Abstract
The widespread use of radiological imaging (ultrasound, computed tomography and magnetic resonance imaging) has resulted in a steady increase in the incidental diagnosis of small renal masses. While open partial nephrectomy (OPN) remains the reference standard for the management of small renal masses, laparoscopic partial nephrectomy (LPN) continues to evolve. LPN is currently advocated to be at par with OPN oncologically. The steep learning curve and technical demand of LPN make it challenging to establish this as a new procedure. We present a detailed up-to-date review on the previous, current and planned technical considerations for the use of LPN, highlighting important surgical techniques, including single-port and robotic surgery, techniques on improving intra-operative haemostasis and the management of complications specific to LPN.
Keywords: Laparoscopic partial nephrectomy, renal cancer
INTRODUCTION
Renal cancer (RC) accounts for 3% of adult cancers and 2% of cancer mortality in the United States of America (USA).[1,2] In Europe, approximately 63,300 new cases of RC are diagnosed annually, accounting for nearly 3% of all cancers.[3] Current data indicate an estimated annual increase of 2% in the diagnosis of RC.[4]
With the use of widespread imaging techniques such as ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI) in the community, the incidence of the diagnosis of small renal masses has risen, and is predicted to rise further in due course.[5] As of result of the above, smaller, and hence low-stage renal masses, are more amenable to partial nephrectomy as the surgical treatment of choice and nephron-sparing surgery (NSS) is considered. The classic surgical approach of radical nephrectomy (RN) is deemed excessive in the surgical excision of small renal masses. Urologists currently perform NSS, i.e. either open partial nephrectomy (OPN) or laparoscopic partial nephrectomy (LPN).[4]
NSS is advocated in patients with a high risk of postoperative renal insufficiency. These patients include those with bilateral renal tumours, tumours in a solitary functioning kidney or with a compromised contralateral kidney.[6] Several studies have demonstrated the surgical and oncological safety of NSS in terms of local recurrence, long-term cancer-specific survival and the overall survival, which is comparable to RN.[7,8]
Lau et al.[9] compared 164 patients undergoing RN and 164 undergoing NSS in patients with a single unilateral renal cell carcinoma (RCC) and a normal contralateral kidney. At a mean follow-up of 10 years, the early complication and recurrence rates were comparable, with no significant differences in the cancer-specific or overall survival. More importantly, the authors demonstrated a significant decrease in the risk of developing renal failure in the NSS cohort (11.6% vs. 22.4%, P = 0.01).
With rapid and progressive improvements in minimally invasive technology and expanding laparoscopic surgical expertise, LPN continues to develop as an emerged and a viable alternative to OPN.
MATERIALS AND METHODS
The PubMed and Medline databases were searched for publications up to June 2010, using the terms “laparoscopic partial nephrectomy”, “nephron-sparing surgery”, “laparoscopic nephron-sparing surgery”, “open partial nephrectomy”, “robotic partial nephrectomy”, “series”, “complications” and “outcomes”. Articles published in the English language that were relevant to the aim of this review were included.
Approach, technical challenges and surgical outcomes
OPN continues to be the reference standard for the surgical management of small (≤4 cm) exophytic renal masses.[4,10,11] Dates from a large published series have demonstrated comparable oncological outcomes for LPN and OPN, with a 5-year overall and cancer-specific survival rate of 86% and 100%, respectively[12] Surgical outcomes of the published series are summarized in Table 1.
Table 1.
Surgical outcomes of laparoscopic partial nephrectomy
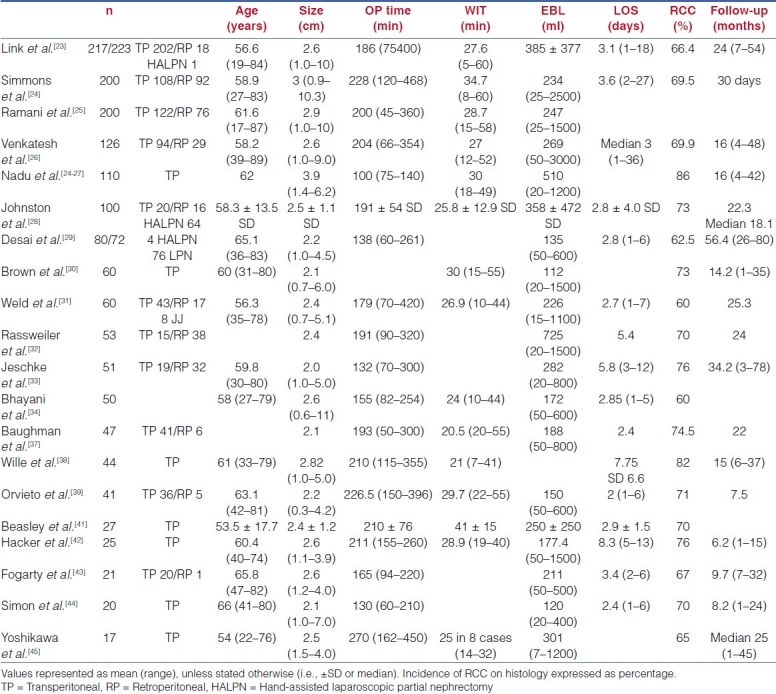
LPN is a technically challenging procedure and was initially limited to a few highly specialised institutions with large laparoscopic urological experience as the technique is evolving and the surgical indications are expanding. These current clinical issues specific to LPN that we aim to address and highlight in the paper include:
-
(1)
Selection criteria for either the transperitoneal or the retroperitoneal approach.
-
(2)
Minimizing the impact of the learning curve.
-
(3)
Impact of vascular control and ischemia time on peri-operative complications and postoperative renal function.
-
(4)
Effect of intra-operative surgical aids such as haemostatic agents and tissue sealants to reduce the risk of intra-operative bleeding and postoperative haemorrhage.
-
(5)
Identification of the risk factors and management of the procedure-specific complications, which include pseudoaneurysm formation and urinary leak.
-
(6)
Newer techniques including robotic and laparoendoscopic single-site (LESS) LPN.
Laparoscopic approach: Transperitoneal versus retroperitoneal laparoscopic partial nephrectomy
In 1992, Winfield et al.[13] performed the first transperitoneal LPN in a woman with a lower-pole caliceal diverticulum containing a stone. Two years later, Gill et al.[14] reported the first case of LPN using the retroperitoneal approach and also described the technique. Although multiple modifications and refinements have been made over the years, the principles and technical challenges for these two approaches remain essentially the same. The final choice of the type of approach is dictated by the tumour location and the surgeon preference.
Ng et al.[15] published results comparing 63 retroperitoneal and 100 transperitoneal procedures. The retroperitoneal approach was associated with a shorter warm ischaemia (28 vs. 31 min), operating time (2.9 vs. 3.5 h) and hospital stay (2.2 vs. 2.9 d). However, the tumours were larger in the transperitoneal cohort (3.2 vs. 2.5 cm). No differences were reported in terms of blood loss, peri-operative complications and postoperative functional and histological outcomes.
Kieran et al.[16] compared the intra-operative and peri-operative complications of 27 retroperitoneal and 45 transperitoneal LPNs, and reported a significant reduction in the operative time (160 vs. 192 min), blood loss (100 vs. 225 ml) and length of stay (1.0 vs. 2.0 d) in retroperitoneal LPNs. The rates of collecting system entry, positive margins and complications were comparable. In this series the size of the excised tumours in the retroperitoneal approach were significantly smaller (2.1 vs. 2.7 cm, P = 0.03).
Wright et al.[17] compared 32 retroperitoneal and 19 transperitoneal LPNs. Based on tumour location as the selection criteria, the retroperitoneal approach was associated with shorter operative time (3.5 vs. 5.4 h), reduced blood loss (192 vs. 403 ml) and reduced length of hospital stay (3.5 vs. 5.4 days).
When comparing the intra-operative haemodynamic and ventilatory parameters during laparoscopic nephrectomy, Nadu et al.[18] observed a significantly greater increase in heart rate, blood pressure, peak inspiratory and plateau pressures as well as requirements for pressure-controlled ventilation (P < 0.05) for the transperitoneal approach.
Currently, the transperitoneal approach has been associated with a wider working space, and this facilitates intra-operative suturing. This approach is currently advocated as the most appropriate for anterior and laterally located tumours as well as for larger or infiltrating tumours requiring heminephrectomy.[15,19] The limited retroperitoneal space and reduced triangulation makes retroperitoneal LPN technically more demanding. The obvious advantage of the retroperitoneal approach is direct access to the posterior and posteromedial renal masses.[16,17]
Yin et al.[20] successfully performed retroperitoneal LPN in 22 patients. They concluded that this technique was feasible and safe for selective patients with renal tumours, especially in patients with previous abdominal surgery. Zheng et al.[21] performed an LPN in 56 patients with a solitary kidney. In this large series, the mean operation time was 95 ± 14.5 min, with no recurrence and metastasis seen at 20 ± 4 months of follow-up.
The current literature suggests that the type of approach for LPN, i.e. either transperitoneal or retroperitoneal, is largely dependent on the preference and the experience of the surgeon. The location of the tumour alone does also ultimately dictate the type of surgical approach. In patients with previous abdominal surgery, the retroperitoneal approach for LPN should be considered.[20,21]
LPN is at the cutting edge of being developed into the procedure of choice in the excision of renal masses amenable to partial nephrectomy. The procedure is technically challenging and is associated with a steep learning curve, even in the hands of a competent laparoscopic urologist. There continues to be a debate on the exact number of LPNs a urologist should perform to be deemed component enough to perform individual operation.
Kapoor et al.[22] found the learning curve for LPN to be steep, with the optimal number of cases likely in the 10–20 range. The author recommended that urologists should select patients with nonhilar and exophytic T1a lesions for the initial commencement of LPN.
Weld et al.[31] chronologically classified 60 consecutive patients undergoing LPN into six groups of 10 patients each. The authors found that surgical experience, technical enhancement and implementation of novel surgical aids with progress in the learning process were all finally translated into a reduction in the number of peri - and postoperative complications. In this series, the first 30 cases had an incidence of four urinary leaks (13.3%) and two haemorrhagic complications (6.7%). The incidence of postoperative complications in the next 30 patients reduced, with only one patient having a urinary leak (3.3%).
Nadu et al.[27] compared the initial 30 cases with the last 110 patients from a cohort of 140 consecutive LPNs. They identified significant differences in tumour size (2.6 vs. 3.9 cm, P < 0.05), conversion rates (10% vs. 2.7%, P < 0.05), postoperative complication rates (urine leakage, 10% vs. 1.4% and re-operation, 6% vs. 1.8%, P < 0.05) and positive margins (10% vs. 3.6%, P < 0.05). Overall, seven cases (5%) had focally positive surgical margins, including three (10%) in the initial cohort and four (3.6%) in the later series.
The Cleveland Clinic recently published results from a contemporary series of 200 cases (24) and retrospectively compared it with their own initial series of 200 procedures.[25] While the cohorts were similar in age, body mass index (BMI), estimated blood loss and tumour size, the latest cohort had a 48% (P < 0.01) higher number of central tumours and a 20% (P = 0.03) increase in the depth of tumour invasion. Twenty-one percent (P < 0.01) of the patients had a higher incidence of pelvicaliceal system repair. Despite the increase in tumour complexity, the authors reported a 44% (P = 0.02) reduction in the incidence of overall complications, with a 56% (P = 0.04) and 53% (P = 0.04) reduction in urological and haemorrhagic complications, respectively.
A stepwise linear regression analysis by Link et al.[23] demonstrated a statistically significant reduction in the operative time, with increased experience. This publication highlighted the importance and impact of the learning curve on the final outcome of LPN.
The acquisition of surgical skills remains the core principle of surgical training. In the laparoscopic sphere and, more specifically, in laparoscopic NSS, it has been proven that specialised intensive training is associated with an ability to overcome technically complex procedures. The development of laparoscopic fellowships in highly through-put specialised centres has had a demonstrable positive impact in the reduction of the learning curve, which is evidenced in a recent report by Bhayani et al.[34] The authors compared the surgical outcomes of two consecutive series of 25 patients each, performed by a postfellowship trained surgeon. The overall complication rate was 16%, with no significant differences in the surgical outcomes of the two cohorts, with the exception of length of stay.
With the introduction of robotic LPN, the effect on the learning curve is still being evaluated. Haseebuddin et al.[35] evaluated the surgical parameters of robotic LPN in a fellowship-trained laparoscopic surgeon with extensive prior experience with LPN. The results from 38 consecutive patients suggested that neither tumour size nor previous experience in robotic LPN affected the outcome. The paper concluded that the learning curve for robotic LPN is short for surgeons already experienced with LPN.
Boylu et al.[36] have recently developed a successful animal model for LESS LPN. Although the technique is feasible, a further refinement of instrumentation is needed to decrease the ischaemia time and to optimize the procedure.
HAEMOSTASIS AND THE IMPACT OF ISCHAEMIC TIME
The achievement of haemostasis and closure of the renal parenchyma during an LPN continue to be important steps during the procedure. Closing the collecting system and preserving a short duration warm-ischaemic time (WIT) also remains a challenging step during LPN. On one hand, the laparoscopist is keen to reduce WIT and on the other hand, to secure strict haemostasis and integrity of the collecting system. Adhering to the principles of open surgery, LPN has been successfully performed using both under warm and cold ischaemia.
Warm renal ischaemia allows a bloodless field, facilitating parenchymal dissection and identification of tumour margins as well as reducing operative time and blood loss. Guillonneau et al.[40] compared the outcomes of 28 consecutive LPNs performed with (n = 16) and without (n = 12) clamping of the renal vessels. Clamping was associated with a significant reduction of the mean operating time (121.5 vs. 179.1 min, P = 0.004) and the intra-operative blood loss (270.3 vs. 708.3 ml, P = 0.014).
Isolated clamping of the renal artery[11,38,39] or “en bloc” clamping of the renal pedicle[21,23] can safely be achieved with endoscopic Satinsky clamps,[24,25,44] bulldogs[38,39] and Rummel tourniquet.[42,45] Although the use of a particular instrument depends on operator preference, the laparoscopic Satinsky clamp has been suggested by the Cleveland Clinic group as a more robust instrument. Hilar clamping with prolonged warm ischaemia carries a potential risk for ischaemic renal damage. As a result of experimental[48] and initial clinical observations,[49,50] a WIT “cut-off point” of 30 min has conventionally been accepted as a safe limit for NSS.
The current safe mean WIT from series reviewed in this article [Table 1] varies from 21 to 41 min.[35,38] Nevertheless, it is evident from the ranges reported in this series that many procedures require longer periods, with WITs of up to 60 min reported by the two largest series.[24,25]
The true impact of warm ischemia on the long-term renal function continues to be evaluated. Experimental animal models[51,52] and clinical series of OPN[53] and LPN[38,40,51–57] have demonstrated minimal impact on the long-term renal function, with WITs of up to 60 min. However, recently published data from the Cleveland Clinic Foundation analysed the functional outcomes after LPN for tumours in solitary functioning kidneys. Nguyen et al.[60] have recently described an early unclamping technique in 50 patients undergoing LPN. This novel approach, where only the initial parenchymal suturing is performed under ischaemia with the remainder of bolstered renorrhaphy performed after unclamping, is associated with a reduction in the WIT by more than 50%. Similarly, Bollens et al.[61] have proposed an “on-demand” clamping approach as a feasible technique to reduce WIT during LPN. However, it is important to remember the risks of poor vascular control at this point. Re-clamping during LPN has been identified as a risk factor for postoperative renal dysfunction.[57]
In an attempt to reduce the WIT during LPN, surgeons have successfully performed laparoscopic NSS under cold ischaemia conditions, and, in certain cases, LPN has been performed without renal ischaemia.
Renal hypothermia/cold ischemic time (CIT) is indicated in large tumours when longer ischaemia periods are expected. This can be achieved by the use of intra-corporeal renal surface cooling with “ice slush” by retrograde pelvicaliceal cooling with a cold serum infusion via a ureteral catheter[64–67,70] and by arterial catheterisation followed by cold perfusion.[70]
Gallucci et al.[71] have recently published results from a cohort of 50 patients with small, solitary and mostly exophytic tumours undergoing LPN following super-selective embolization of tumour vessels. With a mean operative time of 90 min and a mean blood loss of 200 ml, all procedures were successfully completed without hilar control. Complications were reported in two patients. The authors conclude that super-selective embolisation is a valid option for LPN.
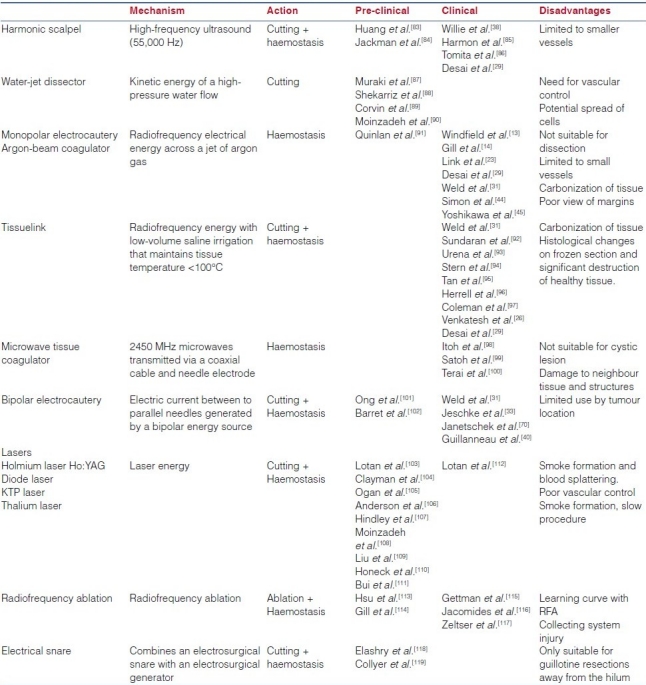
In small and peripherally located tumours, LPN can be accomplished in the absence of hilar control by novel mechanical and biological haemostatic aids. A detailed description of this vast armamentarium of laparoscopic tools is beyond the scope of this article. We provide an up-to-date summary of the current agents being used for haemostasis during LPN. These agents are classified into: (a) biological haemostatic agents and (b) laparoscopic dissector/coagulator instruments, which is presented in Table 2a and b.
Table 2a.
Haemostatic biological and sealant agents for laparoscopic partial nephrectomy
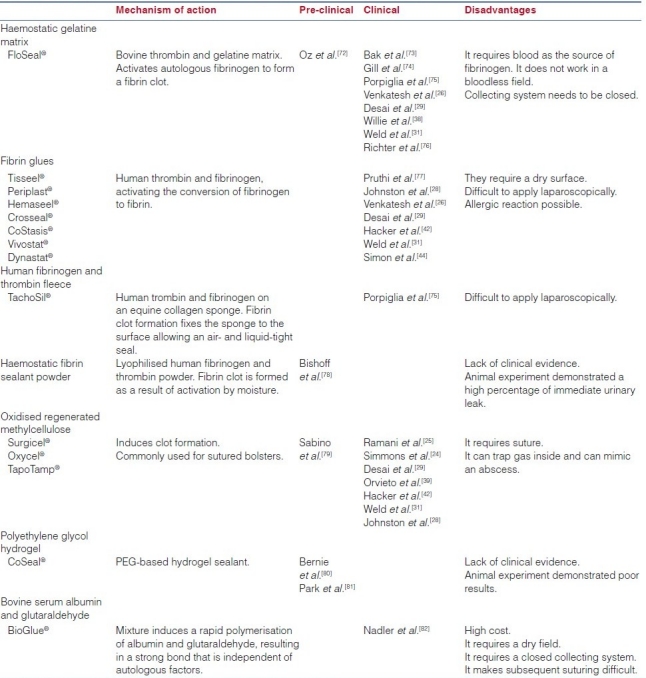
Table 2b.
Instruments and energy sources for laparoscopic partial nephrectomy
With the life expectancy of patients increasing in both the developed and the developing worlds, it is predicted that the number of elderly patients requiring surgery for small renal masses is also likely to increase. Increasing age is a risk factor for increased peri- and postoperative co-morbidity, primarily due to poorer physiological functional reserve.
Rais-Bahrami et al.[120] studied the pre-operative risk factors, intra-operative course and pathologic outcomes of 35 patients from a cohort of 257 LPN who required conversion to laparoscopic RN or open surgery. Patients aged ≥70 years had a 3.8-fold higher risk of conversion. Of all the pre-operative factors studied, age and tumour size were the only risk factors associated with a higher risk of conversion.[114] In a study by Desai et al.[50] a combination of age ≥70 years and serum creatinine ≥1.5 mg/dl correlated with poorer renal function after LPN.
The true impact of age with LPN outcomes continues to be evaluated. The current literature continues to evaluate the potential differences between laparoscopic and open approaches in the elderly patient. Furthermore, one can expect that these patients are more likely to benefit with laparoscopic NSS. Although studying RN and not partial nephrectomy cases, Lai et al.[121] addressed the impact of age in laparoscopic renal surgery in a cohort of 158 consecutive patients. In this study, patients older than 70 years, despite higher co-morbid conditions, demonstrated comparable outcomes to those of younger patients.
OBESE PATIENTS
With obesity sharply increasing in the western world, urologists are now faced with a higher number of obese patients being considered for NSS. While obesity is no longer a relative contraindication for LPN,[19] its impact on complications and outcomes remains unclear.
Anast et al.[122] retrospectively analysed the outcomes of 189 patients undergoing laparoscopic renal surgery, including 44 LPN. Obese patients, representing 29% of the cohort, had a longer operative time and greater blood loss compared with nonobese patients. However, no differences were found in terms of complications, conversion rates, analgesia requirements or length of hospital stay. The authors therefore conclude that LPN is safe and well tolerated in obese patients.
Gill et al.[123] reported excellent results in obese and morbidly obese patients. The peri-operative outcomes of 140 obese (BMI >30 kg/m2) and 238 nonobese patients were compared. Pre-operatively, obese patients had a significantly higher incidence of diabetes and hypertension. Operative parameters such as estimated blood loss (310 vs. 249 ml), operating time (3.4 vs. 3.4 h), mean WIT (31 vs. 32 min), complications (5.7% vs. 8%) and blood transfusion rates (6% vs. 3%) were comparable. Equally, the postoperative complication rate was not significantly different between the two groups (13% vs. 9%, P = 0.77). In this study, the retroperitoneal approach was associated with reduced operative time and length of stay.
Recently, Romero et al.[124] published the results of a direct comparison of a cohort of 56 OPN (28 obese and 28 nonobese) and 112 LPN (56 obese and 56 nonobese). Obese patients undergoing OPN had increased operative time (285.9 vs. 195.2 min), blood loss (484.5 vs. 391.7 ml), WIT (44.4 vs. 28.2 min), intra-operative complications (14.3% vs. 1.8%), postoperative complications (35.7% vs. 17.9%) and hospital stay (6.4 ± 2.8 vs. 3.2 ± 2.2 days) than those undergoing LPN. In this study, a comparison of obese versus nonobese patients who underwent LPN demonstrated comparable peri-operative outcomes, apart from a greater estimated blood loss in the obese patient cohort (391.7 ± 308.6 vs. 280.9 ± 202.1 ml). The above data demonstrate the safety of LPN in obese and morbidly obese patients who can benefit the most from reduced respiratory and cardiovascular morbidities of minimally access surgery.
pT1b tumours
Comparing the outcomes of 91 patients treated with NSS and 841 patients treated with RN for pT1b RCCs, Leibovich et al.[125] found no statistically significant differences in the 5-year cancer-specific survival and distant metastases-free survival. Although on univariate analysis the RN cohort was less likely to have recurrence (risk ratio 0.32, 95% CI 0.12–0.85, P = 0.022).
A retrospective multicentre analysis of 1454 patients undergoing NSS (n = 379) or RN (n = 1075) for T1N0M0 renal tumours, with a mean follow-up period of 62.5 ± 51.8 months, showed no significant differences in local or distant recurrence rates between the two groups for either T1a (P = 0.6) or T1b tumours (P = 0.5). Equally, the cancer-specific death rates between the NSS and the radical groups was comparable for T1a (2.2% vs. 2.6%, P = 0.8) as well as for T1b tumours (6.2% vs. 9%, P = 0.6).[126]
A recently published retrospective multicentre analysis including 1048 patients from eight international academic institutions has evaluated the morbidity of NSS in relation to tumour size and surgical indication.[127] A sub-analysis of 730 elective indications, including 130 tumours >4 cm, demonstrated a significantly higher mean operative time (P = 0.002), mean blood loss (P = 0.01), blood transfusion rate (P = 0.001) and urinary fistula rate (P = 0.01) for tumours >4 cm. However, no significant differences were found in the overall medical (P = 0.4), surgical complication rates (P = 0.6) or length of hospital stay (P = 0.9). More importantly, positive surgical margins, local or distant recurrence rates and cancer-specific survival were not significantly different in tumors ≤4 cm and >4 cm.
Analysis of data from the Cleveland Clinic[19] evaluating the impact of tumour size (<2 vs. 2–4 vs. >4 cm) in LPN outcomes showed comparable results in terms of operative time, estimated blood loss and hospital stay. No correlation was found with positive margin, complication rate or poorer renal functional outcomes, although the WIT was increased in the larger tumour group (30 vs. 32 vs. 38 min).
Supporting these findings, Porpiglia et al.[11] assessed the risk factors in a series of 90 cases with tumours ranging from 1 to 6 cm and demonstrated no correlation of tumour size with complications of LPN. Similar findings were described in a recently published cohort of 200 LPNs for tumours ranging from 0.9 to 10.3 cm, where, on univariate analysis, the tumour size correlated with the WIT but not with the operative time or urological complication rate.[21] This was also demonstrated by Link et al. in their series of 217 LPNs for tumours ranging from 1 to 10 cm.[20] The above studies demonstrate that, in experienced hands, LPN is feasible and safe for T1b tumours, with no increase in surgical complications, and outcomes are comparable to those from open surgery.
≥pT2 tumours
In 2006, The Cleveland Clinic group published their experience with LPN in patients with occult stage pT2, pT3a or pT3b tumours, representing 4% of their LPN cohort. With a mean tumour size of 3.7 cm (range: 1.8–7.4 cm) and employing en block laparoscopic excision of the overlying perirenal fat along with the tumour, mirroring open surgery, surgical margins were negative for cancer in all patients. One patient with pT3b and intra-operatively detected renal vein thrombus required conversion. Another patient with a pT3a sarcomatoid RCC with negative surgical margins developed distant metastasis, and died of the. With a mean follow-up of 29 months (range: 1–58 months), the cancer-specific survival rate was 95%.[128]
CENTRAL AND HILAR TUMOURS
The incidence of centrally located tumours ranges from 6.5% to 48.5%.[123] While it is widely accepted that centrally located tumours are technically more challenging and subject to higher complication rates, a standardised definition is lacking.[130–135]
Porpiglia et al.[129] have recently published an assessment of risk factors for complications of LPN in a series of 90 cases. With a 24.4% complication rate, only tumour growth pattern was found to correlate with the number of complications.
Having demonstrated the feasibility and safety of the procedure in hilar tumours,[130] Gill et al.[131] compared the outcomes of 154 centrally and 209 peripherally located tumours undergoing LPN. Central masses were larger (3 vs. 2.4 cm, P < 0.001) and had larger surgical specimens (43 vs. 22 g, P < 0.001), longer WIT (33.5 vs. 30 min, P < 0.001), operative time (3.5 vs. 3 h, P = 0.008) and hospital stay (67 vs. 60 h, P < 0.001). The intra-operative and late postoperative complications, renal functional outcomes and positive margin rates were comparable. However, early postoperative complications were more frequent in centrally located tumours (6% vs. 2%, P = 0.05).
Lattouf et al.[133] reported the outcomes on 18 LPNs for hilar tumours, which they defined as “a lesion suspicious for renal cell carcinoma in contact with a major renal vessel on pre-operative cross-sectional imaging”. With a mean tumour size of 3 cm (range: 2–4.5 cm), two (11%) entries into major vessels and one postoperative re-exploration for urinary leak were reported. One (7.1%) patient had a positive surgical margin. With a median follow-up of 26 months (range: 1–59 months), no local recurrence or systemic progression was observed.
In a retrospective review of LPN for 123 renal masses completed by seven urologists, Venkatesh et al.[23] analysed the impact of tumour location in LPN outcomes and observed a higher complication rate and higher malignancy rate in hilar tumours. Urinary leak in particular was reported in 50% of the patients undergoing LPN for hilar tumours. Similarly, Reisiger et al.[132] reported nine postoperative complications in six of eight patients undergoing LPN for hilar tumours, including four cases of urinary leak.
Despite the surgical complexity, LPN is feasible for central and hilar tumours. Increased experience has translated in a significant reduction of complications and improved surgical outcomes[21] as well as expansion towards higher stage tumours.[134] Technique modifications such as the introduction of a running haemostatic suture and thrombin sealant, with avoidance of bolstered renorrhaphy, has now been reported in selected patients,[135] allowing a faster procedure with a significant reduction of the WITs.
SOLITARY KIDNEY
LPN in a solitary kidney represents the greatest challenge to the laparoscopic surgeon. Balance between maximal parenchymal preservation and oncological safety as well as the potential risk for renal loss with the prospect of dialysis is challenging for both the patient and the surgeon. A suspect renal malignant mass in a solitary kidney continues to be an absolute indication for LPN.[136]
In 2006, Gill et al.[137] reported the initial experience in 22 patients undergoing LPN for tumour in a solitary kidney, where the mean tumour size was 3.6 cm (range: 1.4–8.3 cm). Two cases were electively converted to open surgery. Duplicating open principles (hilar clamping, cold cut tumour excision and sutured reconstruction), a mean operative time of 3.3 h (range: 2.2–4.5 h), a median blood loss of 200 ml (range: 50–500 ml) and a mean WIT of 29 min (range: 14–55 min), no positive margins or recurrences were identified. Major and minor complications were reported in three (15%) and seven (32%) patients, respectively. The median pre- and postoperative serum creatinine and estimated glomerular filtration rate (GFR) reflected a change of 33% and 27%, respectively. One patient (4.5%) required temporary haemodialysis. With a median follow-up of 2.5 years (range: 0.5–4.5 years), the cancer-specific and overall survival was 100% and 91%, respectively.
However, the prolonged WIT and its potential impact on renal function has recently been highlighted by this same group. Comparison of functional outcomes from 169 OPN and 30 LPN performed for ≤7 cm tumours in a solitary functioning kidney has recently been published by the Cleveland Clinic. The GFR decreased by 21% and 28% in the open and laparoscopic groups, respectively (P = 0.24). Postoperative dialysis was required acutely in 0.6% of OPNs versus 10% of LPN (P = 0.01), and dialysis-dependent end-stage renal failure within 1 year occurred in 0.6% of OPN versus 6.6% of LPNs (P = 0.06). The WIT was 9 min longer (P < 0.0001) and the chance of postoperative complications was 2.54-fold higher (P < 0.05) with LPN, with longer WIT (>20 min) and pre-operative GFR being associated with poorer postoperative GFR. In view of these findings, the authors conclude that, at present, OPN may be the preferred nephron-sparing approach for these patients at high risk for chronic kidney disease.
COMPLICATIONS OF LAPAROSCOPIC PARTIAL NEPHRECTOMY
LPN has become an attractive alternative to open NSS. While complication rates are still subject to scrutiny among the urological community, validation of surgical safety against its open reference standard is paramount for the potential future widespread implementation of LPN.
Risk factor analysis has identified prolonged WIT, increased blood loss, tumour location and solitary functioning kidney status to be the major risk factors of complications after LPN.[11,138]
Complications, positive margin rates and recurrence rates from published series are summarized in Table 3 and those of open partial nephrectomy are shown in Table 4.
Table 3.
Complications, positive margin rate and local recurrence rate of laparoscopic partial nephrectomy
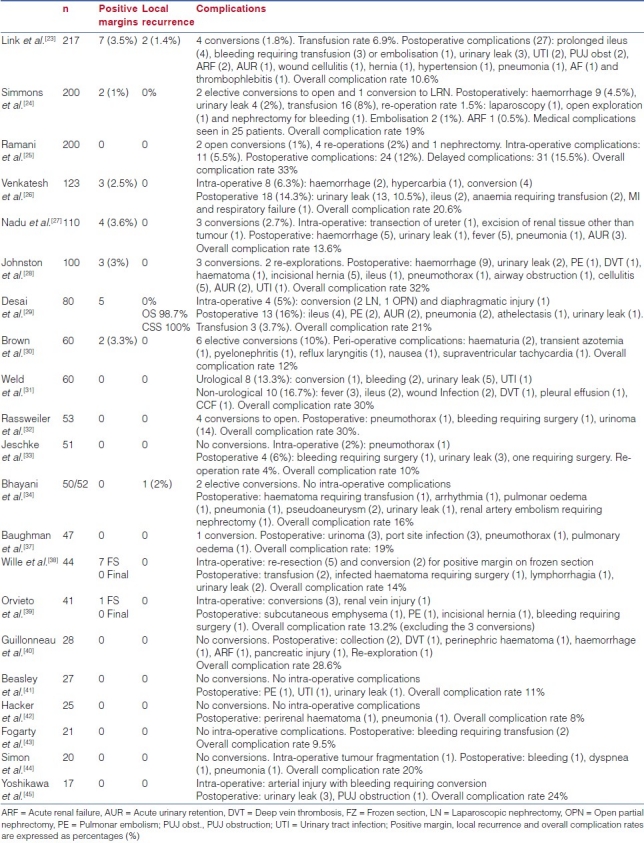
Table 4.
Complication rates in the open partial nephrectomy series
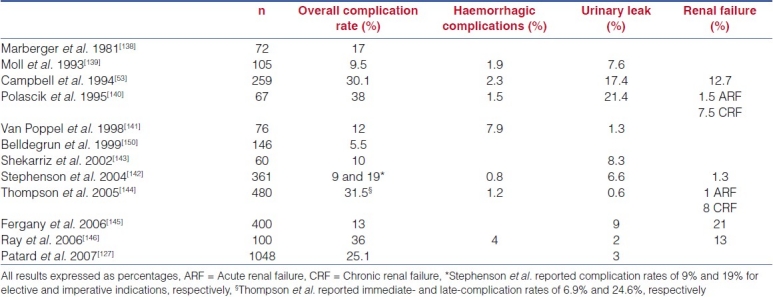
With more than 1,600 cases published by different series, the overall complication rate varies from 8% to 33% [Table 3]. These rates are comparable to those from published OPN series, ranging from 5.5% to 38%.[51,125,136] The urological complications include haemorrhage, urinary leak and impaired renal function. However, the reader should always take into consideration the lack of standardisation in published series. Different selection criteria, pre-operative comorbid status and the definition of complications reported by different cohorts is currently not standardised.
Among published series, the reported hemorrhagic complication rates range from 2% to 9.5%, with a reported blood transfusion rate of 6.9%[20] and 8% in the largest series.[24,25] Technical modifications, with the widespread use of hilar clamping[40] and adherence to the principles of open surgery, have impacted on peri-operative haemostasis.[147] More importantly, operator experience has clearly resulted in a reduction in morbidity, which is evidenced in this large series. When comparing two consecutive series of 200 patients each, Gill et al. observed a reduction in the overall complication rate from 33% to 19%, with haemorrhagic complications reduced from 9.5% to 4.5%. The impact of novel biological haemostatic agents still remains controversial. Gill et al. compared the outcomes of LPN with Floseal (n = 63) and without Floseal (n = 62), and demonstrated a reduction in the overall (16% vs. 36.8%, P = 0.008) and haemorrhagic (3.2% vs. 11.8%, P = 0.08) complication rates; other authors have however not observed a real advantage with the use of these novel agents.[11]
Urinary leak rates after LPN have been reported, ranging from 0% to 10.5%, comparable to those ranging from 0.6% to 17.4% found in published OPN series.[53,142] The impact of experience is less noticeable here due to the progressive inclusion of central and hilar tumours as the surgeons become more proficient in the technique. In a retrospective review of 123 LPN, Venkatesh et al.[26] reported a higher incidence of urinary leaks in endophytic (26.3%) and hilar (50%) tumours compared with 6.1% in exophytic masses (P < 0.001). In the more central tumours, intra-operative use of a ureteral catheter and retrograde injection of dye, in order to reduce urinary leaks, has been recommended by the most experienced surgeons.
The renal functional outcome after LPN is of upmost importance. Risk factors for poor renal functional outcomes following LPN include pre-operative renal impairment, age over 70 years with WIT >30 min, reclamping of the renal artery and a WIT >60 min.[55] Although the impact on renal function is not addressed by all published series, acute renal failure has been reported in 0.9% and 0.5% of the cases by the two largest published series,[23,24] results that compare favourably with those from open surgery, where impaired renal function has been reported in up to 21% of the patients.[8] However, the issue of functional safety is less clear in the case of solitary functioning kidney.
Gill et al.[148] recently published a comparison of early postoperative outcomes in 1800 patients with a single renal tumour ≤7 cm undergoing OPN (n = 1028) and LPN (n = 771). Based on multivariate analysis, LPN was associated with shorter operative time (P < 0.0001), decreased operative blood loss (P < 0.0001) and shorter hospital stay (P < 0.0001). The chance of intra-operative complications was comparable in the two groups. However, LPN was associated with longer ischaemia time, more immediate postoperative complications, particularly urological, and an increased number of subsequent procedures. In this study, renal functional outcomes were similar 3 months after open and laparoscopic surgery, with 99.6% and 97.9% of the renal units retaining function, respectively. However, the majority of patients in this study had a functionally normal contralateral kidney. Subsequent analysis of patients with tumours in a single functioning kidney demonstrated a greater proportion of patients requiring temporary or permanent dialysis in the LPN group, highlighting the need for further scrutiny of the impact of WIT and its implications when selecting the open or laparoscopic approach in patients with a high risk of renal impairment.[149]
In an attempt to overcome the technical difficulties of laparoscopic NSS for small renal tumours, two novel approaches have developed in parallel to LPN. Hand-assisted partial nephrectomy represents an attempt to combine the surgical advantages of laparoscopy with the hand control offered by open surgery. More recently, experienced surgeons in the field of robotic surgery are attempting to translate the advantages of the robotic interface to laparoscopic NSS. Although the clinical experience is limited and for many still controversial, the authors consider it appropriate to review these techniques, and the published series are reviewed below.
HAND-ASSISTED LAPAROSCOPIC PARTIAL NEPHRECTOMY
Hand-assisted laparoscopic nephrectomy (HALPN) has been advocated as an intermediate approach to NSS, which is less invasive than open surgery and technically less demanding than LPN. The potential advantages of HALPN are better control of the renal pedicle, easier compression of renal parenchyma, haemostasis as well as faster dissection and suturing, while maintaining a less-morbid incision. Moreover, the use of the hand as a working instrument results in a shorter learning curve.
In 2001, Stifelman et al.[151] reported results from 11 HALPNs for tumours with a mean size of 1.9 cm, demonstrating the feasibility of this procedure. No patient required hilar occlusion. With a mean operating time of 273 min and a mean estimated blood loss of 320 ml, no patient required transfusion. The mean hospital stay was 3.3 days. No positive surgical margins or recurrences were reported. One patient required conversion to open surgery.
In 2004, Hamasaki et al.[152] reported their initial experience in seven patients undergoing HALPN for small peripheral renal masses. With a mean tumour size of 2.7 cm (range: 2–4 cm) and routine use of a microwave tissue coagulator, the mean operating time was 382.7 min (range: 160–580 min) and a mean estimated blood was 379.3 ml (range: 50–1140 ml). The authors reported vascular injury in the initial three cases as well as one case of urinary leak. No positive margins were reported in this cohort. Pruthi et al.[77] published results form 15 HALPNs combining hand parenchymal compression with monopolar electrocauterization and argon-beam coagulation followed by application of fibrin sealant via a laparoscopic applicator. There were no immediate or delayed complications in any case.
The same year, Brown et al.[153] published their results in 30 patients and compared the results of 22 peripheral versus eight centrally located masses. The hand-assisted technique consisted of mobilization and manual parenchymal compression, without vascular occlusion or ureteral stent placement. Argon-beam coagulation and a fibrin glue bandage were used for haemostasis. The mean tumour size was 2.6 cm (range: 1.0–4.7 cm). The mean operative time was 199 and 271 min and the estimated blood loss was 240 and 894 ml for peripheral and central lesions, respectively. In the postoperative period, two peripheral (9.1%) and four central (50%) tumours required transfusion, while six (20%) had a urinary leak. Patients with centrally located tumours were more likely to have postoperative complications than those with peripheral or polar lesions.
In 2005, Schiff et al.[154] retrospectively analysed and compared the outcomes of 66 HALPNs and 59 OPNs. The mean tumour size was 2.2 versus 3.4 cm, the mean operating time was 144 vs. 239 min (both P < 0.001) and the mean estimated blood loss 236 versus 363 ml (P = 0.09). Serum creatinine levels were measured before and 1 and 2 days after surgery, and were 88, 88 and 97 micromol/L for the HALPN group and 97, 106 and 106 micromol/L for the OPN group. The complication rates were comparable among the two cohorts, with significantly shorter length of hospital stay for the HALPN group (3.4 vs. 5.3 days, P < 0.001).
ROBOTIC-ASSISTED LAPAROSCOPIC PARTIAL NEPHRECTOMY
Expertise gained with the rapidly expanding robotic radical prostatectomy has moved surgeons to translate this novel technology to the field of partial nephrectomy.
After describing their technique for robotic-assisted laparoscopic partial nephrectomy (RALPN),[155,156] Caruso et al.[153] published in 2006 the initial experience with 10 RALPNs (mean age 58 years, mean tumour size 2 cm) and compared the results with 10 conventional LPNs (mean age 61 years, mean tumour size 2.18 cm). Demographic data, intra-operative parameters and postoperative outcomes were statistically similar. Two intra-operative complications were reported in the RALPN group, with no clinical advantage demonstrated with the robotic approach.
Gettman et al.[158] reported data from a cohort of 13 patients undergoing RALPN with tumour excision and intracorporeal suturing performed entirely with telerobotics. With a mean tumour size of 3.5 cm (range: 2.0–6.0 cm) and a mean operative time of 215 min (range: 130–262 min), the reported WIT and estimated blood loss were 22 min (range: 15–29 min) and 170 ml (range: 50–300 ml), respectively. Postoperative ileus was reported in one patient and one patient had a positive margin. With a follow-up of up to 11 months, no recurrences were observed.
In 2007, Kaul et al.[159] published their results on 10 patients undergoing robotic partial nephrectomy. The mean age was 59 years, the mean tumour size was 2.0 cm and histology demonstrated RCC in eight cases, all with negative margins. The median hospital stay was 1.5 days. With a mean follow-up of 15 months (range: 6–28 months), no recurrences were reported.
As urologists have become more familiar with the robotic surgical interface, the indications have extended to more complex tumours. In 2008, Rogers et al.[160] have published their technique and initial experience in eight patients with 14 complex masses, including hilar, endophytic and multiple tumours. With a mean tumour size of 3.6 cm (range: 2.6–6.4 cm) and estimated blood loss of 230 ml (range: 100–450 ml), no complications or positive margins were reported. Hilar clamping was used, with a mean WIT of 31 min (range: 24–45 min). At 3-months follow-up, there was no evidence of recurrence.
Robotic technology offers theoretical advantages over conventional LPN, namely tridimensional vision, computer-aided elimination of tremor and six degrees of freedom at the distal ends of instruments, facilitating intracorporeal suture and potentially reducing the operative time and the WIT. However, the limited clinical evidence available has so far failed to demonstrate any clear advantage over conventional LPN, as shown by Caruso et al.[157] and further evidenced in two recently reported comparisons between robotic assisted and LPN.[159,160]
Deane et al.[161] compared the outcomes of 12 standard LPNs and 11 RALPNs performed at the University of Illinois. The mean tumour size was 2.3 cm (range: 1.7–6.2 cm) for LPN and 3.1 cm (range: 2.5–4 cm) for RLPN. No statistical differences were found between LPN and RLPN on mean operating time (289.5 vs. 228.7 min, P = 0.102), mean estimated blood loss (198 vs. 115 ml, P = 0.169) or mean WIT (35.3 vs. 32.1 min, P = 0.501).
The Cleveland Clinic has made public a comparison of robotically assisted partial nephrectomy, using a matched-pair analysis, with LPN. With 12 matched patients in each group, no differences were identified in peri-operative variables (WIT, estimated blood loss, operating time, length of hospital stay). Equally, renal functional outcomes, transfusion rate and complication rates were comparable. Two RPN cases required conversion to standard LPN. More importantly, in a subset of patients who underwent early unclamping, the WIT was shorter with LPN (14 vs. 21 min, P = 0.05), despite the larger tumours in this cohort (3 vs. 2.4 cm, P < 0.01).[161]
RALPN is a developing technique.[162] The available data demonstrate its feasibility as well as its functional and oncological safety. However, no clear clinical advantage has been evidenced when compared with conventional LPN. Pending further validation against current standards, the main limiting factor is still its high cost.
SINGLE-PORT LAPAROSCOPIC PARTIAL NEPHRECTOMY
Aron et al.[163] reported the use of LESS partial nephrectomy. In the technique described, the procedure was performed through the umbilicus using a multiport channel posr; R-port (Advanced Surgical Concepts, Ireland) and a 2-mm grasper inserted via a 2-mm miniport. The authors controlled the renal artery and vein during the procedure using an en bloc laparoscopic Satinsky clamp (Karl Stroz, Germany). The median WIT was 20 (11–29) min. The postoperative complications included bleeding and pulmonary embolism.
Kaouk et al.[164] performed six LPN using LESS in a series of 100 patients. All the tumours were excised using the harmonic scalpel with no renal ischemic time and with normal renal perfusion. All surgical margins were negative. Minimal pain was noted at discharge following both laparoscopic and robotic single-port surgery. No complications were reported. This publication highlights the challenges faced during LESS, including suturing, which has been succesfully overcome with the use of the daVinci robotic system.
With the development of further techniques to enhance LESS for LPNs, the current publications[163,164] stress the importance of patient selection. Larger patient series and longer follow-up is required to validate the patient and oncological success of the newly evolving technique.
SUMMARY
Widespread use of imaging techniques has resulted in a significant increase in the detection of incidental small renal masses for which RN represents an unnecessary overtreatment. While OPN remains as the reference standard for these small tumours, LPN is now widely accepted as a feasible and safe alternative.
Oncological safety of this procedure has been demonstrated in published series [Table 3] reporting positive margin rates (0–3.6%) and local recurrence rates (0–2%) comparable, if not better, to those reported in open series ranging from 0% to 14%[20] and 0% to 10%,[10] respectively. Time has further evidenced the oncological safety of LPN, with reports from the largest series demonstrating a 5-year overall and cancer-specific survival rate of 86% and 100%, respectively.[12]
Furthermore, with more than 1,600 published cases, clinical evidence has confirmed surgical outcomes comparable to those from open NSS. Gill et al.[148] compared the surgical outcomes of 1800 laparoscopic and open NSS procedures for single renal masses. On multivariate analysis, LPN was associated with a significantly shorter operative time, decreased operative blood loss and shorter hospital stay. The chance of intra-operative complications was comparable in the two groups. However, LPN was associated with longer ischaemia time (P < 0.0001). More importantly, although renal functional outcomes were similar in this study, and experimental as well as clinical evidence suggests a minimal impact of warm ischaemia periods of up to 60 min, the potential impact of prolonged warm ischaemia in the solitary kidney remains a crucial issue.[149,165]
Standardisation of the laparoscopic technique, familiarisation and rational use of novel biological agents and energy sources and, more importantly, specialised intensive training in the context of laparoscopic fellowships will improve surgical outcomes even further.
Gained expertise has moved to the more experienced surgeons to expand the indications of LPN, which now include the obese patient, multiple tumours, pT1a, pT1b and pT2 tumours, central and hilar masses, concomitant en bloc adrenalectomy, tumour in horseshoe kidney, cystic tumours and more questionable tumours in a solitary functioning kidney.[19] A randomised clinical trial is needed to validate and compare the advantages and disadvantages of LPN over OPN. However, the absolute current indications for LPN in the current literature include suspected renal mass in a solitary kidney, bilateral renal tumours and patients with chronic renal impartment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jemal A, Sr, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Hock LM LJ, Balaji KC. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: An analysis of surveillance, epidemiology and end results program data. J Urol. 2002;167:57–60. [PubMed] [Google Scholar]
- 3.Ferlay JA, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–92. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg BH, Kuczyk MA, Merseburger AS, Mulders PF, Patard JJ, Sinescu IC. Guidelines on renal cell carcinoma. EAU Gudelines. 2007 doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 5.Chow WH DS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 6.Lam JS SO, Pantuck AJ. Changing concepts in the surgical management of renal cell carcinoma. Eur Urol. 2004;45:692–705. doi: 10.1016/j.eururo.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Lee CT KJ, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm.or less in a contemporary cohort. J Urol. 2000;163:730–6. [PubMed] [Google Scholar]
- 8.Fergany AF HK, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442–5. [PubMed] [Google Scholar]
- 9.Lau WK BM, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000;75:1236–42. doi: 10.4065/75.12.1236. [DOI] [PubMed] [Google Scholar]
- 10.Uzzo RG NA. Nephron sparing surgery for renal tumors: Indications, techniques and outcomes. J Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 11.Porpiglia F VA, Billia M, Renard J, Scarpa RM. Assessment of risk factors for complications of laparoscopic partial nephrectomy. Eur Urol. 2008;53:590–6. doi: 10.1016/j.eururo.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 12.Lane BR GI. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol. 2007;177:70–4. doi: 10.1016/j.juro.2006.08.093. [DOI] [PubMed] [Google Scholar]
- 13.Winfield HN DJ, Godet AS, Clayman RV. Laparoscopic partial nephrectomy: Initial case report or benign disease. J Endourol. 1993;7:521–6. doi: 10.1089/end.1993.7.521. [DOI] [PubMed] [Google Scholar]
- 14.Gill IS DM, Munch LC. Laparoscopic retroperitoneal partial nephrectomy. J Urol. 1994;152:1539–42. doi: 10.1016/s0022-5347(17)32465-5. [DOI] [PubMed] [Google Scholar]
- 15.Ng CS, Ramani AP, Steinberg AP, Spaliviero M, Abreu SC, Kaouk JH, et al. Transperitoneal versus retroperitoneal laparoscopic partial nephrectomy: Patient selection and perioperative outcomes. J Urol. 2005;174:846–9. doi: 10.1097/01.ju.0000169259.49754.02. [DOI] [PubMed] [Google Scholar]
- 16.Kieran K MJ, Daignault S, Roberts WW, Wolf JS., Jr Comparison of intraoperative parameters and perioperative complications of retroperitoneal and transperitoneal approaches to laparoscopic partial nephrectomy: Support for a retroperitoneal approach in selected patients. J Endourol. 2007;21:754–9. doi: 10.1089/end.2007.0337. [DOI] [PubMed] [Google Scholar]
- 17.Wright JL. Laparoscopic partial nephrectomy: Comparison of transperitoneal and retroperitoneal approaches. J Urol. 2005;174:841–5. doi: 10.1097/01.ju.0000169423.94253.46. [DOI] [PubMed] [Google Scholar]
- 18.Nadu AE, Szold A, Friedman A, Nakache R, Cohen Y, Matzkin H, et al. Ventilatory and hemodynamic changes during retroperitoneal and transperitoneal laparoscopic nephrectomy: A prospective real-time comparison. J Urol. 2005;174:1013–7. doi: 10.1097/01.ju.0000169456.00399.de. [DOI] [PubMed] [Google Scholar]
- 19.Turna BA, Gill IS. Expanding indications for laparoscopic partial nephrectomy. Urology. 2008;72:481–7. doi: 10.1016/j.urology.2008.01.056. [DOI] [PubMed] [Google Scholar]
- 20.Yin M, Yang XQ, Li RB, Yang YQ, Yang M. Retroperitoneal laparoscopic nephron-sparing surgery for renal tumors. Zhonghua Yi Xue Za Zhi. 2009;89:1983–5. [PubMed] [Google Scholar]
- 21.Zheng JH, Xu YF, Peng B, Zhang HM, Yan Y, Gao QR, et al. Retroperitoneal laparoscopic partial nephrectomy for renal-cell carcinoma in a solitary kidney: Report of 56 cases. J Endourol. 2009;23:1971–4. doi: 10.1089/end.2008.0653. [DOI] [PubMed] [Google Scholar]
- 22.Kapoor A. Laparoscopic partial nephrectomy: A challenging operation with a steep learning curve. Can Urol Assoc J. 2009;3:119. doi: 10.5489/cuaj.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Link RE, Allaf ME, Varkarakis I, Inagaki T, Rogers C, Su LM, et al. Exploring the learning curve, pathological outcomes and perioperative morbidity of laparoscopic partial nephrectomy performed for renal mass. J Urol. 2005;173:1690–4. doi: 10.1097/01.ju.0000154777.24753.1b. [DOI] [PubMed] [Google Scholar]
- 24.Simmons MN. Decreased complications of contemporary laparoscopic partial nephrectomy: Use of a standardized reporting system. J Urol. 2007;177:2067–73. doi: 10.1016/j.juro.2007.01.129. [DOI] [PubMed] [Google Scholar]
- 25.Ramani AP, Steinberg AP, Ng CS, Abreu SC, Kaouk JH, Finelli A, et al. Complications of laparoscopic partial nephrectomy in 200 cases. J Urol. 2005;173:42–7. doi: 10.1097/01.ju.0000147177.20458.73. [DOI] [PubMed] [Google Scholar]
- 26.Venkatesh RW, Ames CD, Figenshau SR, Sundaram CP, Andriole GL, Clayman RV, et al. Laparoscopic partial nephrectomy for renal masses: Effect of tumor location. Urology. 2006;67:1169–74. doi: 10.1016/j.urology.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 27.Nadu AM, Laufer M, Winkler H, Kleinmann N, Kitrey N, Ramon J. Laparoscopic partial nephrectomy: Single center experience with 140 patients-evolution of the surgical technique and its impact on patient outcomes. J Urol. 2007;178:435–9. doi: 10.1016/j.juro.2007.03.143. [DOI] [PubMed] [Google Scholar]
- 28.Johnston WK, 3rd, Seifman BD, Hollenbeck BK, Wolf JS., Jr Fibrin glue v sutured bolster: Lessons learned during 100 laparoscopic partial nephrectomies. J Urol. 2005;174:47–52. doi: 10.1097/01.ju.0000162041.64143.08. [DOI] [PubMed] [Google Scholar]
- 29.Desai PJ, Ferrigni RG, Castle EP. Laparoscopic partial nephrectomy at the Mayo Clinic Arizona: Follow-up surveillance of positive margin disease. Urology. 2008;71:283–6. doi: 10.1016/j.urology.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 30.Brown GA. Laparoscopic partial nephrectomy: Experience in 60 cases. J Endourol. 2007;21:71–4. doi: 10.1089/end.2006.0166. [DOI] [PubMed] [Google Scholar]
- 31.Weld KJ, Huang J, Landman J. Evolution of surgical technique and patient outcomes for laparoscopic partial nephrectomy. Urology. 2006;67:502–6. doi: 10.1016/j.urology.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 32.Rassweiler JJ, Janetschek G, Jeschke K. Laparoscopic partial nephrectomy: The European experience. Urol Clin North Am. 2000;27:721–36. doi: 10.1016/s0094-0143(05)70121-x. [DOI] [PubMed] [Google Scholar]
- 33.Jeschke KP, Wakonig J, Schellander L, Bartsch G, Henning K. Laparoscopic nephron-sparing surgery for renal tumors. Urology. 2001;58:688–92. doi: 10.1016/s0090-4295(01)01357-7. [DOI] [PubMed] [Google Scholar]
- 34.Bhayani S. Laparoscopic partial nephrectomy: Fifty cases. J Endourol. 2008;22:313–6. doi: 10.1089/end.2007.0128. [DOI] [PubMed] [Google Scholar]
- 35.Haseebuddin M, Benway BM, Cabello JM, Bhayani SB. Robot-assisted partial nephrectomy: Evaluation of learning curve for an experienced renal surgeon. J Endourol. 2010;24:57–61. doi: 10.1089/end.2008.0601. [DOI] [PubMed] [Google Scholar]
- 36.Boylu U, Oommen M, Thomas R, Lee BR. Transumbilical single-port laparoscopic partial nephrectomy in a pig model. BJU Int. 2010;105:686–90. doi: 10.1111/j.1464-410X.2009.08916.x. [DOI] [PubMed] [Google Scholar]
- 37.Baughman SM. Laparoscopic partial nephrectomy: A novel transperitoneal technique. Contemp Urol. 2005;17:34–43. [Google Scholar]
- 38.Wille AH, Roigas J, Loening SA, Deger S. Laparoscopic partial nephrectomy in renal cell cancer--results and reproducibility by different surgeons in a high volume laparoscopic center. Eur Urol. 2006;49:337–42. doi: 10.1016/j.eururo.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Orvieto MA, Tolhurst SR, Rapp DE, Steinberg GD, Mikhail AA, Brendler CB, et al. Simplifying laparoscopic partial nephrectomy: Technical considerations for reproducible outcomes. Urology. 2005;66:976–80. doi: 10.1016/j.urology.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Guillonneau BB, Gholami S, El Fettouh H, Gupta R, Adorno Rosa J, Baumert H, et al. Laparoscopic partial nephrectomy for renal tumor: Single center experience comparing clamping and no clamping techniques of the renal vasculature. J Urol. 2003;169:483–6. doi: 10.1097/01.ju.0000045225.64349.bf. [DOI] [PubMed] [Google Scholar]
- 41.Beasley KA, Shaikh A, Bochinski D, Khakhar A, Izawa JI, Welch RO, et al. Laparoscopic versus open partial nephrectomy. Urology. 2004;64:458–61. doi: 10.1016/j.urology.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 42.Häcker A AA, Jauker W, Ziegerhofer J, Albquami N, Jeschke S, Leeb K, et al. Nephron-sparing surgery for renal tumours: Acceleration and facilitation of the laparoscopic technique. Eur Urol. 2007;51:358–65. doi: 10.1016/j.eururo.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 43.Fogarty JD, Hoenig DM, Ghavamian R. Laparoscopic nephron-sparing surgery for the small exophytic renal mass. JSLS. 2005;9:199–204. [PMC free article] [PubMed] [Google Scholar]
- 44.Simon SD, Novicki DE, Lamm DL, Swanson SS, Andrews PE. Mayo Clinic Scottsdale experience with laparoscopic nephron sparing surgery for renal tumors. J Urol. 2003;169:2059–62. doi: 10.1097/01.ju.0000058407.28232.38. [DOI] [PubMed] [Google Scholar]
- 45.Yoshikawa YO, Hattori R, Gotoh M, Yoshino Y, Katsuno S, Katoh M, et al. Laparoscopic partial nephrectomy for renal tumor: Nagoya experience. Urology. 2004;64:259–63. doi: 10.1016/j.urology.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Verhoest GM, Bensalah K, Vincendeau S, Rioux-Leclercq N, Guillé F, Patard JJ. Laparoscopic partial nephrectomy with clamping of the renal parenchyma: Ainitial experience. Eur Urol. 2007;52:1340–6. doi: 10.1016/j.eururo.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 47.Rosales AS, De Graeve N, Angerri O, Villavicencio H. Clamping of the renal artery in laparoscopic partial nephrectomy: An old device for a new technique. Eur Urol. 2005;47:98–101. doi: 10.1016/j.eururo.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 48.Ward J. Determination of the Optimum temperature for regional renal hypothermia during temporary renal ischaemia. Br J Urol. 1975;47:17–24. doi: 10.1111/j.1464-410x.1975.tb03913.x. [DOI] [PubMed] [Google Scholar]
- 49.Novick A. Renal hypothermia: In vivo and ex vivo. Urol Clin North Am. 1983;10:637–44. [PubMed] [Google Scholar]
- 50.Desai MM, Ramani AP, Spaliviero M, Rybicki L, Kaouk JH. The impact of warm ischaemia on renal function after laparoscopic partial nephrectomy. BJU Int. 2005;95:377–83. doi: 10.1111/j.1464-410X.2005.05304.x. [DOI] [PubMed] [Google Scholar]
- 51.Laven BA, Chuang MS, Ritch CR, Murray P, Harland RC, Inman SR, et al. Renal tolerance to prolonged warm ischemia time in a laparoscopic versus open surgery porcine model. J Urol. 2004;172:2471–4. doi: 10.1097/01.ju.0000138158.16968.8d. [DOI] [PubMed] [Google Scholar]
- 52.Baldwin DD, Berger KA, Desai PJ, Zuppan CW, Zimmerman GJ, Winkielman AM, et al. Laparoscopic warm renal ischemia in the solitary porcine kidney model. Urology. 2004;64:592–7. doi: 10.1016/j.urology.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 53.Campbell SC, Streem SB, Klein E, Licht M. Complications of nephron sparing surgery for renal tumors. J Urol. 1994;151:1177–80. doi: 10.1016/s0022-5347(17)35207-2. [DOI] [PubMed] [Google Scholar]
- 54.Bhayani SB, Pinto PA, Ong AM, Allaf ME, Trock BJ, Jarrett TW, et al. Laparoscopic partial nephrectomy: Effect of warm ischemia on serum creatinine. J Urol. 2004;172:1264–6. doi: 10.1097/01.ju.0000138187.56050.20. [DOI] [PubMed] [Google Scholar]
- 55.Foyil KV, Ferguson GG, Weld KJ, Figenshau RS, Venkatesh R, Yan Y, et al. Longterm changes in creatinine clearance after laparoscopic renal surgery. J Am Coll Surg. 2008;206:511–5. doi: 10.1016/j.jamcollsurg.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 56.Shekarriz BS, Upadhyay J. Impact of temporary hilar clamping during laparoscopic partial nephrectomy on postoperative renal function: A prospective study. J Urol. 2004;172:54–7. doi: 10.1097/01.ju.0000132125.78189.93. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi YU, Shima M, Akio H, Miyakita H, Inatsuchi H, Terachi T. Evaluation of renal function after laparoscopic partial nephrectomy with renal scintigraphy using 99mtechnetium-mercaptoacetyltriglycine. Int J Urol. 2006;13:1371–4. doi: 10.1111/j.1442-2042.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- 58.Kane CJ, Meng MV, Anast J, Carroll PR, Stoller ML. Laparoscopic partial nephrectomy with temporary arterial occlusion: Description of technique and renal functional outcomes. Urology. 2004;63:241–6. doi: 10.1016/j.urology.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 59.Nadu AK, Mor Y, Golomb J, Ramon J. Laparoscopic partial nephrectomy: Is it advantageous and safe to clamp the renal artery? Urology. 2005;66:279–82. doi: 10.1016/j.urology.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen MM. Halving ischemia time during laparoscopic partial nephrectomy. J Urol. 2008;179:627–32. doi: 10.1016/j.juro.2007.09.086. [DOI] [PubMed] [Google Scholar]
- 61.Bollens RR, Espinoza BP, De Groote A, Quackels T, Roumeguere T, Vanden Bossche M, et al. Laparoscopic partial nephrectomy with “on-demand” clamping reduces warm ischemia time. Eur Urol. 2007;52:804–9. doi: 10.1016/j.eururo.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 62.Gill IS, Desai MM, Steinberg AP, Ramani AP, Ng C, Banks K, et al. Laparoscopic ice slush renal hypothermia for partial nephrectomy: The initial experience. J Urol. 2003;170:52–6. doi: 10.1097/01.ju.0000072332.02529.10. [DOI] [PubMed] [Google Scholar]
- 63.Wakabayashi YN, Kim CJ, Kawakami T, Yoshiki T, Okada Y. Renal hypothermia using ice slush for retroperitoneal laparoscopic partial nephrectomy. Urology. 2004;63:773–5. doi: 10.1016/j.urology.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 64.Martins AC, Dias-Neto JA, Tucci-Junior S, Suaid HJ. Renal hypothermia: Experience in pigs and clinical trial. J Endourol. 2008;22:61–4. doi: 10.1089/end.2007.0049. [DOI] [PubMed] [Google Scholar]
- 65.Bermudez HG, Gupta R, Adorno Rosa J, Cathelineau X, Fromont G, Vallancien G. Initial experience in laparoscopic partial nephrectomy for renal tumor with clamping of renal vessels. J Endourol. 2003;17:373–8. doi: 10.1089/089277903767923146. [DOI] [PubMed] [Google Scholar]
- 66.Shikanov S, Lifshitz D, Chan AA, Okhunov Z, Ordonez MA, et al. Impact of ischemia on renal function after laparoscopic partial nephrectomy: A multicenter study. J Urol. 2010;183:1714–8. doi: 10.1016/j.juro.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 67.Landman JV, Lee D, Vanlangendonck R, Morissey K, Andriole GL, Clayman RV, et al. Renal hypothermia achieved by retrograde endoscopic cold saline perfusion: Technique and initial clinical application. Urology. 2003;61:1023–5. doi: 10.1016/s0090-4295(02)02566-9. [DOI] [PubMed] [Google Scholar]
- 68.Landman JR, Sundaram CP, Bhayani S, Monga M, Pattaras JG, Gokden N, et al. Renal hypothermia achieved by retrograde intracavitary saline perfusion. J Endourol. 2002;16:445–9. doi: 10.1089/089277902760367386. [DOI] [PubMed] [Google Scholar]
- 69.Matsuda TN, Oguchi N, Yanishi M, Fukui S, Kawa G, Muguruma K. Retroperitoneoscopic partial nephrectomy with transient occlusion of renal artery for treatment of small renal tumors. Urology. 2004;64:26–30. doi: 10.1016/j.urology.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Janetschek GA, Bagheri F, Al-Zahrani H, Leeb K, Gschwendtner M. Laparoscopic partial nephrectomy in cold ischemia: Renal artery perfusion. J Urol. 2004;171:68–71. doi: 10.1097/01.ju.0000101040.13244.c4. [DOI] [PubMed] [Google Scholar]
- 71.Gallucci MG, Carpanese L, Papalia R, Simone G, Forestiere E, Leonardo C. Superselective embolization as first step of laparoscopic partial nephrectomy. Urology. 2007;69:642–5. doi: 10.1016/j.urology.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 72.Oz MC, Shargill NS. FloSeal Matrix: New generation topical hemostatic sealant. J Card Surg. 2003;18:486–93. doi: 10.1046/j.0886-0440.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 73.Bak JB, Shekarriz B. Use of gelatin matrix thrombin tissue sealant as an effective hemostatic agent during laparoscopic partial nephrectomy. J Urol. 2004;171:780–2. doi: 10.1097/01.ju.0000104800.97009.c6. [DOI] [PubMed] [Google Scholar]
- 74.Gill IS, Spaliviero M, Xu M, Finelli A, Kaouk JH, Desai MM. Improved hemostasis during laparoscopic partial nephrectomy using gelatin matrix thrombin sealant. Urology. 2005;65:463–6. doi: 10.1016/j.urology.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 75.Porpiglia FR, Billia M, Morra I, Terrone C, Scarpa RM. Biological glues and collagen fleece for hemostasis during laparoscopic partial nephrectomy: Technique and results of prospective study. J Endourol. 2007;21:423–8. doi: 10.1089/end.2006.0265. [DOI] [PubMed] [Google Scholar]
- 76.Richter FS, Deger S, Trk I, Roigas J, Wille A, Loening SA. Improvement of hemostasis in open and laparoscopically performed partial nephrectomy using a gelatin matrix-thrombin tissue sealant (FloSeal) Urology. 2003;61:73–7. doi: 10.1016/s0090-4295(02)02143-x. [DOI] [PubMed] [Google Scholar]
- 77.Pruthi RS, Richman M. The use of a fibrin tissue sealant during laparoscopic partial nephrectomy. BJU Int. 2004;93:813–7. doi: 10.1111/j.1464-410X.2003.04742.x. [DOI] [PubMed] [Google Scholar]
- 78.Bishoff JT, Perahia B, Seay T, Eliason S, Katus M, Morey A, et al. Laparoscopic heminephrectomy using a new fibrin sealant powder. Urology. 2003;62:1139–43. doi: 10.1016/s0090-4295(03)00783-0. [DOI] [PubMed] [Google Scholar]
- 79.Sabino LA, Faria EF, Ferreira PS, Paz AR, Kalil W, De Figueiredo LP, et al. Evaluation of renal defect healing, hemostasis, and urinary fistula after laparoscopic partial nephrectomy with oxidized cellulose. J Endourol. 2007;21:551–6. doi: 10.1089/end.2005.9999. [DOI] [PubMed] [Google Scholar]
- 80.Bernie JE, Bargman V, Gardner T, Cheng L, Sundaram CP. Evaluation of hydrogel tissue sealant in porcine laparoscopic partial-nephrectomy model. J Endourol. 2005;19:1122–6. doi: 10.1089/end.2005.19.1122. [DOI] [PubMed] [Google Scholar]
- 81.Park EL, Scott KM, Ullrich NP, Linehan JA, French MH, Ho WY, et al. Evaluation of polyethylene glycol based hydrogel for tissue sealing after laparoscopic partial nephrectomy in a porcine model. J Urol. 2004;172:2446–50. doi: 10.1097/01.ju.0000138159.69642.d9. [DOI] [PubMed] [Google Scholar]
- 82.Nadler RB, Rubenstein RA, Vardi IY. Use of BioGlue in laparoscopic partial nephrectomy. Urology. 2006;68:416–8. doi: 10.1016/j.urology.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 83.Huang SH, Lin CH, Liu CJ, Huan SK, Lee BS, Lin WL, et al. Efficacy of ultrasonic tissue dissector and tissue glue for laparoscopic partial nephrectomy in a porcine model. Int Surg. 2003;88:199–204. [PubMed] [Google Scholar]
- 84.Jackman SV, Chen RN, Micali S, Bishoff JT, Lee BR, Moore RG, et al. Utility of the harmonic scalpel for laparoscopic partial nephrectomy. J Endourol. 1998;12:441–4. doi: 10.1089/end.1998.12.441. [DOI] [PubMed] [Google Scholar]
- 85.Harmon WJ, Bishoff JT. Laparoscopic nephron-sparing surgery for solid renal masses using the ultrasonic shears. Urology. 2000;56:754–9. doi: 10.1016/s0090-4295(00)00766-4. [DOI] [PubMed] [Google Scholar]
- 86.Tomita YK, Takahashi K, Tamaki M, Morishita H. Use of the harmonic scalpel for nephron sparing surgery in renal cell carcinoma. J Urol. 1998;159:2063–4. doi: 10.1016/S0022-5347(01)63247-6. [DOI] [PubMed] [Google Scholar]
- 87.Muraki JA, Lastarria E, Eshghi M, Choudhury MS. New Cavitron system (CUSA/CEM): Its application for kidney surgery. Urology. 1993;41:195–8. doi: 10.1016/0090-4295(93)90181-9. [DOI] [PubMed] [Google Scholar]
- 88.Shekarriz HS, Upadhyay J, Comman A, Markert U, Bürk CG, Kujath P, et al. Hydro-Jet assisted laparroscopic cholecystectomy: Initial experience in a porcine model. JSLS. 2002;6:53–8. [PMC free article] [PubMed] [Google Scholar]
- 89.Corvin SO, Adam C, Frimberger D, Zaak D, Siebels M, Hofstetter A. Use of hydro-jet cutting for laparoscopic partial nephrectomy in a porcine model. Urology. 2001;58:1070–3. doi: 10.1016/s0090-4295(01)01447-9. [DOI] [PubMed] [Google Scholar]
- 90.Moinzadeh AH, Spaliviero M, Finelli A, Kilciler M, Magi-Galluzzi C, El Gabry E, et al. Water jet assisted laparoscopic partial nephrectomy without hilar clamping in the calf model. J Urol. 2005;174:317–21. doi: 10.1097/01.ju.0000161587.95033.c9. [DOI] [PubMed] [Google Scholar]
- 91.Quinlan DM, Brendler CB. Application of argon beam coagulation in urological surgery. J Urol. 1992;147:410–2. doi: 10.1016/s0022-5347(17)37252-x. [DOI] [PubMed] [Google Scholar]
- 92.Sundaram CP, Venkatesh R, Lee D, Rageb MM, Kibel A, Landman J. Hemostatic laparoscopic partial nephrectomy assisted by a water-cooled, high-density, monopolar device without renal vascular control. Urology. 2003;61:906–9. doi: 10.1016/s0090-4295(02)02550-5. [DOI] [PubMed] [Google Scholar]
- 93.Urena RM, Woods M, Thomas R, Davis R. Laparoscopic partial nephrectomy of solid renal masses without hilar clamping using a monopolar radio frequency device. J Urol. 2004;171:1054–6. doi: 10.1097/01.ju.0000103927.75499.5d. [DOI] [PubMed] [Google Scholar]
- 94.Stern JA, Ferrigni RG, Andrews PE. Tissue Link device for laparoscopic nephron-sparing surgery. J Endourol. 2004;18:455–6. doi: 10.1089/0892779041271463. [DOI] [PubMed] [Google Scholar]
- 95.Tan YH, L’Esperance JO, Preminger GM, Albala DM. Hand-assisted laparoscopic partial nephrectomy without hilar vascular clamping using a saline-cooled, high-density monopolar radiofrequency device. J Endourol. 2004;18:883–7. doi: 10.1089/end.2004.18.883. [DOI] [PubMed] [Google Scholar]
- 96.Herrell SD. Laparoscopic partial nephrectomy: Use of the TissueLink hemostatic dissection device. J Endourol. 2005;19:446–9. doi: 10.1089/end.2005.19.446. [DOI] [PubMed] [Google Scholar]
- 97.Coleman JS, Pinto P, Phillips J, Pritchard W, Wray-Cahen D, Wood BJ. Radiofrequency- assisted laparoscopic partial nephrectomy: Clinical and histologic results. J Endourol. 2007;21:600–5. doi: 10.1089/end.2006.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Itoh KS, Miuru M, Tsukigi M, Ichiyanagi O, Sasagawa I. Posterior retroperitoneoscopic partial nephrectomy using microwave tissue coagulator for small renal tumors. J Endourol. 2002;16:367–71. doi: 10.1089/089277902760261400. [DOI] [PubMed] [Google Scholar]
- 99.Satoh YU, Nanri M, Nakashima K, Kanou T, Tokuda Y, Fujiyama C, et al. Renal-tissue damage induced by laparoscopic partial nephrectomy using microwave tissue coagulator. J Endourol. 2005;19:818–22. doi: 10.1089/end.2005.19.818. [DOI] [PubMed] [Google Scholar]
- 100.Terai AI, Yoshimura K, Ichioka K, Kamoto T, Arai Y, Ogawa O. Laparoscopic partial nephrectomy using microwave tissue coagulator for small renal tumors: Usefulness and complications. Eur Urol. 2004;45:774–8. doi: 10.1016/j.eururo.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 101.Ong AM, Hsu TH, Pinto PA, Rha KH, Thomas M, Nicol T, et al. Bipolar needle electrocautery for laparoscopic partial nephrectomy without renal vascular occlusion in a porcine model. Urology. 2003;62:1144–8. doi: 10.1016/s0090-4295(03)00689-7. [DOI] [PubMed] [Google Scholar]
- 102.Barret EG, Cathelineau X, Validire P, Vallancien G. Laparoscopic partial nephrectomy in the pig: Comparison of three hemostasis techniques. J Endourol. 2001;15:307–12. doi: 10.1089/089277901750161890. [DOI] [PubMed] [Google Scholar]
- 103.Lotan YG, Lindberg G, Napper CA, Hoopman J, Pearle MS, Cadeddu JA. Laparoscopic partial nephrectomy using holmium laser in a porcine model. JSLS. 2004;8:51–5. [PMC free article] [PubMed] [Google Scholar]
- 104.Clayman R. Laparoscopic partial nephrectomy using holmium laser in a porcine model. J Urol. 2005;173:1200–1. [PubMed] [Google Scholar]
- 105.Ogan KW, Lindberg G, Lotan Y, Napper C, Hoopman J, Pearle MS, et al. Laparoscopic partial nephrectomy with a diode laser: Porcine results. J Endourol. 2002;16:749–53. doi: 10.1089/08927790260472908. [DOI] [PubMed] [Google Scholar]
- 106.Anderson JK, Lindberg G, Cadeddu JA. Large-volume laparoscopic partial nephrectomy using the potassium-titanyl-phosphate (KTP) laser in a survival porcine model. Eur Urol. 2007;51:749–54. doi: 10.1016/j.eururo.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 107.Hindley RG, Walsh K, Petersen A, Poulsen J, Muir GH. Laparoscopic partial nephrectomy using the potassium titanyl phosphate laser in a porcine model. Urology. 2006;67:1079–83. doi: 10.1016/j.urology.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 108.Moinzadeh AG, Rubenstein M, Ukimura O, Aron M, Spaliviero M, Nahen K, et al. Potassium-titanyl-phosphate laser laparoscopic partial nephrectomy without hilar clamping in the survival calf model. J Urol. 2005;174:1110–4. doi: 10.1097/01.ju.0000168620.36893.6c. [DOI] [PubMed] [Google Scholar]
- 109.Liu MR, Zhu G, Petersen A, Muir GH, Poulson J. Laparoscopic partial nephrectomy with saline-irrigated KTP laser in a porcine model. J Endourol. 2006;20:1096–100. doi: 10.1089/end.2006.20.1096. [DOI] [PubMed] [Google Scholar]
- 110.Honeck P, Wendt-Nordahl G, Bolenz C, Peters T, Weiss C, Alken P, Michel MS, Häcker A. Hemostatic properties of four devices for partial nephrectomy: a comparative ex vivo study. J Endourol. 2008 May;22(5):1071–6. doi: 10.1089/end.2007.0236. [DOI] [PubMed] [Google Scholar]
- 111.Bui MH, Gui D, Said J, Schulam P. Less smoke and minimal tissue carbonization using a thulium laser for laparoscopic partial nephrectomy without hilar clamping in a porcine model. J Endourol. 2007;21:1107–11. doi: 10.1089/end.2006.0440. [DOI] [PubMed] [Google Scholar]
- 112.Lotan YG, Ogan K, Baker LA, Cadeddu JA. Clinical use of the holmium:YAG laser in laparoscopic partial nephrectomy. J Endourol. 2002;16:289–92. doi: 10.1089/089277902760102767. [DOI] [PubMed] [Google Scholar]
- 113.Hsu TH, Gill IS. Radiofrequency ablation of the kidney: Acute and chronic histology in porcine model. Urology. 2000;56:872–5. doi: 10.1016/s0090-4295(00)00737-8. [DOI] [PubMed] [Google Scholar]
- 114.Gill IS, Fox RL, Matamoros A, Miller CD, Leveen RF, Grune MT, et al. Laparoscopic and percutaneous radiofrequency ablation of the kidney: Acute and chronic porcine study. Urology. 2000;56:197–200. doi: 10.1016/s0090-4295(00)00607-5. [DOI] [PubMed] [Google Scholar]
- 115.Gettman MT, Su LM, Chan D, Kavoussi LR, Jarrett TW, Cadeddu JA. Hemostatic laparoscopic partial nephrectomy: Initial experience with the radiofrequency coagulation-assisted technique. Urology. 2001;58:8–11. doi: 10.1016/s0090-4295(01)01086-x. [DOI] [PubMed] [Google Scholar]
- 116.Jacomides LO, Watumull L, Cadeddu JA. Laparoscopic application of radio frequency energy enables in situ renal tumor ablation and partial nephrectomy. J Urol. 2003;169:49–53. doi: 10.1016/S0022-5347(05)64032-3. [DOI] [PubMed] [Google Scholar]
- 117.Zeltser IS, Park S, Anderson JK, Cadeddu JA. Intermediate-term prospective results of radiofrequency-assisted laparoscopic partial nephrectomy: A non-ischaemic coagulative technique. BJU Int. 2008;101:36–8. doi: 10.1111/j.1464-410X.2007.07176.x. [DOI] [PubMed] [Google Scholar]
- 118.Elashry OM, Rayala HJ, McDougall EM, Clayman RV. Recent advances in laparoscopic partial nephrectomy: Comparative study of electrosurgical snare electrode and ultrasound dissection. J Endourol. 1997;11:15–22. doi: 10.1089/end.1997.11.15. [DOI] [PubMed] [Google Scholar]
- 119.Collyer WC, Olweny EO, Andreoni C, Kibel A, Andriole GL, Bostwick DG, et al. Laparoscopic partial nephrectomy with a novel electrosurgical snare in a porcine model. J Endourol. 2002;16:673–9. doi: 10.1089/089277902761403032. [DOI] [PubMed] [Google Scholar]
- 120.Rais-Bahrami SL, Varkarakis IM, Romero FR, Trock B, Jarrett TW, Kavoussi LR. Intraoperative conversion of laparoscopic partial nephrectomy. J Endourol. 2006;20:205–8. doi: 10.1089/end.2006.20.205. [DOI] [PubMed] [Google Scholar]
- 121.Lai FC, Ng CS, Fuchs GJ. Laparoscopic nephrectomy outcomes of elderly patients in the 21st century. J Endourol. 2007;21:1309–13. doi: 10.1089/end.2007.9885. [DOI] [PubMed] [Google Scholar]
- 122.Anast JW, Meng MV, Master VA, Mitchell JA, Bassett WW, Kane CJ. Differences in complications and outcomes for obese patients undergoing laparoscopic radical, partial or simple nephrectomy. J Urol. 2004;172:2287–91. doi: 10.1097/01.ju.0000143820.56649.a4. [DOI] [PubMed] [Google Scholar]
- 123.Colombo JR, Jr, Aron M, Xu M, Gill IS. Laparoscopic partial nephrectomy in obese patients. Urology. 2007;69:44–8. doi: 10.1016/j.urology.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 124.Romero FR, Muntener M, Brito FA, Jarrett TW, Kavoussi LR. Laparoscopic partial nephrectomy in obese and non-obese patients: Comparison with open surgery. Urology. 2008;71:806–9. doi: 10.1016/j.urology.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 125.Leibovich BC, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066–70. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 126.Patard JJ, Lam JS, Pantuck AJ, Kim HL, Ficarra V, Cindolo L, et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171:2181–5. doi: 10.1097/01.ju.0000124846.37299.5e. [DOI] [PubMed] [Google Scholar]
- 127.Patard JJ, Crepel M, Lam JS, Bellec L, Albouy B, Lopes D, et al. Morbidity and clinical outcome of nephron-sparing surgery in relation to tumour size and indication. Eur Urol. 2007;52:148–54. doi: 10.1016/j.eururo.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 128.Ukimura OH, Remer EM, Gill IS. Laparoscopic partial nephrectomy for incidental stage pT2 or worse tumors. Urology. 2006;68:976–82. doi: 10.1016/j.urology.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 129.Porpiglia FV, Billia M, Scarpa RM. Laparoscopic versus open partial nephrectomy: Analysis of the current literature. Eur Urol. 2008;53:732–42. doi: 10.1016/j.eururo.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 130.Frank IC, Rubinstein M, Desai M, Kaouk J, Gill IS. Laparoscopic partial nephrectomy for centrally located renal tumors. J Urol. 2006;175:849–52. doi: 10.1016/S0022-5347(05)00346-0. [DOI] [PubMed] [Google Scholar]
- 131.Gill IS, Frank I, Moinzadeh A, Kaouk J, Desai M. Laparoscopic partial nephrectomy for hilar tumors. J Urol. 2005;174:850–3. doi: 10.1097/01.ju.0000169493.05498.c3. [DOI] [PubMed] [Google Scholar]
- 132.Reisiger KV, Figenshau RS, Bae KT, Landman J. Complex laparoscopic partial nephrectomy for renal hilar tumors. Urology. 2005;65:888–91. doi: 10.1016/j.urology.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 133.Lattouf JB, D’Ambros OF, Grüll M, Leeb K, Janetschek G. Laparoscopic partial nephrectomy for hilar tumors: Technique and results. Eur Urol. 2008 doi: 10.1016/j.eururo.2008.04.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 134.Richstone LM, Ost M, Reggio E, Permpongkosol S, Kavoussi LR. Laparoscopic partial nephrectomy for hilar tumors: Evaluation of short-term oncologic outcome. Urology. 2008;71:36–40. doi: 10.1016/j.urology.2007.09.062. [DOI] [PubMed] [Google Scholar]
- 135.Weight CJ, Gill IS. Laparoscopic partial nephrectomy for selected central tumours: Omitting the bolster. BJU Int. 2007;100:375–8. doi: 10.1111/j.1464-410X.2007.06928.x. [DOI] [PubMed] [Google Scholar]
- 136.Gill IS, Moinzadeh A, Finelli A, Ukimura O, Tucker K, Kaouk J, et al. Laparoscopic partial nephrectomy in solitary kidney. J Urol. 2006;175:454–8. doi: 10.1016/S0022-5347(05)00150-3. [DOI] [PubMed] [Google Scholar]
- 137.Turna BF, Kamoi K, Lin YC, Aron M, Desai MM, Kaouk JH, et al. Risk factor analysis of postoperative complications in laparoscopic partial nephrectomy. J Urol. 2008;179:1289–94. doi: 10.1016/j.juro.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 138.Marberger MP, Auvert J, Bertermann H, Costantini A, Gammelgaard PA, Petterson S, et al. Conservation surgery of renal carcinoma: The EIRSS experience. Br J Urol. 1981;53:528–32. doi: 10.1111/j.1464-410x.1981.tb03254.x. [DOI] [PubMed] [Google Scholar]
- 139.Moll VB, Ziegler M. Kidney preserving surgery in renal cell tumors: Indications, techniques and results in 152 patients. J Urol. 1993;150:319–23. doi: 10.1016/s0022-5347(17)35471-x. [DOI] [PubMed] [Google Scholar]
- 140.Polascik TJ, Meng MV, Partin AW, Marshall FF. Partial nephrectomy: Technique, complications and pathological findings. J Urol. 1995;154:1312–8. doi: 10.1016/s0022-5347(01)66845-9. [DOI] [PubMed] [Google Scholar]
- 141.Van Poppel HB, Oyen R, Baert L. Partial nephrectomy for renal cell carcinoma can achieve long-term tumor control. J Urol. 1998;160:674–8. doi: 10.1016/S0022-5347(01)62751-4. [DOI] [PubMed] [Google Scholar]
- 142.Stephenson AJ, Snyder ME, Russo P. Complications of radical and partial nephrectomy in a large contemporary cohort. J Urol. 2004;171:130–4. doi: 10.1097/01.ju.0000101281.04634.13. [DOI] [PubMed] [Google Scholar]
- 143.Shekarriz BU, Shekarriz H, de Assis Mendes Goes F, Jr, Bianco FJ, Tiguert R, Gheiler E, et al. Comparison of costs and complications of radical and partial nephrectomy for treatment of localized renal cell carcinoma. Urology. 2002;59:211–5. doi: 10.1016/s0090-4295(01)01514-x. [DOI] [PubMed] [Google Scholar]
- 144.Thompson RH, Lohse CM, Zincke H, Blute ML. Complications of contemporary open nephron sparing surgery: A single institution experience. J Urol. 2005;174:855–8. doi: 10.1097/01.ju.0000169453.29706.42. [DOI] [PubMed] [Google Scholar]
- 145.Fergany AF, Woo L, Novick AC. Open partial nephrectomy for tumor in a solitary kidney: Experience with 400 cases. J Urol. 2006;175:1630–3. doi: 10.1016/S0022-5347(05)00991-2. [DOI] [PubMed] [Google Scholar]
- 146.Ray ER, Singh R, Chandra A, Cranston DW, O’Brien TS. Open partial nephrectomy: Outcomes from two UK centres. BJU Int. 2006;97:1211–5. doi: 10.1111/j.1464-410X.2006.06093.x. [DOI] [PubMed] [Google Scholar]
- 147.Haber GP GI. Laparoscopic partial nephrectomy: Contemporary technique and outcomes. Eur Urol. 2006;49:660–5. doi: 10.1016/j.eururo.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 148.Gill IS, Lane BR, Blute ML, Babineau D, Colombo JR, Jr, Frank I, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–6. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 149.Lane BR, Babineau D, Fergany AF, Kaouk JH, Gill IS. Comparison of laparoscopic and open partial nephrectomy for tumor in a solitary kidney. J Urol. 2008;179:847–51. doi: 10.1016/j.juro.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 150.Belldegrun AT, deKernion JB, Smith RB. Efficacy of nephron-sparing surgery for renal cell carcinoma: Analysis based on the new 1997 tumor-node-metastasis staging system. J Clin Oncol. 1999;17:2868–75. doi: 10.1200/JCO.1999.17.9.2868. [DOI] [PubMed] [Google Scholar]
- 151.Stifelman MD, Nakada SY, Shichman SJ. Hand-assisted laparoscopic partial nephrectomy. J Endourol. 2001;15:161–4. doi: 10.1089/089277901750134467. [DOI] [PubMed] [Google Scholar]
- 152.Hamasaki TK, Matuzawa I, Tsuboi N, Nishimura T. Laparoscopic partial nephrectomy using a microwave tissue coagulator for treating small peripheral renal tumors. J Nippon Med Sch. 2004;71:392–8. doi: 10.1272/jnms.71.392. [DOI] [PubMed] [Google Scholar]
- 153.Brown JA, Gomella LG, Strup SE. Hand assisted laparoscopic partial nephrectomy for peripheral and central lesions: A review of 30 consecutive cases. J Urol. 2004;171:1443–6. doi: 10.1097/01.ju.0000117962.54732.3e. [DOI] [PubMed] [Google Scholar]
- 154.Schiff JD, Vaughan ED, Jr, Sosa RE, Coll D, Del Pizzo JJ. Laparoscopic vs open partial nephrectomy in consecutive patients: The Cornell experience. BJU Int. 2005;96:811–4. doi: 10.1111/j.1464-410X.2005.05718.x. [DOI] [PubMed] [Google Scholar]
- 155.Phillips CK, Stifelman MD. Robot-assisted laparoscopic partial nephrectomy: The NYU technique. J Endourol. 2005;19:441–5. doi: 10.1089/end.2005.19.441. [DOI] [PubMed] [Google Scholar]
- 156.Stifelman MD, Nieder AM, Taneja SS. Robot-assisted laparoscopic partial nephrectomy. JSLS. 2005;9:83–6. [PMC free article] [PubMed] [Google Scholar]
- 157.Caruso RP, Kau E, Taneja SS, Stifelman MD. Robot assisted laparoscopic partial nephrectomy: Initial experience. J Urol. 2006;176:36–9. doi: 10.1016/S0022-5347(06)00499-X. [DOI] [PubMed] [Google Scholar]
- 158.Gettman MT, Chow GK, Neururer R, Bartsch G, Peschel R. Robotic-assisted laparoscopic partial nephrectomy: Technique and initial clinical experience with DaVinci robotic system. Urology. 2004;64:914–8. doi: 10.1016/j.urology.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 159.Kaul SL, Sarle R, Stricker H, Peabody J, Littleton R, Menon M. da Vinci-assisted robotic partial nephrectomy: Technique and results at a mean of 15 months of follow-up. Eur Urol. 2007;51:186–91. doi: 10.1016/j.eururo.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 160.Rogers CG, Blatt AM, Linehan WM, Pinto PA. Robotic partial nephrectomy for complex renal tumors: Surgical technique. Eur Urol. 2008;53:514–21. doi: 10.1016/j.eururo.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Deane LA, Box GN, Melamud O, Yee DS, Abraham JB, Finley DS, et al. Robotic versus standard laparoscopic partial/wedge nephrectomy: A comparison of intraoperative and perioperative results from a single institution. J Endourol. 2008;22:947–52. doi: 10.1089/end.2007.0376. [DOI] [PubMed] [Google Scholar]
- 162.Aron MK, Kaouk JH, Nguyen MM, Desai MM, Gill IS. Robotic and laparoscopic partial nephrectomy: A matched-pair comparison from a high-volume centre. BJU Int. 2008 doi: 10.1111/j.1464-410X.2008.07580.x. [DOI] [PubMed] [Google Scholar]
- 163.Aron M, Canes D, Desai MM, Haber GP, Kaouk JH, Gill IS. Transumbilical single-port laparoscopic partial nephrectomy. BJU Int. 2009;103:516–21164. doi: 10.1111/j.1464-410X.2008.08007.x. [DOI] [PubMed] [Google Scholar]
- 164.Kaouk JH, Goel RK. Single-port laparoscopic and robotic partial nephrectomy 2009;55:1163-9. Eur Urol. 2009;55:1163–9. doi: 10.1016/j.eururo.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 165.Brandina R, Aron M. Laparoscopic partial nephrectomy: Advances since 2005. Curr Opin Urol. 2010;20:111–8. doi: 10.1097/MOU.0b013e3283362624. [DOI] [PubMed] [Google Scholar]


