Abstract
Antibiotic resistance, a global concern, is particularly pressing in developing nations, including India, where the burden of infectious disease is high and healthcare spending is low. The Global Antibiotic Resistance Partnership (GARP) was established to develop actionable policy recommendations specifically relevant to low- and middle-income countries where suboptimal access to antibiotics - not a major concern in high-income countries - is possibly as severe a problem as is the spread of resistant organisms. This report summarizes the situation as it is known regarding antibiotic use and growing resistance in India and recommends short and long term actions. Recommendations aim at (i) reducing the need for antibiotics; (ii) lowering resistance-enhancing drug pressure through improved antibiotic targeting, and (iii) eliminating antibiotic use for growth promotion in agriculture. The highest priority needs to be given to (i) national surveillance of antibiotic resistance and antibiotic use - better information to underpin decisions on standard treatment guidelines, education and other actions, as well as to monitor changes over time; (ii) increasing the use of diagnostic tests, which necessitates behavioural changes and improvements in microbiology laboratory capacity; (iii) setting up and/or strengthening infection control committees in hospitals; and (iv) restricting the use of antibiotics for non-therapeutic uses in agriculture. These interventions should help to reduce the spread of antibiotic resistance, improve public health directly, benefit the populace and reduce pressure on the healthcare system. Finally, increasing the types and coverage of childhood vaccines offered by the government would reduce the disease burden enormously and spare antibiotics.
Keywords: Agriculture, antibiotic resistance, healthcare access, health policy, hospital, acquired infection, infection control, vaccination, veterinary use
Introduction
Antimicrobial resistance, a global problem, is particularly pressing in developing countries where the infectious disease burden is high and cost constrains the replacement of older antibiotics with newer, more expensive ones. Management of common and lethal bacterial infections has been critically compromised by the appearance and rapid spread of antibiotic-resistant bacteria. The Global Antibiotic Resistance Partnership (GARP) was established to begin the process of developing actionable policy recommendations relevant to low- and middle-income countries. Multidisciplinary working groups in India, Kenya, South Africa, and Vietnam took up this challenge in 2009 and have surveyed the state of antibiotic use and resistance and related factors (cddep.org). In light of this evidence, working groups have begun to define a set of broad policy objectives. This report summarises the India Situation Analysis of 2011 - the most comprehensive survey of undertaken in India - and recommends next steps.
The bacterial disease burden in India is among the highest in the world1; consequently, antibiotics will play a critical role in limiting morbidity and mortality in the country. As a marker of disease burden, pneumonia causes an estimated 410,000 deaths in India each year2, and it is the number-one killer of children3. Many of these deaths occur because patients do not have access to life-saving antibiotics when and where these are needed. At the other extreme, antibiotics are used in situations where these cannot be expected to improve the patient's condition, particularly as treatment for the common cold and uncomplicated cases of diarrhoea (which are appropriately treated with oral rehydration therapy).
‘Drug selection pressure’ is the single most important factor in the evolution of drug resistance in bacteria. The reasons for drug pressure are multifactorial and involve both human and animal use. Although drug resistance is primarily a medical problem, the factors that influence the spread of resistance are ecological, epidemiological, cultural, social, and economic. Patients, physicians, veterinarians, and healthcare facilities and retailers - from large pharmacies to local drug sellers - have little motivation (economic or otherwise) to acknowledge the consequences of their use of antibiotics on others, especially on future generations.
Every time an antibiotic is used - whether appropriately or not, in human beings or in animals- the probability of the development and spread of antibiotic-resistant bacteria is increased4,5. Antibiotic effectiveness is a globally shared resource and a shared responsibility. That responsibility is to maintain antibiotic effectiveness as long as possible, while allowing the maximum possible health benefits to accrue to the world's population. The actions needed to achieve this goal cannot be decided globally. Each nation must adopt strategies tailored to its own conditions. The GARP working groups’ recommendations are based on an understanding of the underlying issues, and the proposed solutions are designed to work in the 21st century.
CURRENT SITUATION IN INDIA
Rising antibiotic use
Antibiotic use has been increasing steadily in recent years (Fig. 1). Between 2005 and 2009, the units of antibiotics sold increased by about 40 per cent. Increased sales of cephalosporins were particularly striking, with sales (in units sold) increasing by 60 per cent over that five-year period, but some increase was seen in most antibiotic classes. In comparison, a pilot survey conducted at private retail pharmacies in 20046 and a survey in the same areas in 2008 found increased use of cephalosporins, but decreased use of macrolides7.
Fig. 1.

Units of antibiotics sold in India, by type. Source: Ref. 8.
The fact that antibiotic use is increasing is not, itself, indicative of a problem, but evidence from studies of prescribing patterns suggests that antibiotics are often used in inappropriate ways.
Resistance to antibiotics
Antibiotic resistance has been a low-priority area in most developing and many developed countries. Compared with the immediate challenges of HIV/AIDS, tuberculosis, malaria, pneumonia, and many other infectious diseases, the loss of antibiotics at some future time does not capture the same attention. Resistance against certain antibiotics is already at high levels in certain places in India (and around the world), but the problem has remained largely unknown because relatively few studies were published and nationwide surveillance was not being carried out. But the issue came to the fore in India when New Delhi metallo-ß-lactamase-1 (NDM-1), first reported in 2009, made front-page news in 2010.
Briefly, NDM-1 is an enzyme produced by the gene blaNDM-1; it is named for New Delhi because the Swedish patient in whom it was first identified had undergone surgery in a New Delhi hospital9. The gene was carried on plasmids and could be transferred between different bacterial species, in this case between Klebsiella pneumoniae and Escherichia coli, and most importantly, conferred broad resistance to most antibiotics, including carbapenems. Later studies reported NDM-1 in a tertiary-care centre in Mumbai10. The controversy heated up when a paper appeared reporting the gene in multidrug-resistant Enterobacteriaceae in hospitals in Chennai and Haryana and in isolates from patients represented in the UK's National Reference Library (a high percentage of whom had travelled to the subcontinent)11. A further study in which NDM-1 was detected in drinking water and seepage water in New Delhi12 added to the concern and the focus on India - whether deserved or not.
NDM-1 may be the most widely known form of antibiotic resistance in India, but a number of studies in recent years have documented significant rates of resistance to a wide range of antibiotics13–30. Many are of hospital-acquired Gram-negative infections with Acinetobacter, Pseudomonas, Klebsiella, E. coli, Salmonella, and Neisseria gonorrhoeae, summarized in Table I.
Table I.
Recent antibiotic resistance studies in India
A World Health Organization (WHO) study in which E. coli was used as an indicator organism at four sites found high levels of resistance, especially in pathogenic isolates31. The study measured both antibiotic resistance and antibiotic use over the course of at least one year at all sites. Resistance rates were highest to those antibiotics in use the longest. However, resistance rates to newer antibiotics, such as fluoroquinolones, were particularly high in India31.
The overall take-home message from studies of resistant infections is that resistance levels have been worryingly high wherever studies have been conducted. Data are not sufficient to clearly delineate trends for specific organisms or specific antibiotics, but clearly outline resistance. This resistance is affecting patients and therapeutic outcomes, with concomitant economic consequences.
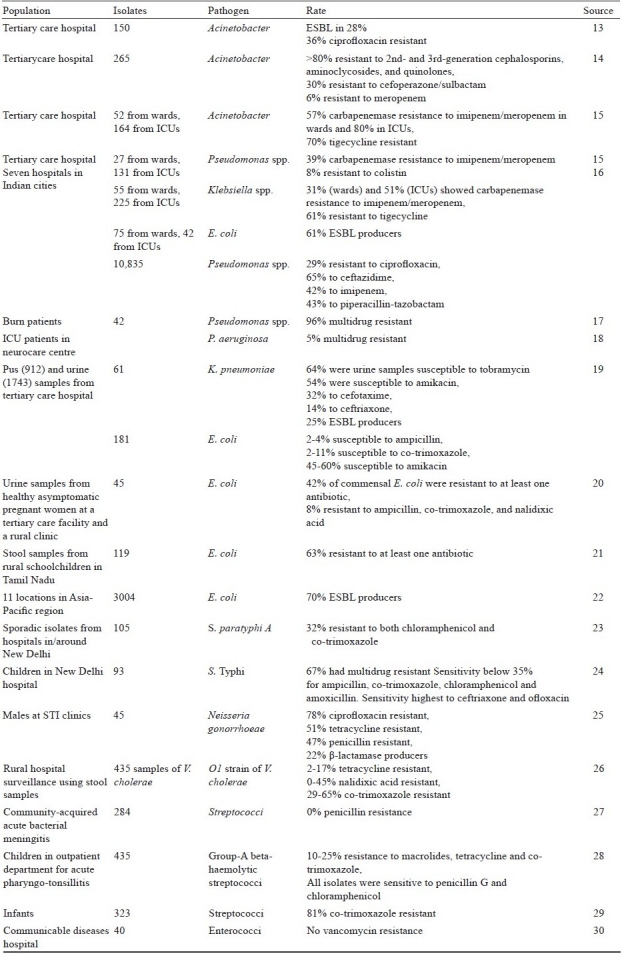
Antibiotic resistance surveillance has been limited to small-scale efforts by the Indian Council of Medical Research (ICMR) and some private agencies on a pilot basis. The Invasive Bacterial Infection Surveillance (IBIS) project produced valuable information on pneumonia in India, though it was unable to meet its goal of establishing a permanent surveillance system for antibiotic resistance32. Evidence that can be pieced together comes from many individual studies, such as studies on methicillin-resistant Staphylococcus aureus (Fig. 2).
Fig. 2.
MRSA resistance rates from various Indian studies vary but appear to increase over time. Source: Ref. 33–51.
Infections acquired in hospitals
Hospital-acquired infections (HAIs) are a particular concern and indicate the level of infection control in a hospital, since many HAIs can be prevented through better hygiene. Staphylococcus aureus and Pseudomonas aeruginosa are among the most common causes of HAIs. Recent findings on the levels of HAIs and the causative organisms in India are similar to those in other parts of the world, and include (i) a study by the International Nosocomial Infection Control Consortium (conducted in 12 intensive-care units in seven hospitals in seven Indian cities), which followed 10,835 patients hospitalized for a total of 52,518 days. The observed patients acquired 476 infections in the hospital (4%), of which 46 per cent were Enterobacteriaceae, 27 per cent Pseudomonas spp., 6 per cent Acinetobacter spp., 8 per cent Candida spp., and 3 per cent S. aureus16; (ii) a prospective study of 71 burn patients at Post Graduate Institute of Medical Education and Research (PGIMER) in Chandigarh found that up to 59 patients (83%) had hospital-acquired infections: 35 per cent of pathogens isolated from wounds and blood were S. aureus, 24 per cent were P. aeruginosa, and 16 per cent were β-haemolytic streptococci52; (iii) a six-month study conducted in the intensive-care units in the All India Institute of Medical Sciences (AIIMS), New Delhi, found that 140 of 1,253 patients (11%) had 152 hospital-acquired infections. P. aeruginosa made up 21 per cent of isolates, 23 per cent were S. aureus, 16 per cent Klebsiella spp., 15 per cent were Acinetobacter baumannii, and 8 per cent. E. coli53; and (iv) a study of 493 patients in a tertiary teaching hospital in Goa found that 103 people (21%) developed 169 infections48.
Patterns of antibiotic overuse
A few hospital- and city-based studies of antibiotic use suggest that antibiotics are often prescribed in irrational or inappropriate ways in India; that is, the drugs are prescribed at an incorrect dose, frequency, or duration, are redundant, or have the potential for adverse interactions with other drugs. Some studies on antibiotic use have employed indicators, such as the average number of drugs prescribed per encounter and the frequency with which fixed-dose combinations are prescribed. Other studies have detailed the reasons for prescribing (or purchasing) antibiotics - in particular, for upper respiratory tract infections, an inappropriate indication. Overprescribing and overuse are seen in all settings: public and private hospitals, clinics and pharmacies7. For example, depending on where they live and the type of practitioner they visit, 45 to 80 per cent of patients with symptoms of acute respiratory infections and diarrhoea are likely to receive an antibiotic, even though it will not be effective if they have a viral illness rather than a bacterial one54,55.
Why this overuse persists is not so easily determined. The possible reasons, as in other parts of the world, include the following: (i) lack of microbiology facilities or unwillingness of patients to undergo tests57; (ii) some doctors’ practice of prescribing antibiotics to any patient with a fever, taking it as a sign of bacterial infection, especially when they are concerned that the patient will not return for follow up57; (iii) the patient's expectation of being given an antibiotic over-the-counter or a prescription for one at the doctor's office57,58; (iv) incentives for pharmacists to make a profit from drug sales59,60; and (v) the public's lack of knowledge about the appropriate use of antibiotics57,61,62 (Figs. 3,4).
Fig. 3.

Prescribing determinants of antibiotics. Sources: Ref. 56.
Fig. 4.
Dispensing determinants of antibiotics. Source: Ref. 56.
All those possible reasons suggest that much of this use could be curtailed without harming health outcomes; in fact, reductions in use could actually improve people's health.
Antibiotic use in animals
Antibiotics are not only used to treat human illness but have also been used in livestock and poultry for more than half a century to control and treat diseases and, in low doses in animal feed, to promote growth and improve production of animal products63,64.
There is no regulation in India of the use of antibiotics in food animals such as poultry, dairy cows, and buffaloes raised for domestic consumption. Prevention of Food Adulteration Rules (1995), Part XVIII: Antibiotic and Other Pharmacologically Active Substances applies only to the use of antibiotics in certain types of seafood63, and the Export Inspection Council of India prohibits the use of certain antibiotics in the feed and medication of poultry intended for export only66.
The precise effect of agricultural antibiotic use on resistance levels in the general population is not known, but the evidence points to a link. In 2003, an expert committee convened by WHO, the UN Food and Agriculture Organization, and the World Organization for Animal Health concluded, ‘there is clear evidence of adverse human health consequences due to resistant organisms resulting from non-human usage of antimicrobials. These consequences include infections that would not have otherwise occurred, increased frequency of treatment failures (in some cases death), and increased severity of infections’67. Furthermore, cattle farming is a common livelihood in many parts of India, and a large proportion of the population is in close contact with livestock, which puts people at risk of acquiring resistant infections from their animals68.
A few studies on antibiotic residues in animal products have been conducted in India, but one on honey was widely publicized69–71. This study, conducted by the Centre for Science and Environment, New Delhi, found that 11 of 12 samples of honey taken from the domestic market were not compliant with standards for export. It concluded that the level of antibiotic residues found was not high enough to trigger an adverse effect in consumers, but it called for regulation and monitoring of antibiotic residues in honey ‘as continuous long-term exposure to low levels of antibiotics could in due course of time lead to antibiotic resistance in pathogenic bacteria making their treatment difficult’72.
Government policy towards antibiotic use and resistance
Regardless of whether NDM-1 turns out to threaten patients’ health in India, the attention directed to this pathogen has spurred the Government to action on antibiotic resistance. As a result, a Ministry of Health and Family Welfare task force announced a new national anti-microbial policy65.
The National Policy for Containment of Antimicrobial Resistance - India covers a range of topics, including curbing antibiotic use in animals, particularly those raised for human consumption; conducting infection surveillance in hospitals; improving hospital surveillance for monitoring antibiotic resistance; promoting rational drug use through education, monitoring, and supervision; researching new drugs; and developing and implementing a standard and more restrictive antibiotic policy65. Under the new Schedule H1 (now called HX), which will regulate antibiotic use, selling antibiotics over-the-counter will be banned. Certain antibiotics, including carbapenems, will be available at only tertiary hospitals65.
Discussion
Knowing that antibiotic resistance is a reality in India and knowing that the prevalence of resistant bacteria will rise over time, no serious constituencies can oppose acting to slow its spread. As with all interventions, preventing the spread of antibiotic resistance will have costs as well as benefits and will not be achieved without deliberate effort. Fortunately, the interventions that lead the list of recommendations from the GARP - India working group will have substantial immediate benefits to individuals in addition to their largely societal benefits in terms of antibiotic resistance. Vaccinations to prevent various illnesses and hospital infection control fall into this category. Setting aside the anti-vaccine arguments, these are win-win interventions, but their ‘antibiotic-sparing’ effects are often overlooked because these are of secondary importance. Their effects on reducing antibiotic use and, through a logic chain, the spread of antibiotic-resistant bacteria, could however, be enormous. Restricting the use of antibiotics in livestock and poultry for nontherapeutic uses (particularly growth promotion) is similarly beneficial: it is known conclusively from other countries that animal health is not harmed, and the effectiveness of antibiotics is thereby preserved for treating disease (in both humans and animals).
A second important tier of recommendations involves reducing antibiotic use by eliminating irrational or inappropriate use. There is no doubt that people benefit from not being treated for what does not ail them. They do not pay for drugs that are not needed, they avoid potential adverse reactions, and they might then be treated for the illness they do have. However, it is sometimes not possible to tell whether a child is in the early stages of pneumonia or has a common viral cold without laboratory testing, which assumes the availability of not only an experienced doctor but also a microbiology laboratory. Moreover, older antibiotics are inexpensive and cause relatively few side effects. However, some measures designed to rationalize antibiotic use may come with unintended consequences. For instance, enforcing prescription-only laws and eliminating over-the-counter antibiotic purchases could cut off antibiotic access for some segments of the population, such as the rural poor. As long as this possibility is factored into decisions, ways to mitigate any negative effects can also be brought to bear. But this has not been considered in most cases, either in India or in other countries where over-the-counter sales of antibiotics are common.
The GARP process has benefitted from the work of the Ministry of Health and Family Welfare task force report, which has recommended policies to deal with antibiotic resistance. That task force was empanelled as a direct result of the furore that erupted over accounts published in 2010 about the NDM-1 genes found in bacterial isolates purportedly originating in India. The report and recommendations have propelled action at a level that would have been difficult, if not impossible, to generate in the absence of a crisis atmosphere. What is needed now is to capitalize on that start without waiting for the next crisis. The ongoing GARP process is designed to do just that, though it is not officially part of the government and does not work under its authority. It is important to recognize that antibiotic resistance is a changing and ongoing issue, with developments in India and around the world occurring continually, not usually in crisis mode.
GARP - India has the advantage of being part of a growing global network. We have already seen sharing of information among the four initial GARP countries (India, Vietnam, Kenya and South Africa) and expect that this network will grow. Partner countries have shared research protocols and findings and will be sharing analyses, decisions on interventions, and as interventions are implemented, lessons learned. Although much of the information published on antibiotic use and resistance - including basic science, epidemiology, and interventions - comes from high-income countries, generating complementary information and filtering published information through the lens of the low- and middle-income country experience is likely to prove extremely valuable, particularly as the GARP network expands to more countries and experience deepens in founding countries. The GARP activities form a large part of the agenda of the 1st Global Forum on Bacterial Infections, taking place in New Delhi in October 2011 and involving participants largely from low- and middle-income countries. The Global Forum will raise awareness about the pressing nature of this problem, and about the wide variety of ways in which it can be tackled.
RECOMMENDATIONS
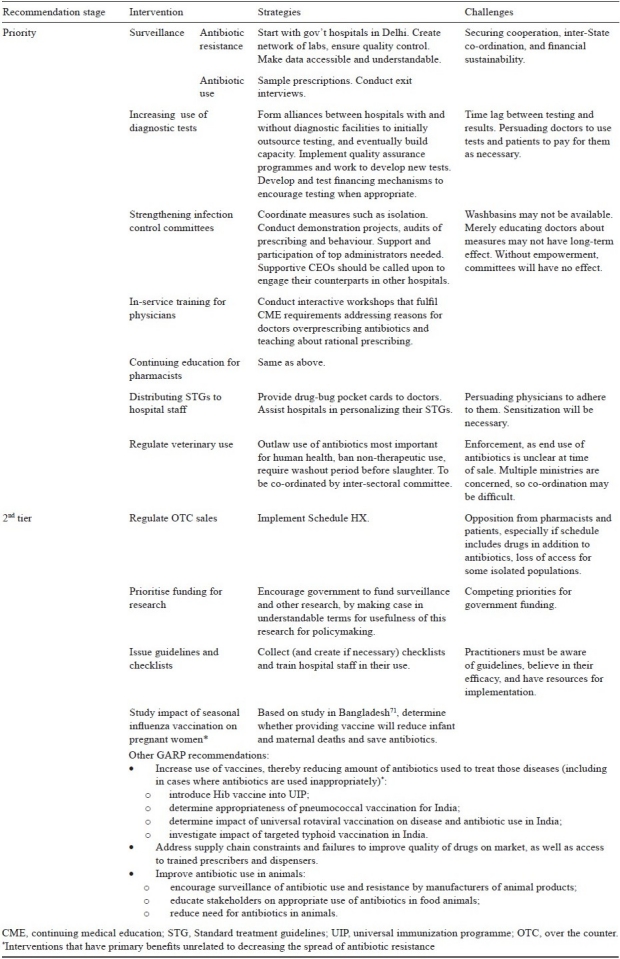
GARP's eventual goal is to use the best available information to develop workable and effective interventions. These include reducing the need for antibiotics by averting disease through vaccination or by averting infection through improved infection control and reducing demand through decreasing antibiotic overuse and eliminating non essential uses (e.g., in livestock growth promotion). The recommendations here must still be considered provisional, though all are considered important and have been informed by the new national policy, discussed above. A first task during the next stage of GARP will be to develop a ‘critical path’ for each recommendation, detailing the pros and cons, the challenges, the costs, the parties of interest, proponents and opponents, the likelihood of success, the size of the potential effect, and a timeline. Small projects could be included to direct implementation plans. In a country as large and complex as India, this is a necessary step and will inform the next phase of GARP. The interventions being considered are described below and summarized in Table II.
Table II.
Summary of GARP - India recommendations
Rationalizing the use of antibiotics in humans
Surveillance: Antibiotic resistance and antibiotic use
Two complementary types of surveillance are recommended: surveillance for antibiotic resistance and surveillance for antibiotic use. This supports a recommendation made in the national policy document. By itself, surveillance of any type will not change antibiotic use or the spread of resistant organisms, but knowing resistance levels and tracking them over time is a powerful tool to support real changes. Once the link between resistance and antibiotic use is accepted, tracking antibiotic use can be used as a surrogate for changes in resistance patterns. To some extent, these patterns can produce evidence for whether interventions are working, and can help identify problem areas, as is the case for antibiotic resistance surveillance. Surveillance results/data can also be fed into standard treatment guidelines and essential drug lists.
The Ministry of Health and Family Welfare task force report65 recommends the development of a laboratory network, beginning in New Delhi and expanding thereafter, as well as prescription and sales monitoring in New Delhi public sector hospitals. Details of co-ordinating bodies, quality assurance, standardization, format, and availability of data and funding are yet to be worked out.
All over the world, surveillance is seen as the backbone of successful programmes to attack the problem of antibiotic resistance. Adequate surveillance for antibiotic resistance and for bacterial infections generally is rare in low- and middle-income countries, and India is no exception. Support has diminished for the two recent large-scale infectious disease surveillance initiatives. The Invasive Bacterial Infection Surveillance project was begun in 1993 to monitor the serotype distribution and antimicrobial resistance of infections caused by Haemophilus influenzae and S. pneumoniae. However, the number of sites in India has dwindled over time, and funding has been fitful. Another programme, the Integrated Disease Surveillance Project was launched by the Ministry of Health and Family Welfare in 2004. It was phased in to nine States beginning in 2004 and was in 33 States and territories by 2007. However, reporting is less complete than anticipated74.
Distributing Standard Treatment Guidelines (STGs)
STGs have been developed at various levels, from the hospital (e.g., for diarrhoea and pneumonia) to national-level programmes (e.g., for TB and HIV/AIDS)75. These guidelines should be tailored to local situations and specific to levels of care. However, employees at all levels in the healthcare system often have little knowledge of the content of these STGs. One means of distributing STGs is through drug-bug ‘pocket cards’: these cards would provide summaries of locally recommended treatments for common conditions, and prescribers would be encouraged to carry and refer to these. The usefulness of these cards should be evaluated, either in a randomized trial or through other means, in different settings. Antibiograms could also be distributed regularly, to make doctors aware of changes in the local pattern of resistance.
Increasing the use of diagnostic tests
This recommendation - which was also made by the Ministry of Health and Family Welfare task force - raises several issues. First, many hospitals (particularly small ones) do not have the required facilities to conduct a range of diagnostic tests. Second, the laboratories that exist may be under-resourced and, therefore, inadequate to the task, a problem that may be either a consequence or a forerunner of a third major issue: physicians do not necessarily value the results from microbiology laboratories even when they exist. This may be because the use of unreliable tests has led to disillusionment among physicians. In India, patients often must pay for these tests and may opt to spend money on presumptive treatment instead. A combination of increasing laboratory capacity (which would involve investing in equipment, supplies, and personnel), partnering between hospitals with diagnostic capacity and those without, and encouraging the development of new rapid diagnostic tests is needed, but the details and critical paths for each remain to be worked out.
Infection control interventions
Hospitals create their own ecology in the bacterial-human interface. The use of antibiotics is much more intense in hospitals than in the community, and highly resistant bacteria may be found and spread there. In response, infection control interventions have been developed to contain bacterial infections in hospitals, including increased hand-washing, isolation rooms, reminders to limit catheter use, and use of gloves and gowns. The Ministry of Health and Family Welfare task force recommends that all hospitals create an infection control plan, committee, and team. It further recommends that clinical microbiologists conduct audits, such as by spot-checking prescribing sheets in wards75.
As uncontroversial as infection control may seem, the infrastructure required (such as washbasins and isolation rooms) is often lacking in hospitals. Microbiology laboratories and trained staff may be unavailable, and in some situations, staff members and administrators may be uninterested in co-operating76. The greatest challenge, then, is empowering the infection control committee and making hospital staff aware of its activities and recommendations.
Checklists for surgical procedures
Patients undergoing surgery are at high risk of infection at the surgical site, but these infections are largely preventable if simple measures are taken consistently before, during, and after surgery. The use of checklists - such as WHO's Surgical Safety Checklist77 - that ensures adherence to common-sense measures - has been demonstrated to improve outcomes, including surgery-related infection in international studies that include India78. Additional demonstrations, adapted checklists, and implementation are now recommended.
Educational approaches
Continuing education of doctors, nurses, dentists, pharmacists, and veterinarians is a perpetually attractive opportunity for instructing these professionals about antibiotic use and resistance. In India, continuing education is beginning to be required for certain professionals. A new Medical Council of India (MCI) rule that doctors must attend 30 hours of continuing medical education every five years to maintain their licenses will help encourage such courses79. Workshops on antibiotics could be offered as part of this, and similarly for other professions. Furthermore, the establishment of clinical microbiology and infectious diseases post-graduate courses should be encouraged.
It may be relatively easy to conduct courses (compared with other interventions), and these are likely to be beneficial, at least in the short term. It is not clear that such efforts result in permanent changes to behaviour. This area may be ripe for experimentation and evaluation.
Improving antibiotic supply chain and quality
The main recommendation for improving the antibiotic supply chain recognizes the success of the Delhi and Tamil Nadu models, which centralized procurement and focused on drugs that are most critical for the health of the State population80,81. India used a similar model to revise its National List of Essential Medicines in 201182. Twenty-one antibacterial medicines are now included, down from 28 in the 2003 list83. This is a step in the right direction toward curbing antibiotic misuse, preserving powerful antibiotics, and consequentially limiting resistance. The real issue, however, is implementing the list in the various categories of health facilities and health care systems. In other countries, where essential drug programmes have been implemented as part of a procurement plan, antibiotic use has been reduced. The essential drug programmes can bring order to drug procurement plans and ensure that only those drugs that are useful in the country are paid for, but merely making people aware of what drugs are on the list does not seem to have an impact84–86.
Vaccines
Vaccine-preventable diseases exact a high toll on the Indian population in terms of morbidity and mortality. Immunization could benefit the population immensely by improving health. Savings in terms of antibiotic use would be a secondary but potentially large benefit - millions of courses of antibiotic use could be avoided each year. Problems with achieving vaccination goals are deeply rooted in India. One-third of the world's unimmunized children are in India, and well under half of all children in India are fully immunized according to the current schedule87. This, of course, has nothing to do with antibiotics. It is clear that the antibiotic voice is a small one in vaccine debates.
Nonetheless, several steps could be taken to improve health and decrease the use of antibiotics; such as (i) introducing the H. influenzae type b (Hib) vaccine into the Universal Immunization Programme (UIP), (ii) introducing a pneumococcal vaccine into the UIP, after determining which would be most appropriate for India; (iii) introducing a rotavirus vaccine into the UIP to prevent a large number of cases of dehydrating diarrhoea - some of them fatal - and reduce what is largely inappropriate antibiotic use (since antibiotics do not treat rotavirus yet are routinely given to children with watery diarrhoea); (iv) investigating the effect of targeted typhoid vaccination in India; and (v) determining the expected effect on child health from providing pregnant women with seasonal influenza vaccination.
Reducing and controlling antibiotic use in the veterinary sector
India is late in recognizing the critical role of antibiotic use in agricultural animals (livestock and poultry) in promoting antibiotic resistance. Current regulations are limited to rules about antibiotic residues (or presence) in seafood. European countries have banned certain uses of antibiotics and limited antibiotic use in general, and India should now take similar steps. The inter-sectoral co-ordination committee mandated by the new national guidelines will be in a position to configure very specific recommendations to accomplish this, empowered as it is to review data, undertake studies, specify antibiotics for use in livestock, review laws from other countries and evaluate their applicability in India, develop regulations on usage and labelling, and enhance the scope of the Prevention of Food Adulteration Rules Act (1995 Part XVIII).
The inter-sectoral committee must aim at three goals:
-
(i)
outlawing veterinary use of those antibiotics deemed to be most important for human health;
-
(ii)
banning use of antibiotics for non-therapeutic purposes, particularly growth promotion; and
-
(iii)
requiring washout periods between the use of antibiotics and animal slaughter.
If adopted in India, these actions would go a long way toward reducing the use of antibiotics in animals without harming their health or endangering humans. Complementary measures include surveillance, education, and alternative measures that will make it possible to reduce use of antibiotics in animals.
Surveillance of antibiotic use and resistance by animal product manufacturers
In collaboration with the World Organisation for Animal Health (OIE) and the UN Food and Agriculture Organisation (FAO), protocols would be developed for surveillance of antibiotic resistance and use in various countries, in parallel with the procedure developed for surveillance in humans. This would be done so that data can be comparable across countries and shared internationally.
Education of farmers and other stakeholders about Appropriate use of antibiotics
Farmers and others in the livestock industries congregate regularly at markets and could be reached there with information on antibiotics. Incentives could be offered for attendance (e.g., lunch, take-home materials, or animal vitamins). Sessions could take the form of didactic sessions, question-and-answer sessions, or demonstrations. As with formal continuing education for human health professionals, the effect of these efforts would have to be evaluated.
Reducing the need for antibiotics in animals
Approaches include improved hygiene, reducing animal stress, including probiotics or nutritional supplements in feed, and increasing rates of vaccination for common animal diseases. An investigation is needed on animal vaccination rates and the costs and availability of vaccines.
References
- 1.World Health Statistics. France: 2011. World Health Organization. [Google Scholar]
- 2.Mathew JL. Pneumococcal vaccination in developing countries: where does science end and commerce begin? Vaccine. 2009;27:4247–51. doi: 10.1016/j.vaccine.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 3.Levine OS, Cherian T. Pneumococcal vaccination for Indian children. Indian Pediatr. 2007;44:491–6. [PubMed] [Google Scholar]
- 4.Austin DJ, Kristinsson KG, Anderson RM. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1152–6. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laxminarayan R, Malani A, Howard D, Smith D. Washington, DC: 2007. [updated 2007; cited]. Policy responses to the growing threat of antibiotic resistance. Available from: http://www.rff.org/Publications/Pages/PublicationDetails.aspx?PublicationID=9575 . [Google Scholar]
- 6.Kotwani A, Holloway K, Chaudhury RR. Methodology for surveillance of antimicrobials use among out-patients in Delhi. Indian J Med Res. 2009;129:555–60. [PubMed] [Google Scholar]
- 7.Kotwani A, Holloway K. Trends in antibiotic use among outpatients in New Delhi, India. BMC Infect Dis. 2011;11:99. doi: 10.1186/1471-2334-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharuch B. Personal Communication: Data from IMS Health Information and Consulting Services-India. In: Laxminarayan R, editor. Courtesy of Pfizer. 2009. [Google Scholar]
- 9.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrobial Agents. 2009;53:5046–54. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshpande P, Rodrigues C, Shetty A, Kapadia F, Hedge A, Soman R. New Delhi Metallo-beta lactamase (NDM-1) in Enterobacteriaceae: treatment options with carbapenems compromised. J Assoc Physicians India. 2010;58:147–9. [PubMed] [Google Scholar]
- 11.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11:355–62. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 13.Sinha M, Srinivasa H, Macaden R. Antibiotic resistance profile & extended spectrum beta-lactamase (ESBL) production in Acinetobacter species. Indian J Med Res. 2007;126:63–7. [PubMed] [Google Scholar]
- 14.Gaur A, Garg A, Prakash P, Anupurba S, Mohapatra TM. Observations on carbapenem resistance by minimum inhibitory concentration in nosocomial isolates of Acinetobacter species: an experience at a tertiary care hospital in North India. J Health Popul Nutr. 2008;26:183–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Wattal C, Goel N, Oberoi JK, Raveendran R, Datta S, Prasad KJ. Surveillance of multidrug resistant organisms in tertiary care hospital in Delhi, India. J Assoc Physicians India. 2010;58(Suppl):S32–6. [PubMed] [Google Scholar]
- 16.Mehta A, Rosenthal VD, Mehta Y, Chakravarthy M, Todi SK, Sen N, et al. Device-associated nosocomial infection rates in intensive care units of seven Indian cities.Findings of the International Nosocomial Infection Control Consortium (INICC) J Hosp Infect. 2007;67:168–74. doi: 10.1016/j.jhin.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Shahid M, Malik A. Resistance due to aminoglycoside modifying enzymes in Pseudomonas aeruginosa isolates from burns patients. Indian J Med Res. 2005;122:324–9. [PubMed] [Google Scholar]
- 18.Kumari HB, Nagarathna S, Chandramuki A. Antimicrobial resistance pattern among aerobic gram-negative bacilli of lower respiratory tract specimens of intensive care unit patients in a neurocentre. Indian J Chest Dis Allied Sci. 2007;49:19–22. [PubMed] [Google Scholar]
- 19.Shahid M, Malik A, Akram M, Agrawal LM, Khan AU, Agrawal M. Prevalent phenotypes and antibiotic resistance in Escherichia coli and Klebsiella pneumoniae at an Indian tertiary care hospital: plasmid-mediated cefoxitin resistance. Int J Infect Dis. 2008;12:256–64. doi: 10.1016/j.ijid.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Mathai E, Chandy S, Thomas K, Antoniswamy B, Joseph I, Mathai M, et al. Antimicrobial resistance surveillance among commensal Escherichia coli in rural and urban areas in Southern India. Trop Med Int Health. 2008;13:41–5. doi: 10.1111/j.1365-3156.2007.01969.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaul S, Brahmadathan KN, Jagannati M, Sudarsanam TD, Pitchamuthu K, Abraham OC, et al. One year trends in the gram-negative bacterial antibiotic susceptibility patterns in a medical intensive care unit in South India. Indian J Med Microbiol. 2007;25:230–5. doi: 10.4103/0255-0857.34764. [DOI] [PubMed] [Google Scholar]
- 22.Hawser SP, Bouchillon SK, Hoban DJ, Badal RE, Hsueh PR, Paterson DL. Emergence of high levels of extended-spectrum-beta-lactamase-producing gram-negative bacilli in the Asia-Pacific region: data from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program, 2007. Antimicrobial agents Chemother. 2009;53:3280–4. doi: 10.1128/AAC.00426-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandel DS, Chaudhry R. Drug-resistant salmonella enterica Serotype Paratyphi A in India. Emerg Infect Dis. 2000;6:420–1. doi: 10.3201/eid0604.000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar R, Gupta N. Multidrug-resistant typhoid fever. Indian J Pediatr. 2007;74:39–42. doi: 10.1007/s12098-007-0024-z. [DOI] [PubMed] [Google Scholar]
- 25.Sethi S, Sharma D, Mehta SD, Singh B, Smriti M, Kumar B, et al. Emergence of ciprofloxacin resistant Neisseria gonorrhoeae in north India. Indian J Med Res. 2006;123:707–10. [PubMed] [Google Scholar]
- 26.Narang P, Mendiratta DK, Deotale VS, Narang R. Changing patterns of Vibrio cholerae in sevagram between 1990 and 2005. Indian J Med Microbiol. 2008;26:40–4. doi: 10.4103/0255-0857.38856. [DOI] [PubMed] [Google Scholar]
- 27.Mani R, Pradhan S, Nagarathna S, Wasiulla R, Chandramuki A. Bacteriological profile of community acquired acute bacterial meningitis: a ten-year retrospective study in a tertiary neurocare centre in South India. Indian J Med Microbiol. 2007;25:108–14. doi: 10.4103/0255-0857.32715. [DOI] [PubMed] [Google Scholar]
- 28.Jain A, Shukla VK, Tiwari V, Kumar R. Antibiotic resistance pattern of group-a beta-hemolytic streptococci isolated from north Indian children. Indian J Med Sci. 2008;62:392–6. [PubMed] [Google Scholar]
- 29.Coles C, Rahmathullah L. Nasopharyngeal carriage of resistant pneumococci in young South Indian infants. Epidemiol Infect. 2002;129:491–7. doi: 10.1017/s0950268802007586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekar R, Srivani R, Vignesh R, Kownhar H, Shankar EM. Low recovery rates of high-level aminoglycoside-resistant enterococci could be attributable to restricted usage of aminoglycosides in Indian settings. J Med Microbiol. 2008;57:397–8. doi: 10.1099/jmm.0.47604-0. [DOI] [PubMed] [Google Scholar]
- 31.Holloway K, Mathai E, Sorensen TL, Gray A. Report on five pilot projects. Geneva: World Health Organization; 2009. (US Agency for International Development). Community-Based Surveillance of Antimicrobial Use and Resistance in Resource-Constrained Settings. [Google Scholar]
- 32.Thomas K, Lalitha MK, Arora NK, Das B, Awasthi S, Amita J, et al. Invasive Bacterial Infectious Surveillance II (IBIS 2) Final Report. Vellore. [cited 2011 5 August]. Available from: http://www.inclentrust.org/resources/iphid/iidi/Annex 1-IBISI2final.pdf .
- 33.Pulimood TB, Lalitha MK, Jesudason MV, Pandian R, Selwyn J, John TJ. The spectrum of antimicrobial resistance among methicillin resistant Staphylococcus aureus (MRSA) in a tertiary care centre in India. Indian J Med Res. 1996;103:212–5. [PubMed] [Google Scholar]
- 34.Shankar CU, Harish BN, Kumar PMU, Navaneeth BV. Prevalence of methicillin resistant Staphylococcus aureus in JIPMER hospital. A preliminary report. Indian J Med Microbiol. 1997;15:137–8. [Google Scholar]
- 35.Tyagi A, Kapil A, Singh P. Incidence of methicillin resistant Stahylococcus aureus (MRSA) in pus samples at a tertiary care hospital, AIIMS, New Delhi. J Indian Acad Clin Med. 2008;9:33–5. [Google Scholar]
- 36.Vidhani S, Mehndiratta PL, Mathur MD. Study of methicillin resistant S. aureus (MRSA) isolates from high risk patients. Indian J Med Microbiol. 2001;19:13–6. [PubMed] [Google Scholar]
- 37.Verma S, Joshi S, Chitnis V, Hemwani N, Chitnis D. Growing problem of methicillin resistant staphylococci - Indian scenario. Indian J Med Sci. 2000;54:535–40. [PubMed] [Google Scholar]
- 38.Tahnkiwale SS, Roy S, Jalgaonkar SV. Methicillin resistance among isolates of Staphylococcus aureus: antibiotic sensitivity pattern & phage typing. Indian J Med Sci. 2002;56:330–4. [PubMed] [Google Scholar]
- 39.Hanumanthappa AR, Chandrappa NR, Rajasekharappa MG. Prevalence of methicillin resistant Staphylococcus aureus in Karnataka. Indian J Pathol Microbiol. 2003;46:129–32. [PubMed] [Google Scholar]
- 40.Anupurba S, Sen MR, Nath G, Sharma BM, Gulati AK, Mohapatra TM. Prevalence of methicillin resistant Staphylococcus aureus in a tertiary referral hospital in eastern Uttar Pradesh. Indian J Med Microbiol. 2003;21:49–51. [PubMed] [Google Scholar]
- 41.Mohanty S, Kapil A, Dhawan B, Das BK. Bacteriological and antimicrobial susceptibility profile of soft tissue infections from Northern India. Indian J Med Sci. 2004;58:10–5. [PubMed] [Google Scholar]
- 42.Krishna BV, Patil AB, Chandrasekhar MR. Community-acquired methicillin-resistant Staphylococcus aureus infections in a south Indian city. Southeast Asian J Trop Med Public Health. 2004;35:371–4. [PubMed] [Google Scholar]
- 43.Anbumani N, Kalyani M, Mallika M. Prevalence of Methicillin-Resistanct Staphylococcus aureus in a tertiary referral hospital in Chennai, South India. Ind J Pract Doc. 2006;3:8–9. [Google Scholar]
- 44.Deep A, Goel N, Sikka R, Chaudhary U, Yadav S.A.G, et al. Quinupristin-dalfopristin resistance in gram-positive bacteria: experience from a tertiary care referral center in North India. J Infect Dis Antimicrob Agents. 2008;25:117–21. [Google Scholar]
- 45.Tiwari HK, Sapkota D, Sen MR. High prevalence of multidrug-resistant MRSA in a tertiary care hospital of northern India. Infec Drug Resist. 2008;1:57–61. doi: 10.2147/idr.s4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murugan S, Mani KR, Uma Devi P. Prevalence of methicillin resistant Staphylococcus aureus among diabetes patients with foot ulcers and their antimicrobial susceptibility pattern. J Clin Diagn Res. 2008;2:979–84. [Google Scholar]
- 47.Majumder D, Bordoloi JS, Phukan AC, Mahanta J. Antimicrobial susceptibility pattern among methicillin resistant staphylococcus isolates in Assam. Indian J Med Microbiol. 2001;19:138–40. [PubMed] [Google Scholar]
- 48.Kamat US, Ferreira A, Savio R, Motghare DD. Antimicrobial resistance among nosocomial isolates in a teaching hospital in Goa. Indian J Community Med. 2008;33:89–92. doi: 10.4103/0970-0218.40875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arora B, Prabhat Ranjan K, Arora DR. Prevalence of methicillin-resistant Staphylococcus aureus in post operative infections in a referral hospital in Haryana, India. J Infect Dis Antimicrob Agents. 2008;25:123–7. [Google Scholar]
- 50.Verghese S, Padmaja P, Sudha P, Vanitha V, Mathew T. Nasal carriage of methicillin resistant Staphylococcus aureus in a cardiovascular tertiary care centre and its detection by Lipovitellin Salt Mannitol Agar. Indian J Pathol Microbiol. 1999;42:441–6. [PubMed] [Google Scholar]
- 51.Mehndiratta PL, Vidhani S, Mathur MD. A study on Staphylococcus aureus strains submitted to a reference laboratory. Indian J Med Res. 2001;114:90–4. [PubMed] [Google Scholar]
- 52.Taneja N, Emmanuel R, Chari PS, Sharma M. A prospective study of hospital-acquired infections in burn patients at a tertiary care referral centre in North India. Burns. 2004;30:665–9. doi: 10.1016/j.burns.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Mathur P, Kapil A, Das B. Nosocomial bacteraemia in intensive care unit patients of a tertiary care centre. Indian J Med Res. 2005;122:305–8. [PubMed] [Google Scholar]
- 54.Kotwani A, Roy Chaudhury R, Holloway K. Conference Paper: Prescribing antibiotics for acute respiratory tract infections by primary care physicians in New Delhi, India. International Society of Pharmacoeconomics and Outcomes Research. 2010 [Google Scholar]
- 55.Kumar R, Indira K, Rizvi A, Rizvi T, Jeyaseelan L. Antibiotic prescribing practices in primary and secondary health care facilities in Uttar Pradesh, India. J Clin Pharm Ther. 2008;33:625–34. doi: 10.1111/j.1365-2710.2008.00960.x. [DOI] [PubMed] [Google Scholar]
- 56.Radyowijati A, Haak H. Improving antibiotic use in low-income countries: an overview of evidence on determinants. Soc Sci Med. 2003;57:733–44. doi: 10.1016/s0277-9536(02)00422-7. [DOI] [PubMed] [Google Scholar]
- 57.Kotwani A, Wattal C, Katewa S, Joshi PC, Holloway K. Factors influencing primary care physicians to prescribe antibiotics in Delhi India. Fam Pract. 2010;27:684–90. doi: 10.1093/fampra/cmq059. [DOI] [PubMed] [Google Scholar]
- 58.Sivagnanam G, Thirumalaikolundusubramanian P, Mohanasundaram J, Raaj AA, Namasivayam K, Rajaram S. A survey on current attitude of practicing physicians upon usage of antimicrobial agents in southern part of India. Med Gen Med. 2004;6:1. [PMC free article] [PubMed] [Google Scholar]
- 59.Dua V, Kunin CM, White LV. The use of antimicrobial drugs in Nagpur, India. A window on medical care in a developing country. Soc Sci Med. 1994;38:717–24. doi: 10.1016/0277-9536(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 60.Kotwani A, Wattal C, Joshi PC, Holloway K. Irrational use of antibiotics and role of the pharmacist: an insight from a qualitative study in New Delhi. J Clin Pharm Ther. doi: 10.1111/j.1365-2710.2011.01293.x. (In press) [DOI] [PubMed] [Google Scholar]
- 61.Promoting awareness amongst school children on rational use of drugs. Delhi: 2009. Delhi Society for Promotion of Rational Use of Drugs. (WHO/ SEARO) [Google Scholar]
- 62.Sharma R, Verma U. Self-medication among urban population of Jammu city. Indian J Pharmacol. 2005;37:37–45. [Google Scholar]
- 63.Manna SK, Brahmane MP, Manna C, Batabyal K, Das R. Occurrence, virulence characteristics and antimicrobial resistance of Escherichia coli 157 in slaughtered cattle and diarrhoeic calves in West Bengal, India. Lett Appl Microbiol. 2006;43:405–9. doi: 10.1111/j.1472-765X.2006.01975.x. [DOI] [PubMed] [Google Scholar]
- 64.Khachatourians GG. Agricultural use of antibiotics and the evolution and transfer of antibiotic-resistant bacteria. Can Med Assoc J. 1998;159:1129–36. [PMC free article] [PubMed] [Google Scholar]
- 65.Ministry of Health & Family Welfare. National Policy for Containment of Antimicrobial Resistance - India. 2011. [updated 2011; cited]. Available from: http://www.nicd.nic.in/ncdc_new/ab_policy.pdf .
- 66.Approval and monitoring of processing/storing establishments for export: Egg products. New Delhi: 2007. [updated 2007; cited 2011 20 June]. Export Inspection Council of India. Available from: http://www.eicindia.org/eic/inspection/egg.pdf . [Google Scholar]
- 67.Non-Human Antimicrobial Usage and Antimicrobial Resistance. Geneva: 2003. [updated 2003; cited 2011 June 20]. FAO/OIE/WHO. Available from: http://www.who.int/foodsafety/publications/micro/en/amr.pdfhttp://www.who.int/foodsafety/publications/micro/en/amr.pdf . [Google Scholar]
- 68.Arya G, Roy A. Serogroups, atypical biochemical characters, colicinogeny and antibiotic resistance pattern of shiga toxin-producing Escherichia coli isolated from diarrhoeic calves in Gujarat, India. Zoonoses Public Health. 2008;55:89–98. doi: 10.1111/j.1863-2378.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- 69.Times News Network. Antibiotics in most honey brands: Study. The Times of India. 2010. Sep 16, Available from: http://articles.timesofindia.indiatimes.com/2010-09-16/india/28225876_1_honey-brands-antibiotics-honey-samples .
- 70.Special Correspondent. Honey! It’s sweet, yet comes with a sting. The Hindu. 2010. Sep 16, Front Page. Available from: http://www.hindu.com/2010/09/16/stories/2010091661730900.htm .
- 71.Honey, these popular brands are contaminated with antibiotics. Deccan Herald. 2010. Sep 15, National. Available from: http://www.deccanherald.com/content/96888/honey-popularbrands-contaminated-antibiotics.html .
- 72.Johnson S, Jadon N, Mathur HB, Agarwal HC. New Delhi: Centre for Science and Environment; 2010. Antibiotic residues in honey. [Google Scholar]
- 73.Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 74.New Delhi: Ministry of Health and Family Welfare; 2008. Integrated Disease Surveillance Project Annual Report, Final. [Google Scholar]
- 75.Srivastava RK, Roy Chaudhury R, Ganguly NK, Bramhachari SK, Singh S, Guleria R, et al. India: Ministry of Health & Family Welfare; 2011. National Policy for Containment of Antimicrobial Resistance. [Google Scholar]
- 76.Rhinehart E, Goldmann DA, O’Rourke EJ. Adaptation of the Centers for Disease Control guidelines for the prevention of nosocomial infection in a pediatric intensive care unit in Jakarta, Indonesia. Am J Med. 1991;91:S213–20. doi: 10.1016/0002-9343(91)90371-4. [DOI] [PubMed] [Google Scholar]
- 77.WHO. Surgical Safety. 1st ed. [cited 2011 5 August]. Available from: http://www.who.int/patientsafety/safesurgery/tools_resources/SSSL_Checklist_finalJun08.pdf.
- 78.Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat A-HS, Dellinger EP, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–9. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 79.Sinha K. MCI plans to send docs back to lecture halls. Times of India. 2011 Apr 5; [Google Scholar]
- 80.Narayanan D. Tamil Nadu's efficient drug procurement system offers clues to fixing the glitches in the national health system. Forbes India. 2010 Jul 27; [Google Scholar]
- 81.Kotwani A, Levinson L. London: DFID; 2007. Price components and access to medicines in Delhi, India. [Google Scholar]
- 82.Gupta YK, Sharma SK, Sidhu TS, Harit AK, Kanungo D, Shekhar C, et al. National List of Essential Medicines of India. 2011. [updated 2011; cited 2011 August 4]. Available from: http://cdsco.nic.in/National List of Essential Medicinefinalcopy.pdf.
- 83.Directorate General of Health Services, Ministry of Health & Family Welfare. National List of Essential Medicines. 2003. [updated 2003; cited 2011 5 August]. Available from: http://www.searo.who.int/LinkFiles/Essential_Drugs_and_Medicines_India.pdf.
- 84.Hogerzeil HV, Bimo MD, Ross-Degnan D, Laing RO, Ofori-Adjei D, Santoso B, et al. Field tests for rational drug use in twelve developing countries. Lancet. 1993;342:1408–10. doi: 10.1016/0140-6736(93)92760-q. [DOI] [PubMed] [Google Scholar]
- 85.Hogerzeil HV, Walker GJ, Sallami AO, Fernando G. Impact of an essential drugs programme on availability and rational use of drugs. Lancet. 1989;1:141–2. doi: 10.1016/s0140-6736(89)91152-5. [DOI] [PubMed] [Google Scholar]
- 86.Lindtjorn B. Essential drug list in a rural hospital.Does it have any influence on drug prescription? Trop Doct. 1987;17:151–5. doi: 10.1177/004947558701700404. [DOI] [PubMed] [Google Scholar]
- 87.Laxminarayan R, Ganguly NK. India's vaccine deficit: Why more than half of Indian children are not fully immunized, and what can - and should - be done. Health Aff (Millwood) 2011;30:1096–103. doi: 10.1377/hlthaff.2011.0405. [DOI] [PubMed] [Google Scholar]


