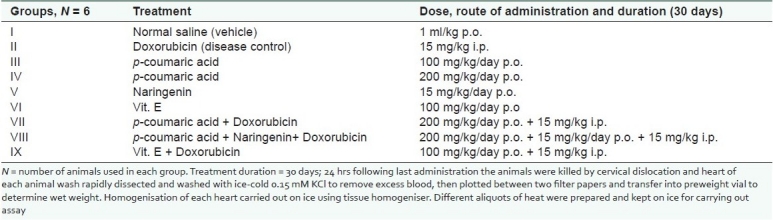
Animals were divided into nine groups with six animals in each group. It is a 30-day study in which Group I (vehicle control) receive 1 ml saline solution daily, Group II receive saline water up to 29th day and at 30th day a single dose of DOX 15 mg/kg body weight (i.p.), Groups III and IV receive PC daily 100 mg/kg/day p.o. and 200 mg/kg/day p.o., respectively, for 30 days. Group V receive NR15 mg/kg/day p.o. for 30 days. Group VI receive a reference drug, i.e. Vitamin E 100 mg/kg/day p.o., Group VII receive PC daily 200 mg/kg/day p.o. up to 29th day and at 30th day a single dose of DOX 15 mg/kg/day i.p. was given. To Group VIII, NR 15 mg/kg/day and PC 200 mg/kg/day p.o. up to 29th day were given and at 30th day a single dose of DOX was given. To Group IX Vitamin E 100 mg/kg/day was given up to 29th day followed by one single dose of DOX 15 mg/kg/day i.p. at 30th day.
Treatment schedule
The treatment schedule is described in Table 1.
Table 1.
Treatment schedule-number of groups with their treatment schedule is presented
Estimation of superoxide dismutase
Twenty milligrams of heart tissue homogenised in potassium phosphate buffer (50 mM/l, pH 7.4) at 10,000 rpm at 4° C in cooling centrifuged for 20 min of supernatant use to measure the activity of SOD. SOD activity was determined by assessing inhibition of pyrogallol auto-oxidation, pyrogallol (24 mM) was prepared in 10 mM HCl and kept at 4°C before use. Aliquots of supernatant were added to Tris–HCl buffer (pH 8.5) containing 25 μl pyrogallol and then mixed thoroughly and changes in the absorbance were recorded at 1 min interval for 3 min.[19–21]
Estimation of catalase
A heart tissue homogenate was prepared in potassium phosphate buffer (50 mM/l, pH 7.4) with the ratio of 1:10 w/v. The homogenate was centrifuged at 10,000 rpm at 4°C in the cooling centrifuged for 20 min. CAT activity in the tissue homogenate was assayed according to the method of Clairborne (1985). In this method, the decomposition of H2O2 (19 mM/l) due to the CAT activity was assayed by the decrease in absorbance of H2O2 at 240 nm.[22,23]
Estimation of glutathione
GSH accounts for the majority of soluble reduced sulfhydryl in the cell.[23,24] GSH in cardiac tissue was determined by measuring the total soluble sulfhydryl content. GSH was determined by utilising 5,5’-dithio-bis(2-nitrobenzoic acid) (Ellman reagent). The homogenised heart tissue in 0.02 M EDTA was mixed with water and 50% tri-chloro acetic acid, the tube was shaken for 10–15 min, centrifuged at 3000 rpm for 15 min. In the supernatant, sulfhydryl concentration was determined photometrically following the method of Ellman.[23,24]
Estimation of lipid peroxidation malondialdehyde (TBARS)
The measurement of heart lipid peroxide by a calorimetric reaction with thio-barbituric acid was done as described by Okhawa et al. In this, 10% tissue homogenate, 30% tri-chloroacetic acid, and 0.8% thio-barbituric acid were added, this tube was covered with aluminium foil and kept in a shaking water bath for 30 min at 80°C, then kept that tube in ice cold water at 30°C and centrifuged at 3000 rpm for 15 min.[25,26]
Absorbance of the supernatant was noted at room temperature against a appropriate blank (blank consist of 1 ml distilled water, 0.5 ml 30°C tri-chloroacetic acid and 0.5 ml of 0.8% thio-barbituric acid).
Statistical analysis
All the experimental results are given as the mean ± SEM. Comparison between experimental and control groups were performed by ANOVA, followed by Dunnett's test for post hoc comparison, when appropriate. A value of P < 0.05 was considered to be significant, while P > 0.05 was non-significant.
RESULTS
Effect of p-coumaric acid and naringenin on doxorubicin-induced tissue superoxide dismutase activity
The SOD activity showed a significant (P < 0.01) decrease in Group II when compared with Group I. Groups IV and VIII showed a less significant (P < 0.05) increase where as Groups VI and IX showed a significant (P < 0.01) increase in SOD activity when compared with Group I. However, Groups III, IV and VII showed a less significant (P < 0.05) increase and significant (P < 0.01) increase in SOD activity of Groups VI, VIII and IX when compared with Group II.
Effect of p-coumaric acid and naringenin on doxorubicin-induced tissue catalase activity
Group II showed a significant decrease in CAT activity when compared with Group I (P < 0.01). Groups III, VII, VIII and IX showed a less significant (P < 0.05) increase in CAT activity where as Groups IV and VI showed a significant (P < 0.01) increase in CAT activity when compared with Group I. Groups III and VIII showed a less significant (P < 0.05). Groups IV, VI, VII and IX however showed the significant (P < 0.01) activity when compared with Group II.
Effect of p-coumaric acid and naringenin on doxorubicin-induced tissue glutathione activity
The blood GSH levels of disease control, i.e. DOX-treated group (Group II) showed a significant (P < 0.01) decrease when compared with normal control (Group I). Groups IV and VI exhibit a less significant (P < 0.05) increase in the GSH level when compared to Group I. While in Groups IV, VIII and IX GSH levels were less significant (P < 0.05) and in Group VI it was restored significantly when compared with Group II.
Effect of p-coumaric acid and naringenin on doxorubicin-induced tissue thiobarbituric acid reactive substance levels
The TBARS concentration of Group II showed a significant (P < 0.01) increase as compared to Group I. VI shows a significant (P < 0.05) decrease in TBARS concentration compare to Groups I and II. Groups IV, VIII and IX showed a less significant (P < 0.05) decrease in TBARS concentration while Group VI exhibited a significant (P < 0.01) decease when compared with Group II.
DISCUSSION
DOX is a broad spectrum antibiotics used as a chemotherapeutic drug for the treatment of different forms of human neoplastic disease.[27] However, the clinical use of anticancer drug is greatly limited by its dose-dependent cardiotoxicity.[28] Free radicals generation and lipid peroxidation have been suggested to be responsible for DOX-induced cardiac toxicity.[29,30] These oxygen derived radicals causes severe damage to plasma membrane and interferes with cytoskeleton assembly.[31]
Free radicals ROS and RNS are generated by our body by various endogenous systems, exposure to different physiochemical conditions, or pathological states. A balance between free radicals and antioxidants is necessary for proper physiological function. If the free radicals overwhelm the body's ability to regulate them, a condition known as oxidative stress ensues. Free radicals thus adversely alter lipids, proteins, and DNA and trigger a number of human diseases. Hence, application of an external source of antioxidants can assist in coping this oxidative stress.[32]
Among all the therapeutic modalities adopted to attenuate DOX cardiac myopathy provide the most promising results from combining the drug with a myriad of antioxidants in an attempt to abate oxidative damage in heart tissue and hence to abrogate the cardiac injury.
The present work is designed to investigate the potential cardioprotective effect of the combination of PC and NR against DOX-induced cardiotoxicity.
DOX-induced cardiotoxicity includes one electron reduction of DOX lead to the formation of corresponding semi-quinone free radicals in cardiac monocytes by myocardial CYP-450 and flavin monoxigenase. In the presence of oxygen, these free radicals rapidly donate their electron to oxygen or react with molecular oxygen and initiate cascade of reaction producing ROS. Free radical generation and lipid peroxidation have been suggested to be responsible for DOX-induced cardiac toxicity.[29,30] Moreover, heart tissue is especially susceptible to the free radical injury because of the low level of free radical detoxifying enzymes such as SOD, CAT, and GSH and less oxygen reserve. Further, DOX also has a high affinity for the phospholipids component of mitochondrial membrane in cardiac myocytes, leading to accumulation of DOX in heart tissue. The cellular GSH level is closely related to lipid peroxidation and disturbances of Ca++ influx induced by toxic agents. DOX administration induced oxidative stress in cardiac tissue as manifested by the alteration observed in the cardiac antioxidant defence system both enzymatic and nonenzymatic. Anthracycline drug reduces significantly the cardiac lipid peroxidation as manifested by increased MDA level.
The modulation of antioxidant enzyme activities followed by DOX administration has been discussed in many studies.[6–9] The association between elevated cardiac content of MDA and lowered cardiac content of GSH found in the study strongly proves the oxidative damage caused by DOX. This observation has been supported by the findings of Lazzarino et al. and Gustafson et al. (1986) who reported that the cardiac content of MDA was increased and GSH content was decreased by administration of DOX to rodents.
It is well documented that long-term treatment by DOX causes irreversible, severe, and potentially life-threatening cardiac damage.[33] The mechanism involves in such toxicity have been documented by many investigators. The involvement of oxygen free radicals oxidative stress have been strongly accepted as crucial factors in the pathogenesis of DOX-induced cardiac damage.
p-Coumaric acid is the phenolic compound widely distributed in the plant and forms a part of human diet.[34] The mechanism of PC includes binding of metal ions, scavenging of ROS, RNS, or their precursors, up-regulation of endogenous antioxidant enzymes, or the repair of oxidative damage to biomolecules.[16] Abdel-Wahab et al. explains that PC shows potential cardioprotective effects against DOX-induced oxidative stress in rat's heart.
Naringenin includes in the class of flavonoids that has a multitude of pharmacological effects. Due to their free radical scavenging activity and iron chelation properties, this drug considers as possible potential protector against DOX-induced cardiac toxicity. NR is the aglycon of the natural glycoside and presents abundantly in grapefruits. Other action includes antithrombotic,[35] anti-inflammatory,[36] antiestrogenic[37] as well as chemopreventive action.[38] Hossam et al. (2005) investigated that NR use as cardioprotective against DOX induced cardiotoxicity.
The combined effect of PC and NR is may be because of their synergistic effects. This combination may act as a hydrogen-donating radicals scavenger by scavenging lipid alkoxyl and peroxyl radical and protect myocardium from DOX-induced injury.
With reference to Table 1, prior administration of both PC (200 mg/kg) and NR (15 mg/kg) in combination for 30 days and followed by a single dose of DOX (15 mg/kg) at 30th day was given and then cardioprotective action of combination determined by measuring biochemical parameters such as superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), and malondialdehyde (MDA) as revealed in Figure 1.
Figure 1.
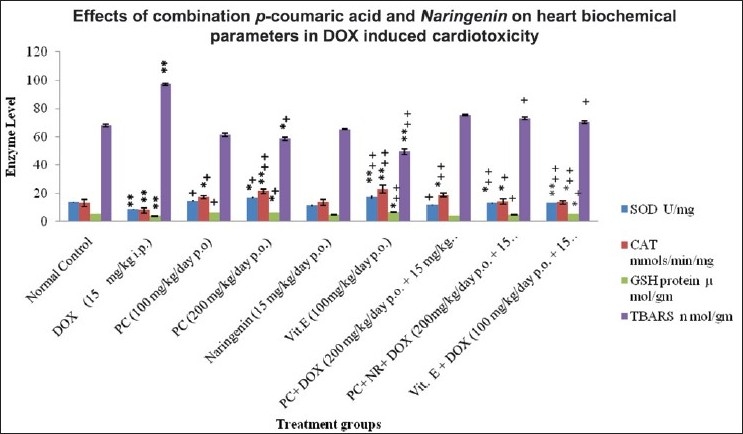
The effects of combination of drug, i.e. p-coumaric acid and naringenin on heart SOD, CAT, GSH, thiobarbituric acid reactive substance (TBARS), in normal and doxorubicin (DOX)-induced myocardial infarction in Swiss albino rats. DOX = Doxorubicin, PC = p-coumaric acid, Vit = Vitamin, NR = Naringenin. *P < 0.05 is less significant as compared to Group I; **P < 0.01 is signifi cant as compared to Group I; +P < 0.05 is less signifi cant as compared to Group II; ++P < 0.01 signifi cant as compared to Group II. Comparison between experimental and control groups were performed by ANOVA followed by Dunnett's test for post hoc comparison, when appropriate.
Pre-treatment of animal with PC and NR modulates oxidative damage induced by DOX administration.
CONCLUSION
In this study, the Swiss albino rats pretreated with combination of drugs, i.e. PC and NR ahead of a single dose of DOX and then determined the effect of this combination of drugs on biochemical parameters such as SOD, CAT, GHS, and MDA.
A result shows that prior administration of drugs leads to ameliorate all biochemical parameters. The study proves the beneficial effects of combination of natural drugs namely PC and NR in protecting the animal against DOX-induced cardiac oxidative damage.
The protecting effect of PC and NR is due to free radical scavenging and iron-chelating properties, hydrogen-donating radicals, scavenger by the scavenging lipid alkoxyl and peroxyl radical. On the basis of our findings, it may be worthy to suggest the concomitant administration of combined dose of PC and NR prior to the DOX use in cancer chemotherapy.
ACKNOWLEDGEMENTS
The authors deeply acknowledge to the Principal and management of the institute for providing laboratory facilities for carrying out this study successfully.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Hitchcock-Bryan S, Gelber RD, Cassady JR, Sallan SE. The impact of induction anthracycline on long-term failure-free survival in childhood acute lymphoblastic leukemia. Med Pediatr Oncol. 1986;14:211–5. doi: 10.1002/mpo.2950140405. [DOI] [PubMed] [Google Scholar]
- 2.Bonadonna G, Zambetti M, Valagussa P. Sequential or alternating doxorubcin and CMF regimens in breast cancer with more than three positive nodes. Ten-year results. JAMA. 1996;273:542–7. [PubMed] [Google Scholar]
- 3.Quiles JL, Huertas JR, Battino M, Mataix J, Ramerez-Tortosa MC. Antioxidant nutrients and adriamycin toxicity. Toxicology. 2002;180:79–95. doi: 10.1016/s0300-483x(02)00383-9. [DOI] [PubMed] [Google Scholar]
- 4.Yagmurca M, Fadillioglu E, Erdogan H, Ucar M, Sogut S, Irmak MK. Erdosteine prevents doxorubicin induced cardiotoxicity in rats. Pharmacol Res. 2003;48:377–82. doi: 10.1016/s1043-6618(03)00185-3. [DOI] [PubMed] [Google Scholar]
- 5.Fantone JC, Ward PA. Polymorphonuclear leukocyte-mediated cell and tissue injury: Oxygen metabolites and their relations to human disease. Hum Pathol. 1985;16:973–8. doi: 10.1016/s0046-8177(85)80273-2. [DOI] [PubMed] [Google Scholar]
- 6.Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: The role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977;197:165–7. doi: 10.1126/science.877547. [DOI] [PubMed] [Google Scholar]
- 7.Mimnaugh EG, Trush MA, Bhatnagar M, Gram TE. Enhancement of reactive oxygen-dependent mitochondrial membrane lipid peroxidation by the anticancer drug Adriamycin. Biochem Pharmacol. 1985;34:847–56. doi: 10.1016/0006-2952(85)90766-x. [DOI] [PubMed] [Google Scholar]
- 8.Sarvazyan NA, Askari A, Huang WH. Effects of doxorubicin on cardiomyocytes with reduced level of superoxide dismutase. Life Sci. 1995;57:1003–10. doi: 10.1016/0024-3205(95)02036-i. [DOI] [PubMed] [Google Scholar]
- 9.Singal PK, Siveski-Iliskovic N, Hill M, Thomas TP, Li T. Combination therapy with probucol prevents adriamycin-induced cardiomyopathy. J Mol Cell Cardiol. 1995;27:1055–63. doi: 10.1016/0022-2828(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 10.Bachur NR, Gordon SL, Gee MV, Kon H. NADPH-cytochrome P450 reductase activation of quinone anticancer agents to free radicals. Proc Natl Acad Sci U S A. 1997;76:945–57. doi: 10.1073/pnas.76.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura Y, Othtaki S, Makino R, Tanaka T, Ishimura Y. Superoxide anion in the initial product in the hydrogen peroxide formation catalyzed by NADPH oxidase in porcine thyroid plasma membrane. J Biol Chem. 1989;264:4759–61. [PubMed] [Google Scholar]
- 12.Odom AL, Hatwing CA, Stanley JS, Benson AM. Biochemical determinants of adriamycin toxicity in mouse liver, heart and intestine. Biochem Pharmacol. 1992;43:831–6. doi: 10.1016/0006-2952(92)90250-m. [DOI] [PubMed] [Google Scholar]
- 13.Quiles JL, Huertas JR, Battino M, Mataix J, Ramerez-Tortosa MC. Antioxidant nutrients and adriamycin toxicity. Toxicology. 2002;180:79–95. doi: 10.1016/s0300-483x(02)00383-9. [DOI] [PubMed] [Google Scholar]
- 14.Scalbert A, Williamson G. Dietary intake and bioavilability of polyphenols. J Nutr. 2000;130(Suppl 8):S2073–85. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 15.King A, Young G. Characteristics and occurrence of phenolic phytochemicals. J Am Diet Assoc. 1999;99:213–8. doi: 10.1016/S0002-8223(99)00051-6. [DOI] [PubMed] [Google Scholar]
- 16.Ursini F, Tubaro F, Rong J, Sevanian A. Optimization of nutrition: Polyphenols and vascular protection. Nutr Rev. 1999;57:241–9. doi: 10.1111/j.1753-4887.1999.tb06951.x. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 18.Hudson EA, Dinh PA, Kokubun T, Simmonds MS, Gescher A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2000;9:1163–70. [PubMed] [Google Scholar]
- 19.Minami M, Yoshikawa H. A simplified assay method of superoxide dismutase activity for clinical use. Clin Chem Acta. 1979;92:237. doi: 10.1016/0009-8981(79)90211-0. [DOI] [PubMed] [Google Scholar]
- 20.Marklund SL. Superoxide dismutase isoenzymes in tissues and plasma from New Zealand black mice. Mutat Res. 1985;148:129–34. doi: 10.1016/0027-5107(85)90216-7. [DOI] [PubMed] [Google Scholar]
- 21.Onasanwo SA, Singh N, Saba AB, Oyagbemi AA, Oridupa OA, Palit G. Anti-ulcerogenic and in vitro antioxidant activities of Lagenaria breviflora (LB) whole fruit ethanolic extract in laboratory animals. Pharmacogn Res. 2011;3:2–8. doi: 10.4103/0974-8490.79108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clairborne A. Catalase activity. In: Greenwald RA, editor. Handbook of Methods for Oxygen Radical Research. Boca Raton, FL, USA: CRC Press; 1986. pp. 383–4. [Google Scholar]
- 23.Prakash JD, Arul Kumar S, Sabesan M. Effect of nanohypericum (Hypericum perforatum gold nanoparticles) treatment on restraint stress induced behavioral and biochemical alteration in male albino mice. Pharmacogn Res. 2010;2:330–4. doi: 10.4103/0974-8490.75450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosower NS, Kosower EM. The glutathione status of cells. Int Rev Cytol. 1978;54:109–60. doi: 10.1016/s0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- 25.Nithiya P, Mohan K. Antioxidative effect of trichosanthes tricuspidata root extract on sildenafil induced migraine in albino mice. Pharmacogn Res. 2009;1:402–5. [Google Scholar]
- 26.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;74:214–26. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 27.Nagi MN, Mansour MA. Protective effects of thymoquinone against doxorubicin induced cardiotoxicity in rat a possible mechanism of protection. Pharmacol Res. 2000;41:283–9. doi: 10.1006/phrs.1999.0585. [DOI] [PubMed] [Google Scholar]
- 28.Al-majed A, Gado A, AL-shbanah O, Mansour MA. Aplha lipoic acid ameliorates myocardial toxicity induced by doxorubicin. Pharmacol Res. 2002;46:499–503. doi: 10.1016/s1043661802002311. [DOI] [PubMed] [Google Scholar]
- 29.Chularojmontri L, Wattanapitayakul SK, Herunsalee A, Charuchongkolwongse S, Niumsakul S, Srichairat S. Antioxidative and cardioprotective effects of Phyllanthus urinaria L on doxorubicin-induced cardiotoxicity. Biol Pharm Bull. 2005;28:1165–71. doi: 10.1248/bpb.28.1165. [DOI] [PubMed] [Google Scholar]
- 30.Xu MF, Tang PL, Qian ZM, Ashraf M. Effects by doxorubicin on the myocardium is mediated by oxygen free radicals. Life Sci. 2001;68:889–901. doi: 10.1016/s0024-3205(00)00990-5. [DOI] [PubMed] [Google Scholar]
- 31.Uchiyama M, Mihara M. Determination of malonaldialdehyde precursor in tissue by thiobarbituric acid test. Anal Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 32.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2011;118:26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hrdina R, Gersl V, Klimtova I, Simunek T, Mach J, Adamcova M. Anthracycline-induced cardiotoxicity. Acta Medica. 2000;43:75–82. [PubMed] [Google Scholar]
- 34.Morton LW, Caccetta RA, Ruddey IB, Croft KB. Chemistry and biological effects of dietary phenolic compounds: Relevance to cardiovascular disease. Clin Exp Pharm Physiol. 2000;27:152–9. doi: 10.1046/j.1440-1681.2000.03214.x. [DOI] [PubMed] [Google Scholar]
- 35.Corvazier E, Maclouf J. Interference of some flavonoids and non-steroidal anti-inflammatory drugs with oxidative metabolism of arachidonic acid by human platelets and neutrophils. Biochim Biophys Acta. 1985;835:315–21. doi: 10.1016/0005-2760(85)90287-5. [DOI] [PubMed] [Google Scholar]
- 36.Middleton E, Jr, Kandaswami C. Effects of flavonoids on immune and inflammatory cell functions. Biochem Pharmacol. 1992;43:1167–79. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- 37.Kao YC, Zhou C, Sherman M, Laughton CA, Chen S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environ Health Perspect. 1998;106:85–92. doi: 10.1289/ehp.9810685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guthrie N, Carroll KK. Inhibition of mammary cancer by citrus flavonoids. Adv Exp Med Biol. 1998;439:227–36. doi: 10.1007/978-1-4615-5335-9_16. [DOI] [PubMed] [Google Scholar]


