Abstract
STUDY OBJECTIVE:
To evaluate the effects of dexmedetomidine on hypoxic pulmonary vasoconstriction (HPV) and oxygenation during one-lung ventilation (OLV) in adults undergoing thoracic surgery.
DESIGN:
Prospective, randomized, double-blinded trial.
SETTING:
Tertiary care, University-based hospital.
PATIENTS:
Nineteen adult patients undergoing thoracic surgery requiring OLV.
INTERVENTIONS:
During inhalational anesthesia with desflurane, patients were randomized to receive either dexmedetomidine (bolus dose of 0.3 μg/kg followed by an infusion of 0.3 μg/kg/hr) or saline placebo.
MEASUREMENTS:
Three arterial blood gas samples (ABG) were obtained to evaluate the effects of dexmedetomidine on oxygenation. Secondary outcomes included differences in hemodynamic parameters (heart rate and mean arterial pressure), end-tidal desflurane concentration required to maintain the bispectral index (BIS) at 40-60, supplemental fentanyl to maintain hemodynamic stability, and phenylephrine to keep the mean arterial pressure (MAP) within 10% of baseline values.
MAIN RESULTS:
Oxygenation during OLV did not change following the administration of dexmedetomidine (PaO2/FiO2 ratio of 188 ± 115 in dexmedetomidine patients versus 135 ± 70 mmHg in placebo patients). There were no differences in hemodynamic variables or depth of anaesthesia between the two groups. With the administration of dexmedetomidine, there was a decrease in the expired concentration of desflurane required to maintain the BIS at 40-60 when compared with the control group (4.5 ± 0.8% versus 5.1 ± 0.8%). In patients receiving dexmedetomidine, fentanyl requirements were decreased when compared to placebo (2.7 μg/kg/patient versus 3.1 μg/kg/patient). However, more patients receiving dexmedetomidine required phenylephrine to maintain hemodynamic stability (6 of 9 patients versus 3 of 10 patients) and the total dose of phenylephrine was greater in patients receiving dexmedetomidine when compared with placebo 10.3 μg/kg/patient versus 1.1 μg/kg/patient).
CONCLUSION:
Dexmedetomidine does not adversely affect oxygenation during OLV in adults undergoing thoracic surgical procedures. The improvement in oxygenation in the dexmedetomidine patients may be related to a decrease in the requirements for inhalational anaesthetic agents thereby limiting its effects on HPV.
Keywords: Dexmedetomidine, hypoxic pulmonary vasoconstriction, one-lung ventilation, thoracoscopy, thoracotomy
INTRODUCTION
One lung ventilation (OLV) is commonly used during open and endoscopic thoracic surgery to improve surgical exposure. Hypoxic pulmonary vasoconstriction (HPV) allows for shunting of blood away from the non-ventilated lung to the non-operative and thus allows for the maintenance of adequate oxygenation.[1,2] The potent inhalational anaesthetics have been shown to inhibit HPV in a dose-dependent manner and thereby alter oxygenation during OLV. In comparison, intravenous anaesthetic agents such as propofol or ketamine have little effect or may actually potentiate HPV and thereby improve oxygenation.[3–5]
Dexmedetomidine is an α2-adrenergic receptor agonist of the imidazole subclass which has seen increasing use both in the intensive care unit and also perioperatively as part of a balanced anaesthetic technique. Dexmedetomidine's effect on the vasculature may be variable with the potential to cause both vasoconstriction through α-2B adrenergic receptor activation on vascular smooth muscle or vasodilation through activation of central α-2 adrenergic receptors resulting in sympatholysis.[6,7] Because of these complex effects on the vasculature, it would be difficult to predict the effects of dexmedetomidine on HPV. As there are currently no data regarding the effects of dexmedetomidine on HPV and its effects on oxygenation during OLV, we prospectively evaluated its impact on oxygenation in a cohort of adult patients requiring OLV for thoracic surgical procedures.
MATERIALS AND METHODS
This was a single-centre, prospective, randomized, double-blinded, placebo-controlled study. This study was approved by the Institutional Review Board of the University of Missouri. It was registered at https://registerclinicaltrials.gov as study number NCT00839605. Written informed consent was obtained from the patients. After informed consent was obtained, a peripheral intravenous cannula was placed and premedication provided with intravenous midazolam (2 mg). All patients were monitored using standard American Society of Anesthesiologists’ monitoring including continuous five-lead electrocardiography, pulse oximetry, non-invasive blood pressure, end-tidal CO2, and temperature. An arterial cannula was placed in either the right or left radial artery. Prior to anaesthetic induction, a thoracic epidural catheter was placed for postoperative pain management, but was not dosed intraoperatively. Anaesthetic induction included intravenous propofol (2-3 mg/kg) followed by cis-atracurium (0.2 mg/kg) to facilitate endotracheal intubation. Maintenance anaesthesia consisted of desflurane titrated to achieve a BIS of 40-60 and fentanyl (2-5 μg/kg) titrated to maintain hemodynamic stability. Lung isolation was performed using either a double lumen endotracheal tube or a bronchial blocker.
Both the patient and the anaesthesiologist were blinded to the identity of the study drug (dexmedetomidine or saline placebo). Randomization was performed via the pharmacy using sealed envelopes. Study drug or placebo was obtained from the pharmacy in a 50 mL bag without identification marked. Dexmedetomidine was prepared as a 4 μg/mL solution. After obtaining the second arterial blood gas (see below), a bolus of 0.075 mL/kg of the solution (0.3 μg/kg of dexmedetomidine or placebo) was administered intravenously over 10 min. After completion of the bolus dose, a continuous infusion of dexmedetomidine or placebo was started at 0.075 mL/kg/h of the solution (0.3 μg/kg/h). The infusion was continued throughout the operative procedure.
Three arterial blood gas samples (ABG) were obtained during the study protocol. The first ABG was obtained 10 min after anaesthetic induction during two-lung ventilation prior to the administration of dexmedetomidine. After positioning the patient in the lateral decubitus position, the operative lung was dropped and OLV started. During OLV, ventilatory management included an FiO2 to maintain oxygen saturation by pulse oximetry ≥ 93%, positive end expiratory pressure of 4 cmH20, peak inflating pressure of 20 cmH2O, I:E ratio of 1:2, and a respiratory rate adjusted to maintain normocarbia (PaCO2 35-40 mmHg). After 10 min of OLV and prior to the start of dexmedetomidine, the second ABG was drawn. The third arterial blood gas was drawn 15 min after completion of the study drug bolus while the continuous infusion was being administered. In addition to evaluating changes in oxygenation during OLV ventilation based on the PaO2/FiO2 ratio, secondary outcomes evaluated between the dexmedetomidine and placebo groups included differences in hemodynamic parameters (heart rate and mean arterial pressure), end-tidal desflurane concentration required to maintain the bispectral index (BIS) at 40-60, supplemental fentanyl to maintain hemodynamic stability, and phenylephrine used to treat hypotension and keep the mean arterial pressure (MAP) within 10% of baseline values.
The demographic data (age and weight) of the two groups were compared using a non-paired t-test for parametric data, while a chi-squared analysis with a contingency table was used for gender. An analysis of variance was used to compare the 3 ABG values that were obtained during the study protocol. A non-paired t-test was used to compare the PaO2/FiO2 PaO2 ratio and the secondary outcomes (desflurane, fentanyl, and phenylephrine use) between patients receiving dexmedetomidine and those receiving placebo. A chi-squared analysis with a contingency table was used to compare the number of patients in each group that required any bolus doses of phenylephrine to maintain hemodynamic stability. Non-parametric data (BIS values) were compared using a Mann-Whitney U test. Parametric data are presented as the mean ± SD, while non-parametric data are presented as the median and the interquartile ranges. A power analysis to determine the sample size needed to detect a 20% difference in oxygenation (PaO2/FiO2) values between the two groups was performed. Assuming a P value of <0.05 as significant and a minimal determined difference of 25% difference, the sample size required was 15.
RESULTS
The study cohort included 19 adult patients who required OLV for thoracic surgery with 9 patients in group A (dexmedetomidine) and 10 patients in group B (placebo). There were no differences in the demographic data of the two groups [Table 1]. The data from the three ABGs are listed in Table 2. No difference was noted in oxygenation during two-lung and one-lung ventilation prior to the administration of dexmedetomidine (ABG numbers 1 and 2). Although it did not reach a statistical significance, oxygenation expressed as the PaO2/FiO2 ratio was greater during OLV in patients receiving dexmedetomidine when compared with placebo (188 ± 115 versus 135 ± 70). The hemodynamic and anaesthetic data are listed in Table 3. No significant difference in hemodynamic variables (HR and MAP) or depth of anaesthesia as measured by the BIS was noted between the two groups at the time that the three ABGs were drawn. With the administration of dexmedetomidine, there was a decrease in the expired concentration of desflurane required to maintain the bispectral index at 40-60 when compared with the control group (4.5 ± 0.8% versus 5.1 ± 0.8%). The fentanyl and phenylephrine requirements between the two groups are listed in Table 4. In patients receiving dexmedetomidine, fentanyl requirements were decreased when compared to placebo (2.7 μg/kg versus 3.1 μg/kg, p=NS). However, more patients receiving dexmedetomidine required phenylephrine to maintain hemodynamic stability (6 of 9 patients versus 3 of 10 patients, P<0.05) and the total dose of phenylephrine was greater in patients receiving dexmedetomidine when compared with placebo (10.3 μg/kg versus 1.1 μg/kg, P<0.05).
Table 1.
Patient demographics

Table 2.
Arterial blood gas data
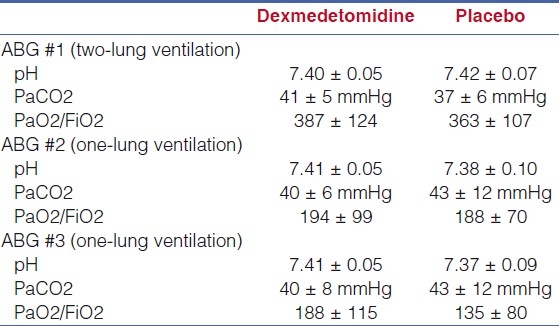
Table 3.
Hemodynamic, bispectral index, and anesthetic data*
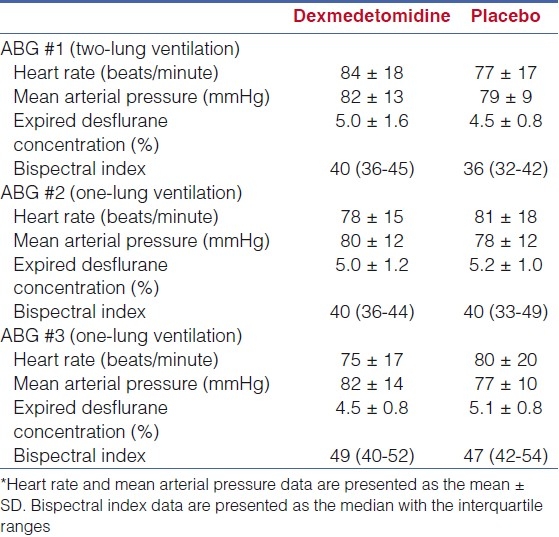
Table 4.
Fentanyl and phenylephrine data

DISCUSSION
With newer thoracic procedures including thoracoscopy, effective OLV has become mandatory to facilitate completion of the procedure and avoid the need for open thoracotomy. OLV is performed using specialized double-lumen endotracheal tubes which provide the ability to ventilate only one lung or by placement of a bronchial blocker which occludes the mainstem bronchus of the operative side. During OLV, HPV shunts blood away from the surgical (non-ventilated) lung to the non-operative lung which is providing oxygenation and ventilation. Even in the presence of effective HPV, intrapulmonary shunting still occurs, resulting in alterations in systemic oxygenation. Agents with vasodilatory properties such as nitroglycerin, sodium nitroprusside, and the potent inhalational anesthetic agents have been shown to attenuate HPV and potentiate intravascular shunting thereby impairing oxygenation. Alternatively, agents with vasoconstrictive properties such as almitrine, a direct acting α-adrenergic agonist, may augment HPV and improve oxygenation in certain clinical scenarios.[8,9]
In healthy adult volunteers, there is a biphasic effect following dexmedetomidine with an initial increase in systolic blood pressure (sBP) and a reflex decrease in HR followed by a stabilization of sBP and HR at a value below the baseline.[6] Stimulation of peripheral postsynaptic α2B-adrenergic receptors results in vasoconstriction and the initial increase in systolic BP, while the eventual decrease in BP and HR results from central presynaptic α2A-adrenergic receptor stimulated sympatholysis.[7] Biochemical data from a cohort of eight adult postoperative patients demonstrates the sympatholytic effects of dexmedetomidine.[10] Following a 60 mindexmedetomidine infusion administered by a computer-controlled infusion protocol to achieve a plasma concentration of 600 pg/mL, the plasma norepinephrine concentration decreased from 2.1 ± 0.8 to 0.7 ± 0.3 nmol/L, and the plasma epinephrine concentration decreased from 0.7 ± 0.5 to 0.2 ± 0.2 nmol/L. Associated hemodynamic changes included a HR decrease from 76 ± 15 to 64 ± 11 beats/minute and a sBP decrease from 158 ± 23 to 140 ± 23 mmHg. The same investigators evaluated changes in plasma and urinary catecholamines in 41 adult patients undergoing vascular surgery.[11] Dexmedetomidine was started intraoperatively and continued for the first 48 postoperative hours. When compared to patients receiving dexmedetomidine, plasma norepinephrine concentrations were two to three times higher at the time of tracheal extubation and at 60 min after arrival in the post-anaesthesia care unit (PACU) in the control group. Urinary normetanephrine levels increased significantly in the placebo group while no change was noted in patients receiving dexmedetomidine. These sympatholytic properties may be expected to inhibit HPV and worsen oxygenation by increasing ventilation-perfusion inequalities.
Alternatively, in an animal model, it has been demonstrated that dexmedetomidine may also lead to transient increases in pulmonary artery pressure related to its direct effects on vascular smooth muscle through α-adrenergic receptors.[12] In six instrumented sheep, dexmedetomidine (2 μg/kg over 1 minute) transiently increased mean pulmonary artery pressure (MPAP) and PVR.[10] PVR increased from a baseline of 81 ± 16 dynes/s/cm5 to a maximum of 141 ± 27 dynes/s/cm5, while MPAP increased from 15 ± 1 to 18 ± 0 mmHg. MAP also increased (86 ± 2 to 93 ± 6 mmHg) as did systemic vascular resistance (1416 ± 83 to 1889 ± 64 dynes/s/cm5). Similar transient pulmonary hemodynamic changes have been reported in healthy human volunteers with graded dexmedetomidine infusions to a plasma concentration of 1.9 ng/mL.[7]
Given the complex hemodynamic effects of dexmedetomidine which may be modified by the patient's co-morbid cardiovascular issues, it may be difficult to predict its effects on HPV. In our cohort of adult patients, we noted no clinically significant effect of a dexmedetomidine loading dose and infusion during OLV. Although the difference in oxygenation did not reach statistical significance, there was a higher PaO2/FiO2 ratio in patients receiving dexmedetomidine thereby further demonstrating the lack of effect on HPV and oxygenation. The improved oxygenation with dexmedetomidine may have resulted from the direct effects of dexmedetomidine on HPV or more likely the anaesthetic-sparing effect of dexmedetomidine thereby allowing for a decrease in the concentration of desflurane and hence a lessening of its effects on HPV.
Although generally well-tolerated in the absence of co-morbid cardiovascular disease, bradycardia and hypotension have been reported in the adult population. In our cohort of adult patients, given their co-morbid conditions and the potential for adverse effects on cardiovascular function especially during the administration of a loading dose, we chose to use a low loading dose and infusion rate on the lower end of the dosing regimens used in clinical anaesthesia practice (loading dose of 0.3 μg/kg and an infusion of 0.3 μg/kg/h). It is feasible that our findings did not reach a statistical significance given the use of this low dose regimen and future studies with higher doses may be warranted. However, even with this low dose regimen, in our population, although the hemodynamic changes were easily controlled by decreasing the desflurane concentration or the administration of phenylephrine, it is worth noting that more patients receiving dexmedetomidine required phenylephrine and the doses required were higher to maintain the MAP within 10% of baseline values.
Given its beneficial physiologic properties, there has been an increasing use of dexmedetomidine in various perioperative scenarios. In thoracic surgery, effective analgesia facilitates fast-tracking with earlier extubation and a decreased incidence of perioperative complications. A major concern with the use of any anaesthetic agent during OLV is its potential impact on HPV and oxygenation during OLV. In the current study, we noted no detrimental effect of the administration of dexmedetomidine on oxygenation. In fact, using dexmedetomidine as part of a balanced anaesthetic technique may actually improve oxygenation by allowing a lower concentration of the inhaled agent thereby limiting its effects on oxygenation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Karzai W, Schwarzkopf K. Hypoxemia during one-lung ventilation. Anesthesiology. 2009;110:402–11. doi: 10.1097/ALN.0b013e31819fb15d. [DOI] [PubMed] [Google Scholar]
- 2.Campos JH. Current techniques for perioperative lung isolation in adults. Anesthesiology. 2002;97:1295–301. doi: 10.1097/00000542-200211000-00036. [DOI] [PubMed] [Google Scholar]
- 3.Benumof JF, Augustine SD, Gibbons JA. Halothane and isoflurane only slightly impair arterial oxygenation during one-lung ventilation in patients undergoing thoracotomy. Anesthesiology. 1987;67:910–4. doi: 10.1097/00000542-198712000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Wang JY, Russell GN, Page RD, Oo A, Pennefather SH. A comparison of the effects of desflurane and isoflurane on arterial oxygenation during one-lung ventilation. Anaesthesia. 2000;55:167–73. doi: 10.1046/j.1365-2044.2000.055002167.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakayama M, Murray PA. Ketamine preserves and propofol potentiates hypoxic pulmonary vasoconstriction compared with the conscious state in chronically instrumented dogs. Anesthesiology. 1999;91:760–71. doi: 10.1097/00000542-199909000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–42. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 7.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans.II: Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Moutafis M, Dalibon N, Colchen A, Fischler M. Improving oxygenation during bronchopulmonary lavage using nitric oxide inhalation and almitrine infusion. Anesth Analg. 1999;89:302–4. doi: 10.1097/00000539-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Silva-Costa-Gomes T, Gallart L, Vallès J, Trillo L, Minguella J, Puig MM. Low- vs high-dose almitrine combined with nitric oxide to prevent hypoxia during open-chest one-lung ventilation. Br J Anaesth. 2005;95:410–6. doi: 10.1093/bja/aei194. [DOI] [PubMed] [Google Scholar]
- 10.Talke P, Richardson CA, Scheinin M, Fisher DM. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Analg. 1997;85:1136–42. doi: 10.1097/00000539-199711000-00033. [DOI] [PubMed] [Google Scholar]
- 11.Talke P, Chen R, Thomas B, Aggarwall A, Gottlieb A, Thorborg P, et al. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg. 2000;90:834–9. doi: 10.1097/00000539-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Kästner SB, Kull S, Kutter AP, Boller J, Bettschart-Wolfensberger R, Huhtinen MK. Cardiopulmonary effects of dexmedetomidine in sevoflurane-anesthetized sheep with and without nitric oxide inhalation. Am J Vet Res. 2005;66:1496–502. doi: 10.2460/ajvr.2005.66.1496. [DOI] [PubMed] [Google Scholar]


