Abstract
Functional constipation is one of the most common gastrointestinal symptoms across the globe. Its high prevalence rate, economic burden, and adverse implications on the quality of life make constipation a major public health issue. Though various treatment options are available for the management of constipation, evidence for their efficacy and safety are limited. An open-label, prospective, interventional, and exploratory clinical trial was carried out to evaluate the efficacy and safety of “TLPL/AY/01/2008” in 34 patients suffering from functional constipation. “TLPL/AY/01/2008” is an Ayurvedic proprietary polyherbal formulation in powder form, containing Isabgol husk, Senna extract, and Triphala extract. Administration of “TLPL/AY/01/2008” for 14 days showed a significant increase in mean weekly bowel movements from 10.19 ± 05.64 to 18.29 ± 05.72 (P<0.05). The mean average time spent on toilet for bowel evacuation reduced significantly from 11.02 ± 05.43 minutes (baseline value) to 08.70 ± 04.72 minutes on day 14 (P<0.05). Mean stool form score assessed on Bristol stool form scale was improved from 02.97 ± 00.48 (baseline value) to 04.61 ± 00.84 (P<0.05) on day 14. A significant improvement (P<0.05) was also noted in straining during defecation, sensation of incomplete evacuation, sensation of anorectal blockage, and other associated symptoms of functional constipation. The significant improvement in most of the above symptoms was endured for a post-treatment observatory period of one week. All the study patients showed an excellent tolerability to the study drug. These findings suggest that “TLPL/AY/01/2008” is an effective, safe, and non-habit-forming herbal laxative formulation for the management of constipation. Comparative clinical studies with larger sample size would be able to confirm the above findings.
Keywords: Clinical trial, functional constipation, Isabgol, laxative, Senna, Triphala
INTRODUCTION
Constipation is a common gastrointestinal complaint in apparently healthy population as well as in patients with various predisposing disorders with approximately 12 to 19% global prevalence.[1] The high prevalence rate, economic burden, and adverse implications on the quality of life and the health state make constipation a major public health issue.[2,3]
Functional constipation is the most common form of constipation. The “Rome III criteria” is a widely accepted format for diagnosis of Functional constipation.[4,5] Treatment of constipation is most often empirical. Simple, helpful measures include patient education, dietary fiber supplementation, adequate fluid intake, and regular physical activity.[6] Patients are evaluated and treated for stress and other psychosocial factors and for problems of chronic abdominal pain.[7] Patients of constipation not benefited by the lifestyle and dietary modifications may benefit from the judicious use of suitable laxative. Various drugs such as bulk-forming agents (polycarbophil and methylcellulose), stimulants (docusate, bile acids, phenolphthalein, bisacodyl, sodium picosulfate, and ricinoleic acid), stool softeners (docusate and docusate calcium), and osmotic agents (magnesium or phosphate salts, lactulose, sorbitol, glycerin suppositories, and polyethylene glycol) are used depending upon the chronicity and severity of the condition.[5,6] Pelvic floor retraining may be helpful in the management of patients with outlet delay. Selective patients with the intractable constipation may benefit from surgery.[6] However, surgery may have serious complications and hence, is least advised.[8]
Though the conventional treatment is well established and safe, it does not provide satisfying improvement for many patients prompting their interest in other therapeutic strategies.[9] Stimulant, osmotic and saline laxatives of chemical origin are known to cause abdominal cramping, hypokalemia, flatulence, abdominal distension, and alteration in electrolyte transportation which limit the long-term use of these drugs.[7]
Hence, there is an impetus to explore the drugs from other systems of medicine such as Ayurveda for potential solutions to the problem of constipation. The formulation used in this study, i.e., “TLPL/AY/01/2008,” is an Ayurvedic proprietary medicine in powder form containing five herbs. All the ingredients of the formulation are being used since thousands of years. The individual therapeutic efficacy of these herbs as laxative has also been reported in an ancient Ayurvedic literature.[10,12,17–19] Scattered references of the efficacy of these herbs are also found in modern literature in conventional scientific formats.[11,13–16,20,22–27] Hence, an open-label, prospective, clinical study was performed to evaluate the efficacy and safety of “TLPL/AY/01/2008” in patients with functional constipation.
MATERIALS AND METHODS
Study design
The study was an open-label, non-comparative, prospective, single-arm, single-center, interventional, and exploratory clinical trial.
Objectives
The primary objective of the study was to evaluate the efficacy of “TLPL/AY/01/2008” in patients with functional constipation by assessing changes in frequency of bowel movements and changes in stool form assessed using the “Bristol stool form scale.” Secondary objectives were to evaluate the efficacy of study drug by assessing changes in symptoms (i.e., straining on defecation, sensation of incomplete evacuation, sensation of anorectal blockage, manual maneuvers required, and average time spent for bowel evacuation), associated symptoms, and overall/global improvement and also to evaluate the safety of the study drug by assessing adverse events and laboratory investigations (viz., hemogram, liver function tests (LFT), renal function tests (RFT), lipid profile, urine and stool examinations).
Investigational product
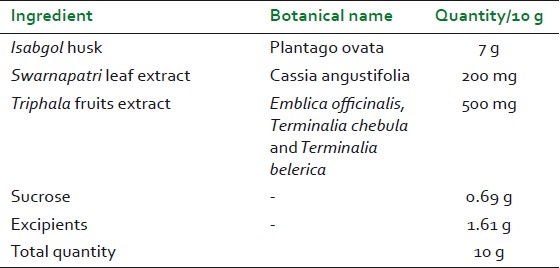
“TLPL/AY/01/2008” is an Ayurvedic proprietary polyherbal formulation in powder form. Composition of 10 g powder of “TLPL/AY/01/2008” is shown in Table 1. TLPL/AY/01/2008 is a standardized formulation wherein Triphala is standardized to gallic acid not less than 25% w/w (in-house method), Swarnapatri is standardized to sennosides not less than 20% w/w (in-house method), and Isabgol is standardized to swelling index not less than 4 (As per British Pharmacopoeia). TLPL/AY/01/2008 is manufactured at Good Manufacturing Practices-approved plant.
Table 1.
Composition of “TLPL/AY/01/2008”
SAMPLE SIZE CALCULATION
Sample size calculation was based on the assumption that a sample size of 25 evaluable cases would provide a 90% power to detect mean change in frequency of bowel movement per week at 5% level of significance.[22,23] Assuming 25% dropout rate, we enrolled 34 patients to get minimum 25 evaluable cases.
Institutional ethics committee approval and regulatory compliance
Before the initiation of the study, the study protocol and related documents were reviewed and approved by Institutional Ethics Committee at T.N. Medical College and B.Y.L. Nair Ch. Hospital, Mumbai. The study was conducted in accordance with Schedule Y of Drugs and Cosmetics act, India, amended in 2005 and ICMR ethical guidelines for biomedical research on human participants 2006.
Patients screening and recruitment
Men and women (age group, 18-70 years) suffering from functional constipation, attending the Outpatient Clinic at T. N. Medical College and B.Y.L. Nair Ch. Hospital and meeting all the inclusion criteria were recruited in the trial. Precautions were taken not to recruit patients from possible vulnerable groups.
Inclusion criteria
Patients meeting the “Rome III diagnostic criteria for functional constipation” [patients presenting with two or more of the following for the last three months with symptom onset at least six months prior to diagnosis: (a) Straining during at least 25% of defecations, (b) Lumpy or hard stools at least 25% of defecations, (c) Sensation of incomplete evacuation at least 25% of defecations, (d) Sensation of anorectal obstruction/blockage at least 25% of defecations, (e) Manual maneuvers to facilitate at least 25% of defecations, e.g., digital evacuation, support of the pelvic floor, (f) Fewer than three defecations per week and those in whom loose stools were rarely present without the use of laxatives] were included. Patients with a stool form score ranging from 1 to 3 on the “Bristol Stool Form Scale” were included. Only those patients willing to give a written informed consent as well as follow the study procedures were included in the study.
Exclusion criteria
Patients on chronic laxative medication (>60 days) and/or who were on medications known to cause constipation (like opioid analgesics, antidepressants, i.e., amitriptyline and imipramine, anticonvulsants, and aluminum-containing antacids) were excluded. Those with functional gastrointestinal disorders other than Functional constipation (i.e., IBS, Belching disorders, etc.) were also excluded. Patients with a history of abdominal or anorectal surgery in the past one year and those with renal or liver dysfunction or colonic inertia or structural abnormalities of gastro-intestinal tract or uncontrolled systemic ailments (like Human immunodeficiency virus, Diabetes mellitus, and Tuberculosis) or neurological problems (like Parkinson's disease, multiple sclerosis, sacral nerve damage, and paraplegia or autonomic neuropathy) were excluded. Pregnant (pregnancy assessed by urine pregnancy test) or lactating women were excluded. Patients allergic to any of the ingredients of the study medication were excluded.
Study procedure
At the screening visit, following written informed consent, patients suspected to be suffering from functional constipation were considered. The diagnosis of functional constipation was confirmed using the Rome-III questionnaire, Bristol stool form scale, and clinical history. Laboratory investigations (viz., - Complete Blood Count, Erythrocyte Sedimentation Rate, Hb%, thyroid function tests, LFT, RFT, lipid profile, blood sugar random, urine routine and microscopic, stool routine and microscopic, HIV test, urine pregnancy test, only for female patients of fertile age) and the ultrasonography of abdomen were carried out to assess the eligibility criteria. In case of patients who failed to give a stool sample due to lack of an urge to defecate, his/her stool examination was done on any day before baseline visit when he/she could produce a stool sample. A washout period of seven days was given and the patients were advised to refrain from any medication for constipation during this period.
At the baseline visit (Day 0), the patient was recruited in the study if he/she fulfilled all the eligibility criteria. The main symptoms and associated symptoms of functional constipation were assessed using the Visual Analogue Scale (VAS scores ranging from 0 to 100 mm) and Bristol stool form scale. The patient was provided with a diary card to note down the details of his/her daily bowel evacuations and other symptoms.
At baseline visit and at first follow-up visit, the patients were provided with the HDPE (High-Density Polyethylene) container containing 100 g powder of the trial medicine (70 g powder for seven days and 30 g additional powder, if the follow up was delayed maximum by three days). Patients were advised to mix 10 g of powder in 200 ml of water, stir well, and consume immediately once a day at bedtime for 14 days. Patients were allowed to take concomitant medicines other than known constipating medicines (like opioid analgesics, antidepressants, i.e., amitriptyline and imipramine, anticonvulsants, and aluminum-containing antacids). During entire study period, specific diet modifications were not advised to the study participants.
The patients were called for follow up on 7th, 14th, and 21st days after the baseline visit. They were allowed to report for a scheduled visit after maximum of three days from the scheduled date. Those reporting later than this grace period were considered as dropouts.
At every follow-up visit, patient's symptoms were assessed using the Bristol stool form scale and VAS. Investigator's global evaluation and the patient's global evaluation for overall improvement were done on the 14th and 21st days. Tolerability of the trial drug was assessed by the investigator as well as by the patient on day 14. Drug compliance was assessed by the investigator on first and second follow-up visits. All the patients were vigilantly monitored for possible adverse events. Post-treatment laboratory investigations (i.e., Complete Blood Count, Erythrocyte Sedimentation Rate, Hb%, LFT, RFT, lipid profile, urine routine and microscopic, and stool routine and microscopic) were performed on 14th day.
The patients were asked to stop trial medication after 14 days of treatment. From 14th to 21st day, patients were observed (without administration of trial medication) for relapse of symptoms of functional constipation.
Statistical analysis
Statistical analysis of the study data was performed by an independent statistician using statistical software SPSS 10.0. Data describing quantitative measures were expressed as median or mean ± SD or SE or the mean with range. Comparison of variables representing categorical data was performed using “Chi-square test” or “Fisher's exact test.” Mean differences of continuous variables from baseline were examined by “t-test” for independent samples or by the “analysis of variance (ANOVA)” if more than 2 subgroups (factor categories) were included. Group means of dependent sample were compared by means of ANOVA (repeated-measures design, generalized linear model procedure) or Wilcoxon sign rank test. Corresponding contrasts were tested using t-test for dependent samples and nonparametric test like “Wilcoxon Sign Rank” Test. All P values are reported based on two-sided significance test and all the statistical tests were interpreted at 5% level of significance.
OBSERVATIONS AND RESULTS
Of the 45 screened patients, eleven did not meet the inclusion criteria and hence were not included in the trial. Of 34 patients included in the trial, 21 were men while 13 were women and the mean age was 41.59 ± 12.43 years. The most common symptoms presented at screening visit were lumpy hard stools, straining during defecation, sensation of incomplete bowel evacuation, and sensation of anorectal blockage. Few patients also reported acidity, flatulence, colic pain, headache, abdominal fullness, nausea, low backache, or a need of manual maneuvers for bowel evacuation.
Thirty-one patients completed the study, while three patients dropped out prematurely. No patient was dropped out or withdrawn due to the adverse event or an adverse reaction. Study treatment did not cause any significant change in vital signs like pulse rate, body temperature, respiratory rate, and the blood pressure.
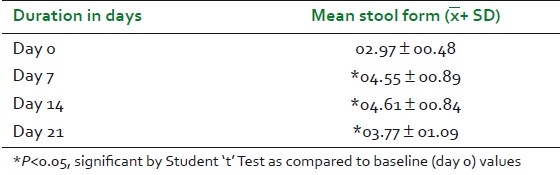
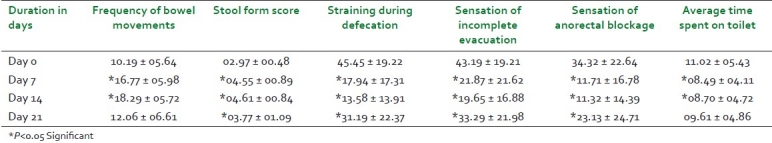
Mean weekly frequency of bowel movements in the study patients at baseline visit was 10.19 + 05.64 and it increased significantly to 16.77 ± 05.98 (64.6%) and 18.29 + 05.72 (79.5%) on day 7 and day 14, respectively. The mean weekly frequency of bowel movements increased from baseline to 12.06 ± 06.61 (18.4%) on day 21 [Table 2 and Figure 1]. Changes in stool form were assessed on the “Bristol Stool Form Scale” and expressed as a mean weekly score ranging from 1 to 7. Mean weekly score of stool form increased significantly on all the three follow-up visits, as shown in Table 3 and Figure 1.
Table 2.
Changes in mean frequency of bowel movements
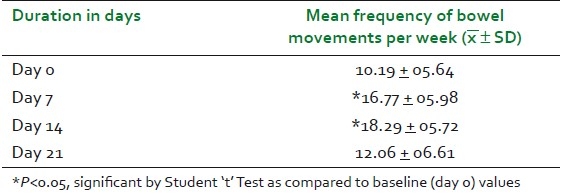
Figure 1.
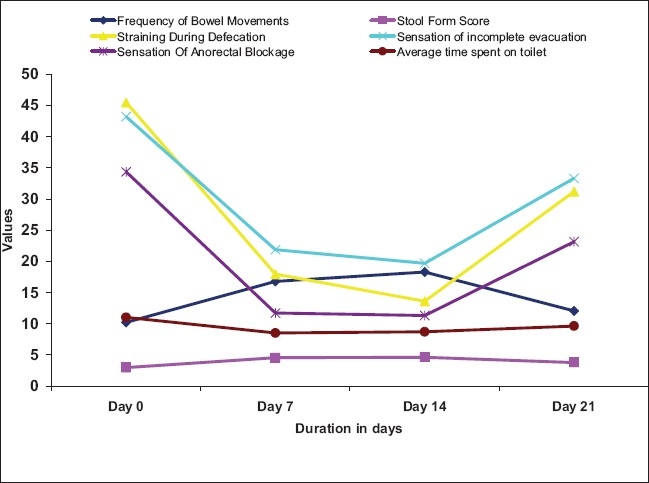
Changes in main symptoms of constipation
Table 3.
Changes in mean score of stool form on Bristol stool form scale
At baseline, the mean score of straining during defecation was 45.45 ± 19.22 and it reduced significantly to 17.94 ± 17.31 (60.5%) and 13.58 ± 13.91 (70.1%) on day 7 and day 14, respectively. On day 21, the mean score of straining during defecation showed a significant reduction from baseline to 31.19 ± 22.37 (31.4%), as shown in Table 9 and Figure 1. Sensation of incomplete evacuation and sensation of anorectal blockage were assessed on the VAS (ranging from 0 to 100 mm). The mean score of “sensation of incomplete evacuation” showed a significant reduction (P<0.05) from baseline value of 43.19 ± 19.21 to 21.87 ± 21.62 (49.4%), 19.65 ± 16.88 (54.5%), and 33.29 ± 21.98 (22.9%) on day 7, day 14, and day 21, respectively. The mean score of “sensation of anorectal blockage” showed significant reduction (P<0.05) from baseline value of 34.32 ± 22.64 to 11.71 ± 16.78 (65.9%), 11.32 ± 14.39 (67.0%), and 23.13 ± 24.71 (32.6%) on day 7, day 14, and day 21, respectively [Table 9 and Figure 1].
Table 9.
Raw Data: Changes in main symptoms of constipation-
The symptom “Manual maneuvers required for bowel evacuation” was assessed as no manual maneuvers required, manual maneuvers required one to two times a week, and manual maneuvers required more than twice a week. On day 14, the number of patients requiring manual maneuvers was reduced significantly, as shown in Table 4.
Table 4.
Number of patients (%) requiring manual maneuvers at different visits (n = 31)

The mean “average time spent on toilet for bowel evacuation” decreased significantly from a baseline value of 11.02 ± 05.43 minutes to 08.49 ± 04.11 minutes and 08.70 ± 04.72 minutes, respectively, after 7 and 14 days of treatment. On day 21, the mean average time decreased from baseline to 09.61 ± 04.86 (12.8%), which was statistically not significant [Table 9].
A significant reduction in VAS scores of all the associated symptoms was observed, as shown in Table 5. No relapse was observed in most of the symptoms and associated symptoms of functional constipation after the observatory period of seven days (i.e., on day 21).
Table 5.
Reduction in VAS scores (in mm) of associated symptoms at different visits
Overall improvement in the signs and symptoms of functional constipation was graded as excellent improvement (>90% remission of the signs and symptoms of functional constipation), good improvement (75 to 90% remission of the signs and symptoms of functional constipation), satisfactory improvement (50 to 74% remission of the signs and symptoms of functional constipation), average improvement (25 to 49% remission of the signs and symptoms of functional constipation), and poor improvement (<25% remission of the signs and symptoms of functional constipation). Findings of the global assessment done by the investigator and by the patients were uniform [Table 6].
Table 6.
Global assessment of overall efficacy (n=31)
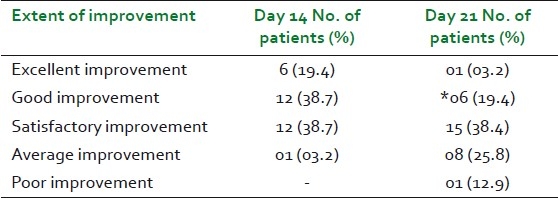
None of the patients reported any adverse events during the entire study duration. Global assessments of the tolerability of the study drug done by the investigator and by the patient are shown in Table 7. Values of post-treatment laboratory investigations were within normal limits and the mean differences between the baseline and end of the study treatment values of the investigations were not significant [Table 8].
Table 7.
Global assessment of drug tolerability assessed by the investigator and patient
Table 8.
Changes in mean values of laboratory parameters at the end of study treatment

DISCUSSION
This study confirms the beneficial effect of “TLPL/AY/01/2008” on functional constipation. The treatment with “TLPL/AY/01/2008” significantly increased (P<0.05) the mean weekly bowel frequency by 64.6% after one week and by 79.5% after two weeks. The increase in mean bowel frequency after “no laxative observatory period” of seven days (i.e., on day 21) was not statistically significant, but the mean score was 18.4% higher than that of baseline value and was clinically significant. Stool form was significantly improved on all the three follow-up visits (P<0.05). There was statistically significant improvement in straining during defecation, sensation of incomplete evacuation, and sensation of anorectal blockage after 14 days of treatment. On 21st day, the mean scores of straining during defecation, sensation of incomplete evacuation, and sensation of anorectal blockage slightly increased as compared with mean values of these symptoms on day 14. Though there was slight increase in the mean scores of these symptoms on day 21, the difference between the baseline values and values on day 21 was clinically significant.
The mean average time spent on toilet for bowel evacuation was decreased significantly after 14 days of treatment. Though the mean average time spent on toilet for bowel evacuation increased slightly on day 21 as compared with mean score on day 14, still there was 12.8% decrease as compared with baseline value. The decrease in mean average time spent on toilet was clinically significant. Only 16.1% study participants required manual maneuvers on 21st day, as compared with 35.5% study participants required it at the time of enrolment.
The present study data show high standard deviations; the probable reasons of high standard deviations could be small sample size and variability in efficacy response.
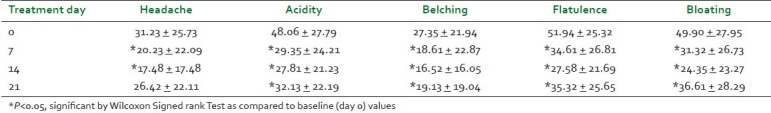
There was statistically significant improvement in mean scores of associated symptoms like acidity, belching, flatulence, and bloating in study patients at all the three follow-up visits. Though there was slight increase in mean score of headache on 21st day as compared with mean score of headache on day 14, it reduced by 15.4% from baseline value on day 21. This change was clinically significant. In global assessment, 58.1% of the patients showed excellent to good overall improvement, while 38.7% of them showed satisfactory improvement after two weeks of study treatment. After an “observatory period” of seven days (i.e., on day 21), the improvement in the symptom of functional constipation was excellent to good in 22.6% patients, satisfactory in 38.4% patients, average in 25.8% patients, and poor in 12.9% patients [Table 6].
No relapse was observed in most of the symptoms of functional constipation after the "laxative free period" of seven days (i.e., day 21).
In the present study, improvement in the frequency of bowel movements and stool form was better than that observed in previous studies done on Isabgol, lactulose, Senna, and combination of “Isabgol and senna.”[14,20,22–25] This superiority in the outcomes of the present study over previous clinical studies done on Senna alone or in combination with Isabgol husk can be due the addition of Triphala to the combination of “Isabgol and senna.” Triphala is prepared by mixing equal parts of Emblica officinalis (Amalaki), Terminalia belerica (Bibhitak), and Terminalia chebula (Haritaki). It is taken in powder form in dosages of 3 to 6 g daily for the treatment of flatulence and constipation. Triphala also helps in digestion and proper assimilation of food.[17] Thus, addition of Triphala to combination of Senna and Isabgol helped in maximizing the laxative effect of the formulation.
Unlike the reports of earlier clinical studies on Isabgol, Senna, or combination of the two, no adverse events were reported in this study.[20,21] All the study patients (i.e., 100%) reported excellent tolerability to the study drug. Also, there were no statistically significant changes in all the safety laboratory parameters at the end of the treatment.
Though the exact mechanism of action of the TLPL/AY/01/2008 is not clearly understood, the synergistic effect of the different types of laxative ingredients has possibly made it a balanced formulation for effective management of constipation. The husk of Isabgol, one of the important ingredients of TLPL/AY/01/2008, has been traditionally used to increase stool bulk and to facilitate the passage of stool. It consists of both polysaccharide and nonpolysaccharide, which on lubrication exude a hydrophilic mucilaginous substance which increase the bulk of intestinal contents. Stool bulk is also increased by additional water-holding properties of Isabgol.[11] Swarnparti or Senna, a mild stimulant laxative, increases the motility of the gastrointestinal tract and in turn the bowel frequency.[13–16] Triphala gently cleanses the colon and relives symptoms like anorectal blockage, sensation of incomplete evacuation, flatulence, and bloating.[17] According to Ayurveda, Isabgol possess Snigddha (helps in easy passage of stool) and Anuloman (mild laxative) properties. Triphala is also having Anuloman property, whereas Swarnapartri acts as Rechan (moderate laxative). Thus, it was thought that the synergistic effect of the ingredients would make the formulation a balanced combination of different types of laxatives for effective management of constipation.
It is known that Senna alone or in combination with Isabgol is habit-forming laxative agent.[26] In contrast to this, TLPL/AY/01/2008 formulation was much better than those of “Isabgol and senna” combination with respect to habit-forming property. This effect also may be due to addition of Triphala to the formulation, which strengthens and tones up the musculature of the bowel and does not cause dependence.[27]
The present investigation was an open-label, uncontrolled, and pilot study and was performed to gather the preliminary reports on efficacy and safety the proprietary Ayurvedic formulation. A randomized, double blind, comparative clinical study of TLPL/AY/01/2008 with placebo or other conventional laxative formulations in larger population may endorse the findings of the current study.
CONCLUSIONS
An Ayurvedic proprietary polyherbal laxative formulation “TLPL/AY/01/2008” is significantly effective in the management of Functional constipation. Two weeks of treatment with the drug also prevented the relapse of most of the symptoms of functional constipation up to one week. These findings suggest that “TLPL/AY/01/2008” is an effective, safe, and non-habit-forming herbal laxative formulation for the management of constipation. Further comparative, double blind studies with large sample size would be able to confirm the above findings.
Conflicts of interest
The authors Kuber V. V., Nipanikar S.U., and Kadbhane K. P. are working with the sponsor Tulip Lab Pvt. Ltd. Other authors have no conflict of interest.
ACKNOWLEDGEMENTS
The authors sincerely thank Mr. Kailas Gandewar, Soham Consultancy, Mumbai, for his kind help in data analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Peppas G, Alexiou VG, Mourtzoukou E, Falagas ME. Epidemiology of Constipation in Europe and Oceania: A Systematic Review. BMC Gastroenterol. 2008;8:5. doi: 10.1186/1471-230X-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talley NJ. Definitions, epidemiology, and impact of chronic constipation. Rev Gastroenterol Disord. 2004;4:S3–10. [PubMed] [Google Scholar]
- 3.Dennison C, Prasad M, Lloyd A, Bhattacharyya SK, Dhawan R, Coyne K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics. 2005;23:461–76. doi: 10.2165/00019053-200523050-00006. [DOI] [PubMed] [Google Scholar]
- 4.Sung IK. Classification and treatment of constipation. Korean J Gastroenterol. 2008;51:4–10. [PubMed] [Google Scholar]
- 5.Rao SS. Constipation: Evaluation and treatment. Gastroenterol Clin North Am. 2003;32:659–83. doi: 10.1016/s0889-8553(03)00026-8. [DOI] [PubMed] [Google Scholar]
- 6.Marshal JB. Chronic constipation in adults: How far should evaluation and treatment go? (54, 57-9, 63).J Postgrad Med. 1990;88:49–51. doi: 10.1080/00325481.1990.11704724. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DA. Treating chronic constipation: How should we interpret the recommendations. Clin Drug Investig. 2006;26:547–57. doi: 10.2165/00044011-200626100-00001. [DOI] [PubMed] [Google Scholar]
- 8.Ahlquist DA, Camilleri M. Diarrhea and Constipation. In: Kasper DL, Braunwald E, Fauci AS, Hauser S, Longo D, Jameson JL, editors. Harrison's Principles of Internal Medicine. Vol. 1. New York: McGraw-hill Medical Publishing Division; 2004. pp. 231–3. [Google Scholar]
- 9.Bongers ME, Benninga MA, Maurice-Stam H, Grootenhuis MA. Health-related quality of life in young adults with symptoms of constipation continuing from childhood into adulthood. Health Qual Life Outcomes. 2009;7:20. doi: 10.1186/1477-7525-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadkarni KM. Indian Materia Medica. Vol. 1. Mumbai: Bombay Popular Prakashan; 2007. pp. 982–5. [Google Scholar]
- 11.European medicines agency, Committee on herbal medicinal products. Assessment report on Plantago ovata Forssk., Seminis tegumentum. Doc. Ref. EMEA/HMPC/165838/2006. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal__HMPC_assessment_report/2010/01/WC500059138.pdf .
- 12.Srikantha Murty KR, editor. Bhavprakash of Bhavmishra. Vol. 1. Varanasi: Chaukhamba Shrikrishna Das Academy; 2008. p. 275. [Google Scholar]
- 13.De Witte P. Metabolism and pharmacokinetics of anthranoids. Pharmacology. 1993;47:86–97. doi: 10.1159/000139847. [DOI] [PubMed] [Google Scholar]
- 14.Kinnunen O, Winblad I, Koistinen P, Salokannel J. Safety and efficacy of a bulk laxative containing senna versus lactulose in the treatment of chronic constipation in geriatric patients. Pharmacology. 1993;47(Suppl 1):253–5. doi: 10.1159/000139866. [DOI] [PubMed] [Google Scholar]
- 15.European medicines agency, Committee on herbal medicinal products. Assessment report on Cassia Senna L. and Cassia angustifolia Vahl, Folium; Doc. Ref. EMEA/ HMPC/51868/2006 Corr. 2007. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal__HMPC_assessment_report/2009/12/WC500018219.pdf .
- 16.Lemli J. The mechanism of action of sennosides. Ann Gastroenterol Hepatol. 1996;32:109–12. [PubMed] [Google Scholar]
- 17.The Ayurvedic Formulary of India, Part 1. 2nd ed. New Delhi: Govt of India, Ministry of Health and Family Welfare, Dept of Indian Systems of Medicine; 2003. Anonymous; p. 110. [Google Scholar]
- 18.Srikantha Murty KR, editor. Bhavprakash of Bhavmishra. Vol. 1. Varanasi: Chaukhamba Shrikrishna Das Academy; 2008. pp. 160–5. [Google Scholar]
- 19.Srikantha Murty KR, editor. Sharangadhar Samhita by Sharangadhar. Varanasi: Chaukhamba Orientalia; 2007. p. 17. [Google Scholar]
- 20.Marlett JA, Li BU, Patrow CJ, Bass P. Comparative Laxation of Psyllium with and without Senna in an Ambulatory Constipated Population. Am J Gastroenterol. 1987;82:333–7. [PubMed] [Google Scholar]
- 21.Fenn GC, Wilkinson PD, Lee CE, Akbar FA. A general practice study of the efficacy of Regulan® in functional constipation. Br J Clin Pract. 1986;40:192. [PubMed] [Google Scholar]
- 22.Passmore AP, Davies KW, Flanagan PG, Stoker C, Scott MG. A Comparison of Agiolax and Lactulose in Elderly Patients with Chronic Constipation. Pharmacology. 1993;47(Suppl 1):249–52. doi: 10.1159/000139865. [DOI] [PubMed] [Google Scholar]
- 23.Passmore AP, Wilson-Davies K, Stoker C, Scott ME. Chronic constipation in long stay elderly patients: A comparison of lactulose and a senna-fibre combination. BMJ. 1993;307:769–71. doi: 10.1136/bmj.307.6907.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouse M, Chapman N, Mahapatra M, Grillage M, Atkinson SN, Prescott P. An open, randomized, parallel group study of lactulose versus ispaghula in the treatment of chronic constipation in adults. Br J Clin Pract. 1991;45:28–30. [PubMed] [Google Scholar]
- 25.Wang HJ, Liang XM, Yu ZL, Zhou LY, Lin SR, Geraint M. A Randomized, Controlled Comparison of Low-Dose Polyethylene Glycol 3350 plus Electrolytes with Ispaghula Husk in the Treatment of Adults with Chronic Functional Constipation. Clin Drug Investig. 2004;10:569–76. doi: 10.2165/00044011-200424100-00002. [DOI] [PubMed] [Google Scholar]
- 26.Foxx-Orenstein AE, McNally MA, Odunsi ST. Update on constipation: One Treatment does not fit all. Clevel Clin J Med. 2008;75:813–24. doi: 10.3949/ccjm.75.11.813. [DOI] [PubMed] [Google Scholar]
- 27.Shiv Prakash, Kilambi Pundarikakshudu., inventors. LAXATIVE FORMULATION. International patent WO 2007/ 013093 A2. 2007. Feb 01,


