Abstract
We evaluated important nontraditional cardiovascular risk factors, endothelial function and oxidative stress (OS) among stable peritoneal dialysis (PD) patients. Their association with carotid intimal medial thickness (CIMT) was also assessed. Thirty-eight adult patients (13 diabetics, 20 males) on PD for >6 months and 15 age and sex-matched controls were studied. Duration of dialysis (DOD), residual urine output (UO), weekly Kt/V urea, detailed biochemical and lipid profile were noted. OS was measured by serum concentration of antioxidants; vitamin C and ferric reducing ability of plasma (FRAP) and pro-oxidant; thiobarbituric acid-reactive substances (TBARS). High-resolution ultrasonography was used to determine CIMT and flow-mediated dilatation of brachial artery [endothelium-dependent dilatation (EDD)] and dilatation subsequent to nitrate spray [endothelium-independent dilatation (EID)]. Mean age, DOD, UO and Kt/V of study population were 49.3 ± 11.6 years, 19.4 ± 11.8 months, 508.2 ± 422.9 ml/day and 1.73 ± 0.24, respectively. As compared to controls PD patients had higher CIMT (0.46 ± 0.05 vs 0.50 ± 0.07 mm, P = 0.003) and TBARS (1.5 ± 0.4 vs 5.1 ± 2.3 nM/ml, P < 0.001) but lower Vitamin C (1.7 ± 0.3 vs 0.6 ± 0.2 mg%, P < 0.001), FRAP (990.8 ± 78.1 vs 328.7 ± 183.5 μM/L, P < 0.001) and EDD (26.2 ± 5.4 vs 9.8 ± 4.6 %, P < 0.001). TBARS correlated positively with DOD and negatively with hemoglobin. Vitamin C and FRAP correlated positively with serum albumin. EDD correlated positively with UO, Kt/V and hemoglobin. CIMT correlated negatively with Kt/V and hemoglobin. Among themselves CIMT correlated negatively with EDD and vitamin C. EDD correlated positively with vitamin C, while FRAP correlated positively with vitamin C and negatively with TBARS. PD patients have higher OS, poorer endothelial function and higher structural atherosclerosis. These parameters are closely linked to each other, hemoglobin, DOD, residual UO, serum albumin and small solute clearances.
Keywords: Antioxidant capacity, flow-mediated dilatation, lipid peroxidation, nontraditional CVD risk factors
Introduction
Cardiovascular disease (CVD) is leading cause of mortality amongst patients of chronic kidney disease (CKD) including those undergoing dialysis.[1,2] However, cause of this increase in CVD in these patients is not explained by traditional risk factors alone[3] and various nontraditional factors including oxidative stress (OS) and endothelial dysfunction have been implicated.
Endothelial dysfunction is an early marker of atherosclerosis.[4] Flow-mediated dilation (FMD), is reliable noninvasive technique to assess endothelial function, which correlates very well with coronary endothelial function.[5]
OS is defined as tissue damage resulting from an imbalance between oxidant compounds and antioxidant defense. OS, by causing NO breakdown,[6] can be a major mechanism of endothelial dysfunction.
Various circulating uremic toxins can be responsible for these abnormalities of endothelial function and OS. Correction of uremia by dialysis should therefore improve OS and endothelial function. However, loss of antioxidants and soluble factors in dialysate, use of bioincompatible fluids and increased advanced glycation end products could increase these abnormalities.
Carotid intimal medial thickness (CIMT) is a reliable, reproducible, noninvasive method of detecting and monitoring progression of atherosclerosis and reflects to a high degree the atherosclerotic situation of the coronary artery.[7]
The present understanding of these important nontraditional cardiovascular risk factors in peritoneal dialysis (PD) patients and their inter-relationship is limited due to small patient numbers and limited number of studies which have simultaneously assessed all these parameters.The present study was designed to assess endothelial function, OS and CIMT in stable patients on PD and to examine their correlates and the relationship if any between them.
Materials and Methods
This cross-sectional study was conducted at a tertiary care hospital in north India. Informed consent was obtained from all participants and the Institute Ethics Committee approved the study. All patients who were on PD for >6 months and were aged 18-65 years were screened for the study. Fifteen healthy and age-matched subjects who were being worked up as renal donors were recruited as controls.
Subjects who smoked, had an episode of peritonitis or received intravenous iron within 6 weeks were excluded. Patients who had evidence of malignancy, autoimmune disease or chronic liver disease, active infection, history of cardiovascular or peripheral vascular disease or were taking drugs that cause release of nitric oxide were also excluded. Forty-five patients were screened and 38 patients who consented and satisfied all criteria were recruited.
Seven days prior to evaluation angiotensin converting enzyme inhibitors (ACE-inhibitors), lipid lowering drugs, angiotensin receptor blocks and folic acid were withheld and appropriate antihypertensive agents were substituted. All subjects were advised to refrain from caffeine containing drinks for at least 12 hours before evaluation.
Detailed demographic data, duration of dialysis (DOD), urine output (UO), total weekly Kt/V urea and biochemical and lipid profile were noted in all patients.
Measurement of oxidants and antioxidants
Ten milliliters of blood collected in plain and EDTA vials and was immediately centrifuged at 2000 rpm for 10 minutes and stored at –80°C for subsequent analysis. Vitamin C and ferric reducing ability of plasma (FRAP) were used as markers of antioxidant capacity. Assessment of thiobarbituric acid-reactive substances (TBARS) that includes malonyl dehydrogenase (MDA) was used as marker of lipid peroxidation. All samples were light protected to retard deterioration from oxidation of substances during storage and were estimated within a month of collection.
TBARS were measured by method of Buege and Aust.[8] Following precipitation of serum by 20% acetic acid, the supernatant was mixed with 0.8% thiobarbituric acid. TBARS resulting from the above reaction was read at 430 nm. Sample, blank and standard were read similarly. The results were compared with standard graph generated by 1, 1, 3, 3-tetraethoxy propane (Sigma, India).
FRAP assay represents total antioxidant capacity of the body. FRAP was assessed by method described by Benzie and Strain.[9] FRAP was evaluated by addition of ferric chloride and tripyridyl-5-triazine to the sample and read at 620 nm on spectrophotometer. Ferrous sulfate curves were used as the standard. This test is based on the principle of the ability of plasma to reduce ferric to ferrous ions as a marker of total antioxidant reserve.
Vitamin C, an important scavenger antioxidant in serum, was measured by method described by Okamura.[10] For estimation of Vitamin C, plasma samples were preserved with metaphosphoric acid and addition of DTCS reagent (dinitrophenyl hydrazones + thiourea + copper sulfate), which were read at 520 nm and total ascorbic acid levels were estimated. The standard ascorbic acid from (Sigma) was used for preparation of the standard graph.
All methods have been standardized at our laboratory and quality control assays were taken to account for the inter and intra-assay variations.
Measurement of brachial flow-mediated dilatation
FMD of brachial artery of limb not having arteriovenous fistula was performed by using high-resolution B-mode ultrasonography system (P-700, Philips) following procedure described the American College of Cardiology.[5]
Ultrasound study was performed in a dark, quiet, temperature-controlled room. Subjects were allowed to rest in the supine position for 10 min before the study. Longitudinal scans of the brachial artery were taken approximately 3.5 cm proximal to the antecubital fossa with the probe positioned so that the best images were obtained. Endothelium-dependent dilatation (EDD): Firstly, baseline brachial artery diameter (D-0) was measured. Endothelium-dependent vasodilatation was determined by the maximal change in the diameter of the brachial artery during reactive hyperemia, which was created by placing a cuff above the antecubital fossa and inflating it to at least 50 mmHg above the systolic pressure for 5 minutes, thereby occluding blood flow to the forearm. The longitudinal image of the brachial artery was continuously recorded from 30 seconds before to 2 minutes after the cuff deflation. The maximal brachial artery diameter after reactive hyperemia (D1) was recorded.
Endothelium-independent dilatation (EID) was determined after a rest of 15 minutes by giving sub-lingual spray of 400 mcg of glyceral trinitrate (GTN) and noting the brachial artery diameter after 3 minutes.
EDD and EID were expressed as:
(Post-procedure diameter – resting diameter/ resting diameter) × 100
All measurements were taken at end-diastole coinciding with R-wave on ECG monitor. An average of three consecutive measurements each taken 10 minutes apart was taken for each subject.
Results of EDD were expressed as percentage of change in diameter of brachial artery in response to hyperemia (D1-D0/D0), but a potential factor that might confound the interpretation of FMD is baseline diameter. If the baseline diameter changes, the resulting percentage change in diameter might be affected. Hence, this study also enlisted the absolute change in diameter after intervention (D1-D0), so as to abolish the above stated confounding factor. Results of EID in response to exogenous GTN, were expressed in similar manner. EID assessment had been done, as an internal control, to detect contribution of vascular smooth muscle function, if any, in the discrepancy found in the dilatation of brachial artery after hyperemia, between study groups.
Measurement of carotid artery intimal thickness
A Philips P -700 ultrasound unit with a 7.5-MHz linear array probe was used for carotid Doppler study. Study was carried out by an experienced radiologist, unaware of patient's history or laboratory findings. Carotid arteries were screened with patients’ in supine position with their neck hyper extended and tilted away from the side to be examined. The evaluation included eight different arterial sites, including the common, internal, external and bifurcation of carotid artery. Mean of intimal media thickness at these eight sites was taken.
Statistical analysis was performed on SPSS ver 15.0 (SPSS Inc. Chicago, IL, USA). All data was calculated as mean ± standard deviation. The Students t-test was used to compare the means between two groups. Pearson's correlation coefficient was calculated for various parameters. Besides this individual stepwise multiple regression analysis was applied for finding predictors of CIMT, FMD, TBARS, FRAP and vitamin C by including in individual model all variables having significance of <0.25. P-value of <0.05 was considered significant.
Results
Thirty-eight patients on PD and 15 age and sex-matched controls were studied. Mean age of study population was 49.3 ± 11.6 years, with 52.5% (20 patients) being male. Cause of renal failure was diabetes in 13 patients (34.1%), chronic interstitial nephritis and chronic glomerulonephritis in seven patients each, ischemic nephritis in four, miscellaneous diseases in three and in four patients cause of renal failure was unknown. Thirty patients were on CAPD, while remaining eight were on CCPD with automated PD at night and day dwell with Icodextrin. Majority of CAPD patients were performing daily 3, 2-liter exchanges of standard lactate-based glucose solutions (Ultra Bag Baxter, Manesar, India), while four patients were performing daily 3, 2.5-liter exchanges of same solution.
Mean duration of PD was 19.4 ± 11.8 months (median 15.5, range 6-44 months), mean UO was 508.2 ± 422.9 ml/day (median 425, range 0-1400 ml/day) and mean weekly Kt/V urea was 1.73 ± 0.24 (median 1.7, range 1.3-2.2).
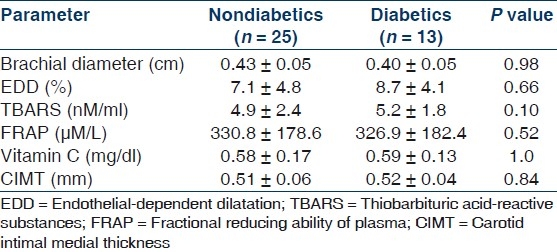
Table 1 shows the important demographic and biochemical parameters in PD patients and their age and sex-matched controls. As expected, patients on PD had higher systolic and diastolic blood pressure, serum creatinine, phosphorus, LDL cholesterol, LDL/HDL cholesterol ratio and triglycerides and lower hemoglobin, serum albumin, calcium and HDL cholesterol. Patients on PD had impaired endothelium-mediated dilatation as indicated by lower absolute and percent change in diameter from the baseline after occlusion but preserved endothelium independent dilatation [Table 2]. Levels of pro-oxidant TBARS were significantly higher, while levels of antioxidant capacity FRAP and vitamin C was significantly lower in PD patients as compared to controls [Table 3]. CIMT was also significantly higher among PD patients as compared to controls. There parameters were not significantly different between diabetics and nondiabetics [Table 4].
Table 1.
Important biochemical parameters and special investigations in the control and PD population
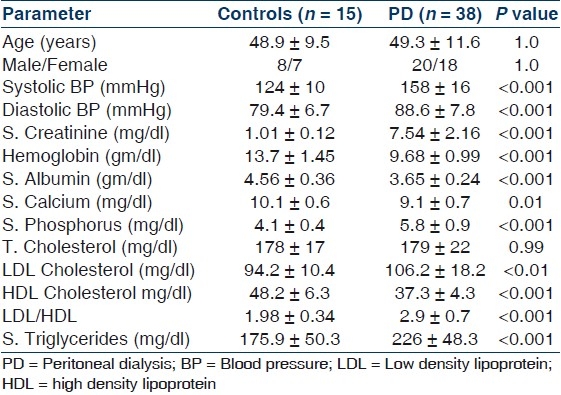
Table 2.
Parameters of flow-mediated dilatation of brachial artery in the control and PD population
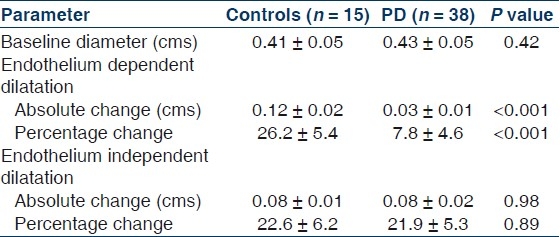
Table 3.
Pro-oxidant, antioxidant parameters and the carotid intimal media thickness in the control and PD population
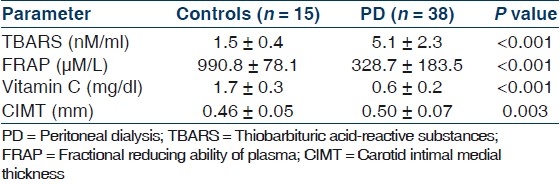
Table 4.
Endothelial-dependent dilatation, pro-oxidant, antioxidant parameters and the carotid intimal media thickness in the nondiabetic and diabetic chronic peritoneal dialysis patients
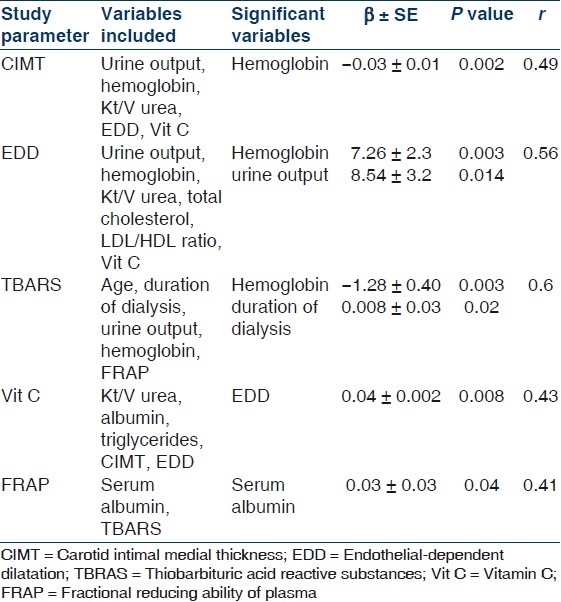
On Pearson's correlation CIMT had significant negative correlation with Kt/V urea (r = –0.371, P = 0.05), hemoglobin (r = –0.356, P = 0.05), EDD (r = –0.474, P = 0.01) and vitamin C (r = –0.456, P = 0.01). EDD of the brachial artery had significant positive correlation with residual UO, Kt/V urea, hemoglobin and vitamin C (r = 0.393, 0.405, 0.385 and 0.408, respectively, P < 0.05 for all). TBARS correlated positively with DOD (r = 0.418, P = 0.012) and negatively with hemoglobin and FRAP level (r = –0.462 and –0.45, respectively, P = 0.05). Vitamin C correlated positively with serum albumin (r = 0.352, P = 0.003) besides CIMT and EDD. While FRAP correlated positively with only serum albumin (r = 0.694, P = 0.05). When stepwise multiple regression model was applied with all variables having significance of <0.25 for a particular study parameter [Table 5], CIMT was predicted only by hemoglobin; EDD by hemoglobin and residual UO; TBARS by hemoglobin and DOD; vitamin C by EDD and FRAP was predicted by serum albumin.
Table 5.
Stepwise multiple regression model of study parmeters with all variables having significance of <0.25
Discussion
Accelerated atherosclerosis is important cause of increased morbidity and mortality in patients on dialysis. OS and endothelial dysfunction are key factors in the development and progression of atherosclerosis. However, only a few studies have looked at these factors, their correlates and their relationship with atherosclerosis simultaneously among patients on PD. We in this study have looked at OS, endothelial function and their correlation with structural atherosclerosis as measured by CIMT in 38 stable chronic PD patients. Our patient population is unique as being from an emerging economy where around two-third of our patients pay for the therapy themselves, the cost of therapy often takes precedence over the quality of dialysis. This is reflected by lower serum hemoglobin and weekly Kt/V urea in our patients as compared to those from the developed world.
We found that patients on PD had higher OS as documented by higher serum concentration of TBARS and lower serum concentrations of antioxidants FRAP and vitamin C as compared to normal controls. We also found no significant difference in these parameters among diabetic and nondiabetic patients and modality of dialysis (CAPD and CCPD). Many authors have similarly reported increased OS among PD patients.[11–16] However, the correlates of increased OS among PD patients are not clearly defined. We found that TBARS correlated positively with DOD and negatively with hemoglobin level, while FRAP correlated positively with serum albumin levels. Similar to our study Ignace et al.,[14] and Sundl et al.,[15] also found a negative correlation of dialysis vintage with lipid peroxidation and AGEs levels in 23 and 37 PD patients, respectively. Kim et al.,[13] also found a direct correlation between total antioxidant capacity and serum albumin, which, however, is not consistent with majority of other studies. Serum albumin in our study was lower than other studies, with 23.4% patients having serum albumin of <3.5 gm/dl and is comparable to 33% of such patients in study by Kim et al. This higher percentage patients having low albumin might have unmasked the association between albumin and total antioxidant capacity. Low serum albumin has been shown to reflect underlying inflammation besides malnourishment and has been shown to be a predictor of long-term mortality in dialysis patients.[17] Although we have not formally assessed the inflammatory status in our patients but higher inflammation in these malnourished patients could be responsible for lower antioxidant capacity in these patients.
We also found that PD patients have markedly impaired endothelial function as documented by impaired FMD of brachial artery (EDD). The nitrate-mediated dilatation (EID) was preserved in these patients indicating that the disease process does not significantly affect the vessel wall. This is supported by majority of studies in literature.[18–21] In addition we found that EDD correlated directly with hemoglobin and residual UO and was unaffected by traditional risk factors like diabetes and lipid abnormalities. Association between EDD and hemoglobin in adult PD patients has not been highlighted previously. It is postulated that anaemia related lower whole blood viscosity reduces physiological laminar shear stress hence decreasing the EDD.[22] Verbeke et al.,[22] found that EDD in HD patients correlated with hemoglobin and partial correction of hemoglobin to 11 gm/dl significantly improved EDD. Dursun et al.,[23] found higher endothelial microparticles, a marker of poor endothelial function in 25 paediatric PD patients, which negatively correlated with hemoglobin. We also found that patients with better residual UO had better EDD. Not many studies have looked at this association before. Wang et al.,[24] reported that every 1 ml/min/1.73 m2 increase in residual GFR is associated with a near 50% reduction in all cause mortality in PD patients. This survival advantage seen in patients has been attributed to lower degree of inflammation, malnutrition and calcification and better middle molecule clearances seen in these patients besides better volume status.[25] Our findings suggest another novel way in which preserved residual renal function by improving endothelial function can decrease CV mortality. Recently Cheng et al.,[21] have reported a negative association between index of volume status and FMD. Although we have not formally assessed volume status in our patients we believe patients with higher residual UO would have better volume status.
We also found an increase evidence of structural atherosclerosis in our PD patients as documented by higher CIMT. This is in concordance with some recently reported studies.[26–30] CIMT has even been shown to be raised in children on PD.[31] We found that CIMT had a significant negative correlation with hemoglobin. Although association of hemoglobin with CIMT in PD patients has not been observed by other studies, a negative correlation between the two has been reported in a large study among non-CKD population. Dijk et al.,[32] in 2514 patients who had manifest arterial disease found that for each mmol/L increase in hemoglobin CIMT was found to be lower by 0.03 mm. It has been postulated that a low hemoglobin by inducing an adaptive response in the vessel wall due to increase in blood flow and shear stress would increase vascular remodelling and hence CIMT.
There are some limitations of our study. Interpretation of our study is limited by small sample size and cross-sectional design of study, due to which establishment of cause and effect relationship between these parameters is not possible. Also we have not studied the effect of these parameters on long term patient outcomes. However, our sample size is still larger than many previous studies. We have also studied uniquely the correlation of two important nontraditional risk factors endothelial dysfunction and OS with each other and with structural atherosclerosis. The initial association of the two with CIMT and lack of its association with established risk factors like diabetes, blood pressure and lipid abnormalities should encourage further prospective multicentric trials enroling larger patient population looking at the relationship and effect of these parameters on long-term patient outcomes and possible role of corrective intervention by use of antioxidants.
To conclude, we demonstrate high OS, poor endothelial function and increased structural atherosclerosis among stable chronic PD patients. These parameters are interrelated and are negatively influenced by increased DOD, poor residual renal function, low hemoglobin, serum albumin and small solute clearance. This highlights the need for achieving the target levels of hemoglobin, albumin and weekly Kt/V urea besides preserving residual renal function among PD patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Hypoalbuminemia, cardiac morbidity and mortality in end-stage renal disease. J Am Soc Nephrol. 1996;7:728–36. doi: 10.1681/ASN.V75728. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt A, Stefenelli T, Schuster E, Mayer G. Informational contribution of noninvasive screening test for coronary artery disease in patients of chronic renal replacement therapy. Am J Kidney Dis. 2001;37:56–63. doi: 10.1053/ajkd.2001.20584. [DOI] [PubMed] [Google Scholar]
- 3.Cheung AK, Sarnak MJ, Yan G. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58:353–62. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990's. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 5.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guideline for the ultrasound assessment of Endothelial-Dependent Flow-Mediated Vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 6.Taddee S, Virdis A, Mattei P, Salvetti A. Vitamin C improves endothelium dependent vasodilation by restoring nitric oxide activity in essential hypertensive patients. Circulation. 1998;97:2222–9. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- 7.Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb. 1991;11:1245–9. doi: 10.1161/01.atv.11.5.1245. [DOI] [PubMed] [Google Scholar]
- 8.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;12:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 9.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power. The FRAP Assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 10.Okamura M. An improved method for determination of L-ascorbic acid and L-dehydroascorbic acid in blood plasma. Clinica Chemica Acta. 1980;103:259–68. doi: 10.1016/0009-8981(80)90144-8. [DOI] [PubMed] [Google Scholar]
- 11.Taylor JE, Scott N, Bridges A, Henderson LS, Stewart WK, Belch JJ. Lipid peroxidation and antioxidants in continuous ambulatory peritoneal dialysis patients. Pderit Dial Int. 1992;12:252–6. [PubMed] [Google Scholar]
- 12.Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–13. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 13.Kim SB, Yang WS, Min WK, Lee SK, Park JS. Reduced oxidative stress in hypoalbuminemic CAPD patients. Perit Dial Int. 2000;20:290–4. [PubMed] [Google Scholar]
- 14.Ignace S, Fouque D, Arkouche W, Steghens JP, Guebre-Egziabher F. Preserved residual renal function is associated with lower oxidative stress in peritoneal dialysis patients. Nephrol Dial Transplant. 2009;24:1685–9. doi: 10.1093/ndt/gfp077. [DOI] [PubMed] [Google Scholar]
- 15.Sundl I, Roob JM, Meinitzer A, Tiran B, Khoschsorur G, Haditsch B, et al. Antioxidant status of patients on peritoneal dialysis: Associations with inflammation and glycoxidative stress. Perit Dial Int. 2009;29:89–101. [PubMed] [Google Scholar]
- 16.Kocak H, Gumuslu S, Sahin E, Ceken K, Gocmen YA, Yakupoglu G, et al. Advanced oxidative protein products are independently associated with endothelial function in peritoneal dialysis patients. Nephrology (Carlton) 2009;14:273–80. doi: 10.1111/j.1440-1797.2008.01062.x. [DOI] [PubMed] [Google Scholar]
- 17.Akdag I, Yilmaz Y, Kahvecioglu S, Bolca N, Ercan I, Ersoy A, et al. Clinical value of the malnutrition-inflammation-atherosclerosis syndrome for long-term prediction of cardiovascular mortality in patients with end-stage renal disease: A 5-year prospective study. Nephron Clin Pract. 2008;108:c99–105. doi: 10.1159/000113526. [DOI] [PubMed] [Google Scholar]
- 18.van Guldener C, Janssen MJ, Lambert J, Steyn M, Donker AJ, Stehouwer CD. Endothelium-dependent vasodilatation is impaired in peritoneal dialysis patients. Nephrol Dial Transplant. 1998;13:1782–6. doi: 10.1093/ndt/13.7.1782. [DOI] [PubMed] [Google Scholar]
- 19.Kocak H, Ceken K, Dinckan A, Mahsereci E, Yavuz A, Yucetin L, et al. Assessment and comparison of endothelial function between dialysis and kidney transplant patients. Transplant Proc. 2006;38:416–8. doi: 10.1016/j.transproceed.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Abdelwhab S, Gandour A. Vascular dysfunction in ESRD patients under replacement therapy. Kidney. 2009;18:304–309. [Google Scholar]
- 21.Cheng LT, Gao YL, Qin C, Tian JP, Gu Y, Bi SH, et al. Volume overhydration is related to endothelial dysfunction in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 2008;28:397–402. [PubMed] [Google Scholar]
- 22.Verbeke FH, Agharazii M, Boutouyrie P, Pannier B, Guérin AP, London GM. Local shear stress and brachial artery functions in end-stage renal disease. J Am Soc Nephrol. 2007;18:621–8. doi: 10.1681/ASN.2006040400. [DOI] [PubMed] [Google Scholar]
- 23.Dursun I, Poyrazoglu HM, Gunduz Z, Ulger H, Yykylmaz A, Dusunsel R, et al. The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease. Nephrol Dial Transplant. 2009;24:2511–8. doi: 10.1093/ndt/gfp066. [DOI] [PubMed] [Google Scholar]
- 24.Wang AY, Wang M, Woo J, Lam CW, Lui SF, Li PK, et al. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol. 2004;15:2186–94. doi: 10.1097/01.ASN.0000135053.98172.D6. [DOI] [PubMed] [Google Scholar]
- 25.Wang AY. The John F. Maher Award Recipient Lecture 2006. The “heart” of peritoneal dialysis: Residual renal function. Perit Dial Int. 2007;27:116–24. [PubMed] [Google Scholar]
- 26.Pawlak K, Pawlak D, Brzosko S, Mysliwiec M. Carotid atherosclerosis is associated with enhanced beta-chemokine levels in patients on continuous ambulatory peritoneal dialysis. Atherosclerosis. 2006;186:146–51. doi: 10.1016/j.atherosclerosis.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Papagianni A, Kokolina E, Kalovoulos M, Vainas A, Dimitriadis C, Memmos D. Carotid atherosclerosis is associated with inflammation, malnutrition and intercellular adhesion molecule-1 in patients on continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2004;19:1258–63. doi: 10.1093/ndt/gfh078. [DOI] [PubMed] [Google Scholar]
- 28.Dursun B, Dursun E, Suleymanlar G, Ozben B, Capraz I, Apaydin A, et al. Carotid artery intima-media thickness correlates with oxidative stress in chronic haemodialysis patients with accelerated atherosclerosis. Nephrol Dial Transplant. 2008;23:1697–703. doi: 10.1093/ndt/gfm906. [DOI] [PubMed] [Google Scholar]
- 29.Kocak H, Gumuslu S, Ermis C, Mahsereci E, Sahin E, Gocmen AY, et al. Oxidative stress and asymmetric dimethylarginine is independently associated with carotid intima media thickness in peritoneal dialysis patients. Am J Nephrol. 2008;28:91–6. doi: 10.1159/000109397. [DOI] [PubMed] [Google Scholar]
- 30.Prasad N, Kumar S, Singh A, Sinha A, Chawla K, Gupta A, et al. Carotid intimal thickness and flow –mediated dilatation in diabetic and nondiabetic continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 2009;29:96–101. [PubMed] [Google Scholar]
- 31.Bakkaloglu SA, Saygili A, Sever L, Noyan A, Akman S, Ekim M, et al. Assessment of cardiovascular risk in paediatric peritoneal dialysis patients: A Turkish Pediatric Peritoneal Dialysis Study Group (TUPEPD) report. Nephrol Dial Transplant. 2009;24:3525–32. doi: 10.1093/ndt/gfp297. [DOI] [PubMed] [Google Scholar]
- 32.Dijk JM, Wangge G, Graaf Y, Bots ML, Grobbee DE, Algra A SMART-Study Group. Hemoglobin and atherosclerosis in patients with manifest arterial disease. The SMART-study. Atherosclerosis. 2006;188:444–9. doi: 10.1016/j.atherosclerosis.2005.11.010. [DOI] [PubMed] [Google Scholar]


