Abstract
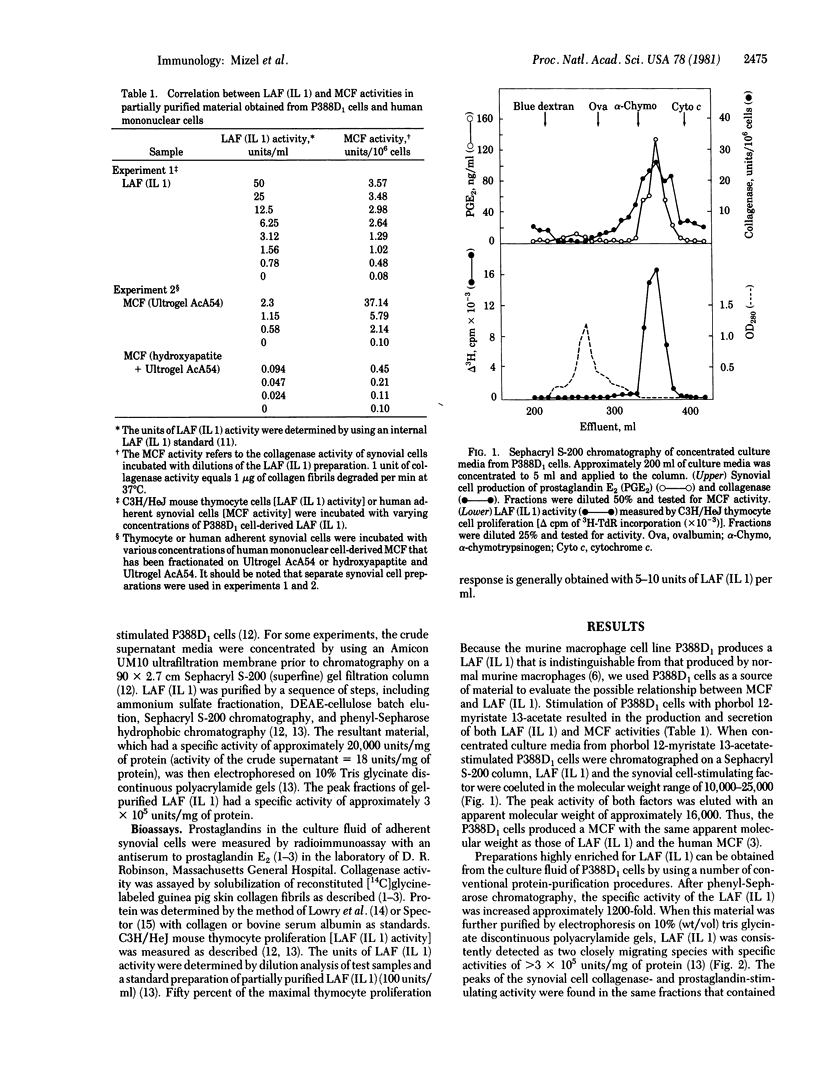
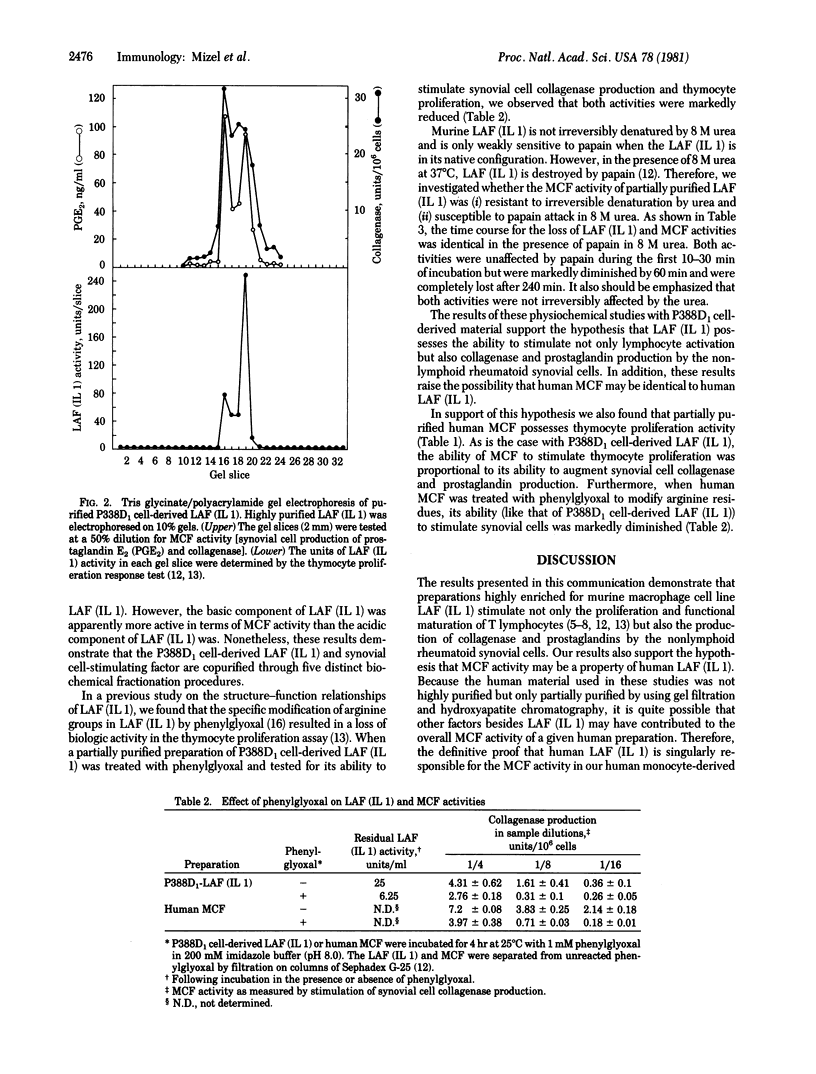
Human macrophages produce in culture a factor termed mononuclear cell factor (MCF) that increases the production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. A factor with similar biologic activity is also produced by the murine macrophage cell line P388D1. By using a sequential purification scheme involving ammonium sulfate fractionation; chromatography on DEAE-cellulose, Sephacryl S-200, and phenyl-Sepharose; and discontinuous polyacrylamide gel electrophoresis, the P388D1 cell-derived, synovial cell-stimulating factor was copurified with the lymphocyte-activating factor [LAF; interleukin 1 (IL 1)]. The specific activity of the partially purified LAF (IL 1) was approximately 15,000-fold higher than that of the LAF (IL 1) in the original P388D1 cell culture supernatant. On the basis of (i) the copurification of the P388D1 cell-derived LAF (IL 1) and synovial cell-stimulating factors; (ii) the similarity in cell of origin, molecular weight, and phenylglyoxal sensitivity of human MCF and murine LAF (IL 1); and (iii) the presence of LAF (IL 1) activity in preparations of partially purified human MCF, we have postulated that LAF (IL 1) may have effects on cell targets that are nonlymphoid in nature and also that human MCF may be similar to, or identical with human LAF (IL 1). The results of these studies have raised the possibility that LAF (IL 1) may play a role in macrophage-mediated activation of synovial cells and lymphocytes which are involved in the inflammatory responses associated with rheumatoid arthritis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dayer J. M., Bréard J., Chess L., Krane S. M. Participation of monocyte-macrophages and lymphocytes in the production of a factor that stimulates collagenase and prostaglandin release by rheumatoid synovial cells. J Clin Invest. 1979 Nov;64(5):1386–1392. doi: 10.1172/JCI109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Graham R., Russell G., Krane S. M. Collagenase production by rheumatoid synovial cells: stimulation by a human lymphocyte factor. Science. 1977 Jan 14;195(4274):181–183. doi: 10.1126/science.188134. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Robinson D. R., Krane S. M. Prostaglandin production by rheumatoid synovial cells: stimulation by a factor from human mononuclear Cells. J Exp Med. 1977 May 1;145(5):1399–1404. doi: 10.1084/jem.145.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh-Phadke K., Lawrence M., Nanda S. Synthesis of collagenase and neutral proteases by articular chondrocytes: stimulation by a macrophage-derived factor. Biochem Biophys Res Commun. 1978 Nov 14;85(1):490–496. doi: 10.1016/s0006-291x(78)80068-0. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Mizel S. B., Farrar J. J. Participation of lymphocyte activating factor (Interleukin 1) in the induction of cytotoxic T cell responses. J Immunol. 1980 Mar;124(3):1371–1377. [PubMed] [Google Scholar]
- Galili U., Rosenthal L., Galili N., Klein E. Activated T cells in the synovial fluid of arthritic patients: characterization and comparison with in vitro activated human and murine T cells in cooperation with monocytes in cytotoxicity. J Immunol. 1979 Mar;122(3):878–883. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mizel S. B., Mizel D. Purification to apparent homogeneity of murine interleukin 1. J Immunol. 1981 Mar;126(3):834–837. [PubMed] [Google Scholar]
- Mizel S. B. Physicochemical characterization of lymphocyte-activating factor (LAF). J Immunol. 1979 Jun;122(6):2167–2172. [PubMed] [Google Scholar]
- Mizel S. B. Studies on the purification and structure-functional relationships of murine lymphocyte activating factor (Interleukin 1). Mol Immunol. 1980 May;17(5):571–577. doi: 10.1016/0161-5890(80)90155-8. [DOI] [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Newsome D. A., Gross J. Regulation of corneal collagenase production: stimulation of serially passaged stromal cells by blood mononuclear cells. Cell. 1979 Apr;16(4):895–900. doi: 10.1016/0092-8674(79)90104-1. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A., Rosenthal A. S. Adherent cell function in murine T-lymphocyte antigen recognition. IV. Enhancement of murine T-cell antigen recognition by human leukocytic pyrogen. J Exp Med. 1979 Sep 19;150(3):709–714. doi: 10.1084/jem.150.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]



