Abstract
IgA nephropathy is being recognized as the commonest glomerular disease worldwide. The prevalence and clinical picture varies from region to region. A retrospective analysis of 400 native renal biopsies performed over a period of 3 years at our center was done to know the prevalence and clinicopathological profile of patients with IgA nephropathy. All the biopsies were processed for both light microscopy and immunofluorescence studies. Patients with predominant IgA deposits were labeled as IgA nephropathy and further classified histopathologically into five subclasses according to the Haas classification. We noted a prevalence of 7.8% (31 cases) of IgA nephropathy. Nephrotic syndrome and chronic renal failure were the most common mode of presentation. Majority of cases fell into subclass III (focal segmental glomerular sclerosis) with 35.5% followed by subclasses IV (diffuse proliferative glomerular sclerosis) and V (global sclerosis) with 25.8% and 22.6% prevalence, respectively. As about 50% cases presented with varying degree of renal insufficiency, many ending with ESRD, IgA nephropathy can be considered as a serious problem in India.
Keywords: IgA nephropathy, end-stage kidney disease, renal biopsy
Introduction
IgA nephropathy is the most commonest primary glomerulonephritis worldwide. It is defined as glomerular disease with IgA-dominant or co-dominant mesanglial immunoglobulin deposits, excluding lupus nephritis.[1] First described by Berger et al. in 1968, IgA still remains elusive because of its highly variable mode of presentation and disease progression. In the past, IgA nephropathy was thought to be a benign disease with a 10-year renal survival rate of greater than 80%. But the emerging data show a different scenario; most patients develop a progressive decline in renal function and about 40% end with ESRD.[2] The exact pathogenesis of the disease remains unknown. A widely stated hypothesis is that mucosal antigenic exposure in a genetically susceptible individual results in the generation of nephritogenic IgA antibodies that form complexes in the circulation and get deposited in glomeruli leading to glomerular injury.[3,4] Environmental and genetic components are considered to contribute significantly in the development and progression of the disease process.[5] Thus the prevalence, clinical course, and outcomes are highly variable when compared between different regions of the world. A higher prevalence has been noted in Asia including India.[6] There are few studies on the prevalence and clinicopathological characteristics of IgA nephropathy especially from the southern part of India.
This retrospective study was undertaken to review the prevalence and clinicopathological profile of IgA nephropathy patients admitted at our tertiary care referral center.
Materials and Methods
All percutaneous renal biopsies submitted to the center from May 2007 to March 2010 were reviewed retrospectively. A total of 400 renal biopsies were included in this study.
The baseline demographics, clinical data along with routine urine examination, and biochemical parameters at the time of presentation/biopsy were also analyzed. glomerular filtration rate (GFR) was estimated in all the patients by the Modification of Diet in Renal Disease (MDRD) formula.
All renal biopsies were examined by light microscopy and immunofluorescence. Tissue was divided for both the studies. For light microscopy, the tissue submitted was fixed in buffered formalin and processed into paraffin blocks; sections were stained with hematoxylin and eosin (H and E), periodic acid Schiff (PAS), and Jones’ silver methanamine. Immunofluorescence was performed on frozen sections labeled with direct fluorescein isocyanate (FITC)-conjugated antibodies against IgG, IgA, IgM, C3, C1q, and fibrinogen.
Biopsies with predominant IgA deposits fulfilling following criteria were labeled as IgA nephropathy: the intensity of IgA staining more than the trace; IgA staining in mesangium; and intensity of other Ig M/G, if present, not greater than IgA (except IgM in sclerotic areas). Lupus was looked for and excluded if the intensity of C1q was more than the trace.
The biopsies were histologically subclassified into five subclasses as per Haas.[7]
Patients suffering with systemic lupus erythomatosus, Henoch–Schönlein purpura, or liver disease were excluded from the study. Based on the clinical mode of presentation, patients who fulfilled the criteria of IgA nephropathy were broadly classified into three clinical groups: (1) nephrotic syndrome, (2) non-nephrotic proteinuria, and (3) chronic renal failure.
Normal renal function was defined as GFR ≥ 90 ml/min/1.73 m2, estimated using the MDRD formula. The definition of hematuria was ≥ 5 RBCs per high-power field in the urinary sediment. Nephrotic syndrome was defined as proteinuria > 3.5 g/day with hypoalbuminemia, edema, and hyperlipidemia. Nephritic syndrome was defined as hematuria (usually with dysmorphic RBCs/ RBCs casts) with proteinuria < 3.0 g/day, hypertension, and elevated serum creatinine. Chronic renal failure was defined as severe irreversible kidney damage and serum creatinine levels persistently above 1.5 mg/dl.[8]
Results
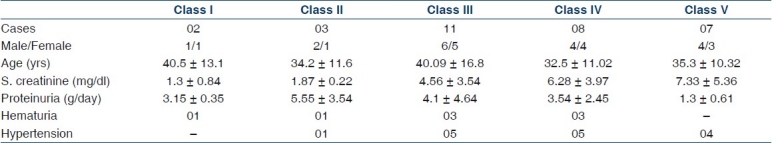
Out of 400 biopsies, 31 cases fulfilled the criteria of IgA nephropathy. The mean age was 36.6±13.02 years with slight male preponderance (17 males to 14 females). Most of the patients presented with varying degree of renal impairment. The commonest mode of presentation was nephrotic syndrome followed by renal failure and nephritic syndrome. Two patients had malignant hypertension, and one of them presented with encephalopathy. Table 1 shows the demographics and laboratory data of the IgA patients.
Table 1.
The demographics and laboratory data in IgA nephropathy patients
Histopathologically majority of cases (48.4%) fell into subclasses IV and V. The prevalence of subclass III was also high with 35.5%, whereas that of subclass I and II lesions was low with 16.1% reflecting a relatively high threshold for kidney biopsy in our referral center. The histological subclassification is shown in Figure 1.
Figure 1.
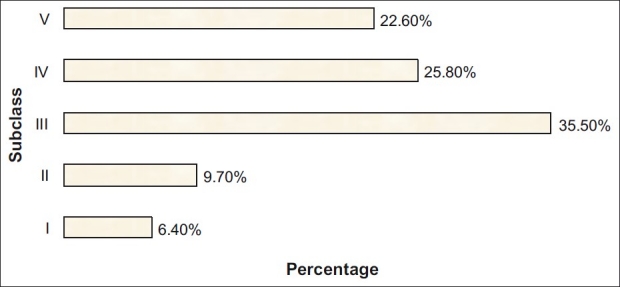
The distribution of subclasses of IgA nephropathy
On immunofluorescence, all the cases demonstrated diffuse, granular mesanglial deposits of IgA with the intensity ranging from 2+ to 4+ [Figures 2 and 3]. Subclass I lesions had minimal changes without glomerular hypercellularity, whereas subclass II lesions showed signs of focal and segmental sclerosis of glomeruli with a mild increase in mesanglial cellularity. In subclass III, glomeruli showed mesanglal expansion with hypercellularity. There were areas of focal and segmental sclerosis with a variable degree of interstitial fibrosis and infiltration. Specimens of subclass IV showed glomeruli predominantly surrounded by circumferential fibrosing crescents leading to near obliteration of glomeruli with patchy fibrosis of the interstitium. Areas of patchy atrophied tubules and blood vessels with medial hypertrophy were also observed. Subclass V showed sclerosed glomeruli with atrophied tubules and interstitial fibrosis.
Figure 2.
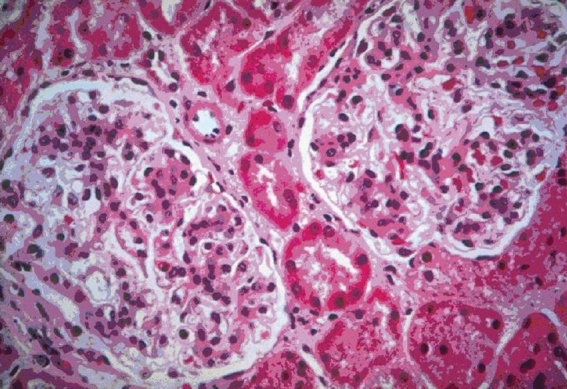
Light microscopy H and E sections show two patent glomeruli with a diffuse increase in the mesagial matrix (100×)
Figure 3.
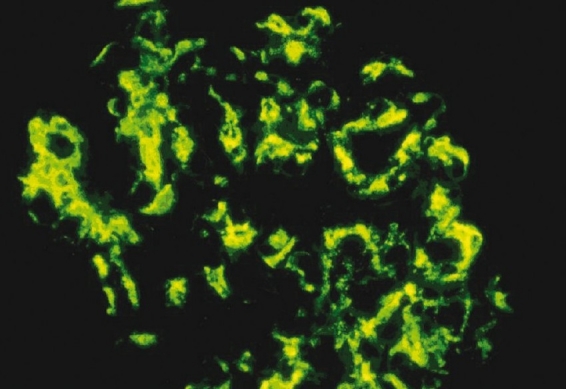
Immunofluorescence shows extensive deposits in the mesangium with deposits also seen focally in the subendothelial region and the capillary (400×)
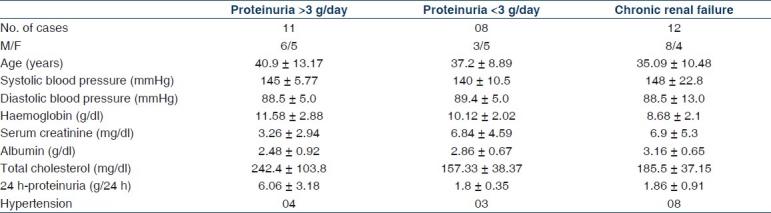
Clinical features of each histologic subclasses of IgA nephropathy are summarized in Table 2. The subclass with the largest fraction of cases is subclass III (35.5%), corresponding to focal proliferative glomerulonephritis. The mean GFR was 25.9±26.1 ml/min/1.73 m2. The highest mean level of serum creatinine was observed in subclass IV and V corresponding to the most active and chronic lesions, respectively. About half of the patients (48%) had hypertension and it was more prevalent in subclass III, IV, and V. All the patients had at least one episode of micro/macro hematuria with a variable amount of proteinuria. Proteinuria was high in subclass II, III, and I and 11 patients had proteinuria > 3 g/day with a mean of 6.0 g/day. Patients belonging to Haas subclass I and II had minimal changes with serum creatinine levels ranging from 0.7 to 2.1 mg/dl and proteinuria of 1.2–7.7 g/24 h.
Table 2.
Clinical data of IgA nephropathy patients in relation to the Haas subclassification
During the course of illness, patients with persistent high blood pressure were managed with a combination of two or three antihypertensives drugs (ACE inhibitor/calcium channel blockers/diuretics). Ten patients were on steroids and two were on immunosuppressive treatment (cyclophosphamide). Additionally, in severe cases two patients were advised immunosuppressive drugs but patients refused the treatment.
Follow-up
As our institute is a tertiary care center, patients come from far and interior regions of South India. Due to economic constraints and distance, some are lost to follow-up. Thus we had follow-up data for 23 patients for a period ranging from 3 to 15 months.
Three patients belonging to subclass I and II were followed for 6 months. During this period, all the three showed reduction in proteinuria to <1 g/day from the initial nephrotic range proteinuria. In addition, in two patients the hematuria persisted.
Of the patients from subclass III and IV, 11 cases were followed for a mean period of 12 months. Eight patients had an about 20% further decrease in the GFR with persistent proteinuria. One of the above-mentioned patients agreed and underwent rebiopsy and was found to have progressed from subclass III to subclass V with > 90% glomeruli sclerosed and severe degree of tubular atrophy and interstitial fibrosis.
All the patients who had reached the stage of ESRD (subclass V) were counseled about renal replacement therapy and among them, two patients had been identified as prospective recipients.
Discussion
This study demonstrates that IgA nephropathy is common with a prevalence of 7.8%, in accordance with previous reports from different parts of India.[9] IgA nephropathy has been reported in all age groups with peak incidence in the second/third decade of life with male preponderance. A similar pattern was observed in our study with a mean age of 36.6 years and a higher prevalence in males.
The typical patterns of clinical presentation described are hematuria, proteinuria, and hypertension. In our study, all our patients had at least one episode of hematuria (micro/macroscopic) with a variable amount of proteinuria. Nephrotic syndrome was the main mode of clinical presentation, followed by chronic renal failure. This is in accordance with most of the studies from Asia, especially from India, China, and Japan.[10–12] This high occurrence of renal failure in Asians has been attributed to undue genetic susceptibility of Asians to IgA nephropathy and its complications.[13,14]
The natural course of the disease is not completely understood and the outcome is generally unpredictable. But according to Mark Haas, IgA nephropathy can be grouped into three groups with different prognosis: an excellent prognosis group (subclasses I and II) corresponding to glomerular lesions without proliferative glomerulonephritis, an intermediate prognosis group (subclass III) corresponding to focal proliferative glomerulonephritis, and a poor prognosis group (subclasses IV and V) corresponding to diffuse proliferative glomerulonephritis and advanced chronic glomerulonephritis.[7] In our study, patients with subclass I and II lesions presented with nephrotic range proteinuria and 60% had nephrotic syndrome. Despite the fact that heavy proteinuria in itself is a negative prognostic indicator for renal survival, patients of both these subclasses did not have any adverse outcome. This is in accordance with earlier studies which have showed that subclass I and II lesions have an excellent outcome.
The largest fraction of cases in our study belonged to subclass III, corresponding to focal proliferative glomerulonephritis, followed by subclass IV. All these patients had impaired renal function and nephrotic syndrome range proteinuria; in addition, 45% of these patients had hypertension. The long-term renal survival among the patients with subclass III and IV lesions for whom follow-up data were available (60%) was poor. Analysis of clinical factors affecting the renal outcome in IgA nephropathy patients revealed that hypertension is a significant prognostic factor. Nearly half of our patients were hypertensive and histologically had progressed to a higher subclass of III, IV, or V.[15,16]
The most significant finding of our study was that about 38% of the patients presented with a severe degree of renal impairment and had progressed toward ESRD. This may be due to the fact that our center is a tertiary care referral center and patients may present late in the course of the disease process. The survival rate is low compared to western countries; patients are gradually lost to follow-up within 3 months, primarily due to economic reasons.[17]
In these patients, renal transplant is the only option but in developing country like India financial constraints are enormous. Still renal disorders are neglected due to the lack of awareness in patients because of the low socioeconomic and educational background. As a consequence, patients present late with advanced disease process. Disparities in the biopsy policies and the requirement of immunofluorescence facilities also compound the problem of diagnosis. So in developing countries like India, with limited resources and an estimated ESRD of 150–200 pmp,[18] a decisive plan of action should be charted and implemented.
Conclusion
As IgA is more common in the younger age group, with no specific treatment and many ending with ESRD, it can be considered as a serious medical problem. As kidney disease is emerging as a major health problem globally and ESRD is showing an alarming growth rate, efforts are needed for the early diagnosis and proper management of these patients. More studies are needed to explore the possible modes of disease progression and treatment especially in a developing country like India for favorable patient outcomes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.J Charles Jennette. IgA Nephropathy and Henoch Schonlein purpura. In: Fogo AB, Bruijn JA, Colvin RB, Jennette JC, editors. Fundamentals of Renal Pathology. Springer; 2006. pp. 61–8. [Google Scholar]
- 2.Donadio JV, Grande JP. IgA nephropathy. New Engl J Med. 2002;347:738–48. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 3.Clarkson AR, Woodroffe AJ, Bannister KM, Lomax-Smith JD, Aarons I. The syndrome of IgA nephropathy. Clin Nephrol. 1984;21:7–14. [PubMed] [Google Scholar]
- 4.Amore A, Emancipator SN, Roccatello D, Gianoglio B, Peruzzi L, Porcellini MG, et al. Functional consequences of the binding of gliadin to cultured rat mesangial cells: bridging immunoglobulin A to cells and modulation of eicosanoid synthesis and altered cytokine production. Am J Kidney Dis. 1994;23:290–301. doi: 10.1016/s0272-6386(12)80987-5. [DOI] [PubMed] [Google Scholar]
- 5.André C, Berthoux FC, André F, Gillon J, Genin C, Sabatier JC. Prevalence of IgA2 deposits in IgA nephropathies: A clue to their pathogenesis. N Eng J Med. 1980;303:1343–6. doi: 10.1056/NEJM198012043032306. [DOI] [PubMed] [Google Scholar]
- 6.Hsu SI, Ramirez SB, Winn MP, Bonventre JV, Owen WF. Evidence for genetic factors in the development and progression of IgA nephropathy. Kid International. 2000;57:1818–35. doi: 10.1046/j.1523-1755.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- 7.Haas M. Histologic subclassification of IgA Nephropathy.A clinicopathologic study of 244 cases. Am J Kid Dis. 1997;29:829–42. doi: 10.1016/s0272-6386(97)90456-x. [DOI] [PubMed] [Google Scholar]
- 8.Rivera F, López-Gómez JM, Pérez-García R. Clinicopathological correlations of renal pathology in Spain. Kidney International. 2004;66:898–904. doi: 10.1111/j.1523-1755.2004.00833.x. [DOI] [PubMed] [Google Scholar]
- 9.Vanikar AV, Kanodia KV, Patel RD, Trivedi HL. Primary immunoglobulin A (IgA) nephropathy in western India. Indian J Nephrol. 2005;15:227–231. [Google Scholar]
- 10.Li L. End stage renal disease in china. Kidney International. 1996;49:287–301. doi: 10.1038/ki.1996.41. [DOI] [PubMed] [Google Scholar]
- 11.Koyama A, Igarashi M, Kobayashi M. Natural history and risk factors for IgA ephropathy in Japan. Am J Kidney Dis. 1997;29:526–32. doi: 10.1016/s0272-6386(97)90333-4. [DOI] [PubMed] [Google Scholar]
- 12.George J, Ninan VT, Thomas PP, Jacob CK, Shastry JC. Primary IgA nephropathy in adults. J Assoc Physicians India. 1993;41:489–91. [PubMed] [Google Scholar]
- 13.De enitchina SS, Shinozaki M, Hirano T, Ando T, Hirakata H, Kiyohara Y, et al. Association of a T-cell receptor constant alpha chain gene polymorphisms with progression of IgA nephropathy in Japanese patients. Am J Kidney Dis. 1999;34:279–88. doi: 10.1016/s0272-6386(99)70356-2. [DOI] [PubMed] [Google Scholar]
- 14.Xia Y, Li Y, Du Y, Yang N, Li C, Leung JC, et al. Association of MEGSIN 2093-C-2180T haplotype at the 3’ centralateral region with disease severity and progression of IgA nephropathy. Nephrol Dial Transplant. 2006;21:1570–4. doi: 10.1093/ndt/gfk096. [DOI] [PubMed] [Google Scholar]
- 15.Chacko B, John GT, Neelakantan N, Balakrishnan N, Meshach G, Kirubakaran M, et al. Primary IgA nephropathy: A ten-year analysis on the renal outcomes and a model for estimating risk of progression. Indian J Nephrol. 2004;14:163–71. [Google Scholar]
- 16.D’Amico G, Minetti L, Ponticelli C, Fellin G, Ferrario F, Barbiano di Belgioioso G, et al. Prognostic indicators in idiopathic IgA mesanglial nephropathy. Q J Med. 1986;59:363. [PubMed] [Google Scholar]
- 17.Yoshimura M, Kida H, Abe T, Takeda S, Katagiri M, Hattori N. Significance of IgA deposition in the glomerular capillary walls in IgA nephropathy. Am J Kidney Dis. 1987;9:404–09. doi: 10.1016/s0272-6386(87)80143-9. [DOI] [PubMed] [Google Scholar]
- 18.Kher V. End-stage renal disease in developing countries. Kidney Int. 2002;62:350–62. doi: 10.1046/j.1523-1755.2002.00426.x. [DOI] [PubMed] [Google Scholar]


