Abstract
Background & objectives:
MMR vaccine in a two dose schedule has successfully eliminated measles, mumps and rubella from many developed countries. In India, it is not a part of national immunization programme but is included in the State immunization programme of Delhi as a single dose between 15-18 months. This prospective study was carried out to assess the extent of seroprotection against these three diseases in immunized children and to study the immune response to a second dose of MMR.
Methods:
Consecutive children aged 4-6 yr, attending the immunization clinic of a tertiary care hospital in Delhi for routine DT vaccination, were enrolled. Second dose of MMR was given and pre- and post-vaccination antibody levels were compared.
Results:
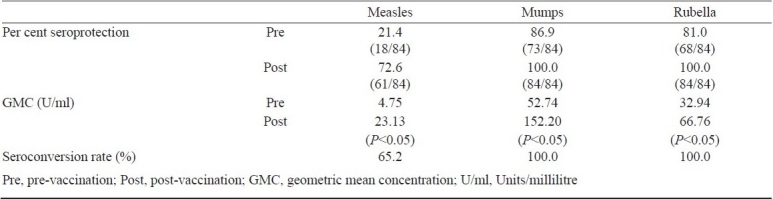
The pre-vaccination percentage seropositivity observed in the 103 children recruited, was 20.4 per cent for measles, 87.4 per cent for mumps and 75.7 per cent for rubella. Amongst the 84 children who were followed up after the second dose, the percentage seroprotection for measles rose from 21.4 (18/84) to 72.6 per cent (61/84) and 100 per cent became seroprotected to mumps and rubella.
Interpretation & conclusions:
The percentage of children protected against measles was found to be alarmingly low which needs to be investigated. Though the observed protection against mumps and rubella was adequate, its durability was not known. The need for re-appraisal of the current MMR immunization policy is called for by carrying out longitudinal studies on a larger sample.
Keywords: Measles, MMR vaccine, mumps, rubella, seroconversion, seroprotection
MMR vaccine has been successfully used by many developed countries in a two dose schedule in preschool children to eliminate measles, mumps and rubella from their population1. In India, children are given measles vaccine (MV) soon after completing 9 months of age considering the large amount of morbidity and mortality caused by the disease. Seroconversion for measles is slightly lower in children who receive the first dose before or at 12 months of age (87% at 9 months, 95% at 12 months and 98% at 15 months) because of persisting maternal antibodies2,3. Infants who receive MV before 12 months of age should be given two additional doses of measles antigen containing vaccine at 12-15 months and 4-6 yr of age2. As far as protection against mumps is concerned, accumulated global experience has shown that 2 doses of mumps antigen containing vaccine are required for a long-lasting protection4. The RA27/3 vaccine for rubella is considered as highly efficacious and the immunity following a single dose is assumed to be life long, although rubella antibodies may fall below detectable levels with time. Following administration of any vaccine, apart from primary non-responders, some of the responders tend to lose their protective immunity over time leading to secondary vaccine failure. Over a period of time the pool of susceptible children accumulates, including the children who missed the opportunity of being vaccinated with the first dose, serving as a fertile ground for an epidemic to take place.
Although MMR vaccine is not a part of the national immunization schedule of India, it was introduced in the State immunization programme of Delhi in1999 as a single dose between 15-18 months (MMR-I). The Indian Academy of Pediatrics (IAP) recommends measles vaccine at 9 months of age. They also recommend to offer MMR vaccine to all parents who can afford it as two dose schedule, one at 15-18 months and second at school entry (4-6 yr of age)5. A two dose MMR programme has also been recommended jointly by the American Academy of Pediatrics (AAP) and the Advisory Committee on Immunization practices (ACIP) in the USA6. We undertook this study to assess the percentage of children having seroprotective levels of antibodies against measles, mumps and rubella in immunized children in Delhi aged 4-6 yr and to study the seroresponse to a second dose of MMR (MMR-II).
Material & Methods
This prospective study was carried out in Guru Teg Bahadur Hospital, a tertiary care hospital in Delhi over a period of one year (May 2007-April 2008). The institutional review board clearance was obtained prior to commencement of the study. The sample size was estimated considering the percentage of children not having antibodies in the protected range to any of the three antigens prior to the second dose of MMR to be around 40 per cent and expecting a 60 per cent reduction in this figure following vaccination. The sample size came out to be around 80 keeping the power of the study to be 90 per cent and a confidence interval of 95 per cent. Consecutive children between 4-6 yr of age attending immunization clinic on two fixed days of the paediatrics OPD for DT booster, having documented evidence (immunization card) of receiving measles vaccine during infancy and MMR vaccine between 12-24 months of age were included in the study after obtaining an informed written consent from the parents. Children receiving prolonged steroid therapy/upto <4 wk after cessation of steroid therapy, having history of convulsions/epilepsy, having received another live vaccine within last 4 wk, having history of administration of blood, plasma transfusion or immunoglobulin within last 3 months, diagnosed with malignancy or immunodeficiency or having history of severe reaction to previous dose of MMR were excluded from the study. MMR-II was administered after collection of blood samples and the children were asked to come for follow up between 4-6 wk after immunization. The details of possible adverse effects were explained to the parents and they were asked to inform the investigator telephonically or at follow up.
Vaccine: MMR vaccine, Tresivac (Serum Institute of India Ltd, Pune) containing equal to or more than 1000, 5000 and 1000 TCID50doses of measles (Edmonston Zagreb), mumps (L-Zagreb) and rubella (RA 27/3) respectively, was given 0.5 ml, subcutaneously.
Collection of blood samples: Pre- and post-vaccination venous blood samples (3-5 ml) were collected in sterile tubes; serum separated by centrifuging the clotted samples and stored at -20°C till antibody level estimation was done.
Estimation of antibody titres: The antibody levels against measles and mumps were estimated using the Demeditec (Germany) ELISA IgG Quantitative estimation kit. According to the manufacturer, antibody level < 8 U/ml was considered as negative, between 8-12 U/ml as equivocal and > 12 U/ml as positive or protective for both measles and mumps. Antibody levels for Rubella was analysed using Nova Tec Immunodiagnostica GmbH (Germany), IgG quantitative ELISA kit. Values <10 IU/ml were considered as negative, 10-15 IU/ml as equivocal and >15 IU/ml as positive for rubella according to the manufacturer. The children having antibody levels above the cut-off for positive were considered as seroprotected.
Seroconversion: It was defined as antibody concentration changing from pre-vaccination negative to post-vaccination positive.
Statistical analysis: The data were analysed using Statistical Package for Social Sciences for windows (SPSS version14.0, Chicago, Illinois, USA). Geometric mean concentrations (GMC) were calculated by taking the antilog of mean logarithmic transformation of data. Pre- and post-vaccination GMC were compared using paired t-test.
Results
Eighty four children of the 103 recruited were followed up between 4-6 wk. The mean age at recruitment was 58.0 months. All the children had either normal nutritional status or were having grade I protein energy malnutrition as per IAP classification7. The age at receiving MV during infancy ranged from 7-11 months (mean = 9.1 months) and the majority had received MMR-I between 15-16 months of age (mean = 16.6 months). The duration since receiving MMR-I at the time of enrolment varied from 28 to 55 months (mean = 41.0 months).
It was observed that prior to giving MMR-II, 20.4% (21/103) children were seroprotected for measles, 87.4 per cent (90/103) for mumps and 75.7 per cent (78/103) for rubella.
On analysing the pre- and post-vaccination samples of the 84 children who were followed up, it was observed that the percentage seroprotection for measles rose from 21.4 per cent (18/84) to 72.6 per cent (61/84) with a high seroconversion rate (65.2%). After MMR-II all the 84 children became seroprotected to mumps and rubella with 100 per cent seroconversion rate. The geometric mean concentration (GMC) of all three antigens rose significantly (P<0.05) compared to pre- vaccination levels (Table I).
Table I.
Seroconversion following the second dose of MMR
No severe systemic or neurological adverse effects were noted in any of the subjects vaccinated. Majority of the adverse effects reported were localized to the site of vaccine administration i.e. pain, redness or swelling (15%). Eleven per cent developed fever for 1-2 days and one of the children reported swelling in parotid region 15 days after vaccination.
Discussion
The percentage seroprotection for measles was around 21 per cent at 4-6 yr which was very low in comparison with all the previous studies in which children had received only one dose of measles antigen containing vaccine i.e. MMR-I (Table II). MMR-I was administered after 12 months of age in all these studies. This indicates that almost four out of every five children, who were fully immunized according to the vaccination schedule practiced in Delhi, were susceptible to measles in spite of receiving two doses of measles antigen containing vaccines. Hence there is a need to investigate the causes of low level of immunity against measles. Sub-optimal levels of measles antibodies could probably be due to immunosuppressant effect of MV received during infancy. Studies have shown that vaccination in the presence of maternal antibody can result in the development of low antibody levels to measles and a reduced immune response to subsequent revaccination that may not be sustained8,9. A study carried out by Stetler et al10 confirmed that the immune response to re-vaccination is altered in infants first vaccinated prior to 10 months of age. A study carried out by Ceyhan et al11 compared the immunogenicity and efficacy of one dose MMR vaccine at 12 months of age with monovalent measles vaccination at 9 months followed by MMR re-vaccination at 15 months of age. The study demonstrated that early vaccination seemed to alter the immune response to re-vaccination as indicated by a higher vaccine failure rate (30.1%), lower antibody levels and lower clinical protection after early measles vaccination followed by MMR vaccination at 12 months of age.
Table II.
Comparison of seroresponse to second dose of MMR vaccine
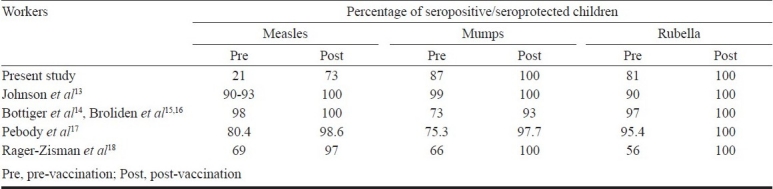
This study revealed that the majority of children were seroprotected against mumps and rubella prior to receiving MMR-II. These results were comparable to the previous studies (Table II) and demonstrate that there is a fair amount of protection against these diseases but its durability is not known. There is a likelihood that these figures may fall further with time due to secondary vaccine failure; hence increasing the pool of susceptibles that might act as a fertile ground for epidemics as observed in Belgium and recently in US with mumps12. Following the trend observed in the developed countries, a shift in the epidemiological profile of these diseases to a higher age group akin to the one observed in developed countries4 may not be ruled out in India. Hence there is a need to boost the immunological responses against these two diseases as well along with measles at this age itself.
We studied the immune response to MMR-II at 4-6 yr of age. GMC increased significantly for all three antigens following vaccination. The GMC/GMT (geometric mean titre) values of the previous studies were not comparable with this study as those were expressed in different units which varied from study to study13–18. Our study has demonstrated that the immune response to MMR-II was good at 4-6 yr of age in Indian children, considering the high seroconversion rates and the significant increase in post-vaccination seropositivity. MMR is considered a safe vaccine to be administered at 4-6 yr as majority of the adverse effects reported were localized to the site of administration and the rates were comparable to previous studies19,20.
There is need to carry out further studies to investigate for the cause of alarmingly low seroprevalence of measles at 4-6 yr of age in children who have already received two doses of measles antigen containing vaccines in the past. The observed protection against mumps and rubella was adequate but its durability was not known. The need for a booster deserves consideration and a re-appraisal of the current MMR immunization policy is called for by carrying out longitudinal large scale multicentric studies.
Acknowledgment
This work was supported by research grant from The Directorate of Family Welfare, Government of NCT Delhi. Authors thank Drs M. K. Aggarwal and D. K. Dewan, State MCH Officers, for their help, co-operation and discussion from time to time.
References
- 1.Peltola H, Heinonen OP, Valle M, Paunio M, Virtanen M, Karanko V, et al. The elimination of indigenous measles, mumps and rubella from Finland by a 12-year, two dose vaccination program. N Engl J Med. 1994;331:1397–402. doi: 10.1056/NEJM199411243312101. [DOI] [PubMed] [Google Scholar]
- 2.Mason WH. Measles. In: Kliegman RM, Behrman RE, Stanton BF, Jenson HB, editors. Nelson textbook of pediatrics. 18th ed. Philadelphia Pa: Saunders Elsevier; 2007. pp. 1331–7. [Google Scholar]
- 3.Job JS, John TJ, Joseph A. Antibody response to measles immunization in India. Bull World Health Organ. 1984;62:737–41. [PMC free article] [PubMed] [Google Scholar]
- 4.Mumps virus vaccines. Wkly Epidemiol Rec. 2007;82:49–60. [No authors listed] [PubMed] [Google Scholar]
- 5.Singhal T, Amdekar YK, Agarwal RK, editors. IAP guidebook on immunisation, IAP Committee on Immunization 2007-2008. New Delhi: Jaypee Brothers Medical Publishers; 2009. Anonymous. Individual vaccines; pp. 16–98. [Google Scholar]
- 6.American Academy of Pediatrics. Committee on Infectious Diseases. Age for routine administration of the second dose of measles-mumps-rubella vaccine. Pediatrics. 1998;101:129–33. [PubMed] [Google Scholar]
- 7.Ghai OP, Paul VK, Bagga A, editors. 7th ed. New Delhi: CBS Publishers; 2009. Essential paediatrics. [Google Scholar]
- 8.Markowitz LE, Albrecht P, Orenstein WA, Lett SM, Pugliese TJ, Farrell D. Persistence of measles antibody after revaccination. J Infect Dis. 1992;166:205–8. doi: 10.1093/infdis/166.1.205. [DOI] [PubMed] [Google Scholar]
- 9.Wilkins J, Wehrle PF. Additional evidence against measles vaccine administration to infants less than 12 months of age: altered immune response following active/passive immunization. J Pediatr. 1979;94:865–9. doi: 10.1016/s0022-3476(79)80203-6. [DOI] [PubMed] [Google Scholar]
- 10.Stetler HC, Orenstein WA, Bernier RH, Herrmann KL, Sirotkln B, Hopfensperger D, et al. Impact of revaccinating children who initially received measles vaccine before 10 months of age. Pediatrics. 1986;77:471–6. [PubMed] [Google Scholar]
- 11.Ceyhan M, Kanra G, Erdem G, Kanra B. Immunogenicity and efficacy of one dose measles-mumps-rubella (MMR) vaccine at twelve months of age as compared to monovalent measles vaccination at nine months followed by MMR revaccination at fifteen months of age. Vaccine. 2001;19:4473–8. doi: 10.1016/s0264-410x(01)00207-9. [DOI] [PubMed] [Google Scholar]
- 12.Vandermeulen C, Leroux-Roels G, Hoppenbrouwers K. Mumps outbreaks in highly vaccinated populations: what makes good even better? Hum Vaccin. 2009;5:494–6. doi: 10.4161/hv.7943. [DOI] [PubMed] [Google Scholar]
- 13.Johnson CE, Kumar ML, Whitwell JK, Staehle BO, Rome LP, Dinakar C, et al. Antibody persistence after primary measles-mumps-rubella vaccine and response to a second dose given at four to six vs. eleven to thirteen years. Pediatr Infect Dis J. 1996;15:687–92. doi: 10.1097/00006454-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Böttiger M. Immunity to rubella before and after vaccination against measles, mumps and rubella (MMR) at 12 years of age of the first generation offered MMR vaccination in Sweden at 18 months. Vaccine. 1995;13:1759–62. doi: 10.1016/0264-410x(95)00143-o. [DOI] [PubMed] [Google Scholar]
- 15.Broliden K, Leven B, Arneborn M, Böttiger M. Immunity to measles before and after MMR booster or primary vaccination at 12 years of age in the first generation offered the 2-dose immunization programme. Scand J Infect Dis. 1998;30:23–7. doi: 10.1080/003655498750002259. [DOI] [PubMed] [Google Scholar]
- 16.Broliden K, Abreu ER, Arneborn M, Böttiger M. Immunity to mumps before and after MMR vaccination at 12 years of age in the first generation offered the two-dose immunization programme. Vaccine. 1998;16:323–7. doi: 10.1016/s0264-410x(97)88332-6. [DOI] [PubMed] [Google Scholar]
- 17.Pebody RG, Gay NJ, Hesketh LM, Vyse A, Morgan-Capner P, Brown DW, et al. Immunogenicity of second dose measles-mumps-rubella (MMR) vaccine and implications for serosurveillance. Vaccine. 2002;20:1134–40. doi: 10.1016/s0264-410x(01)00435-2. [DOI] [PubMed] [Google Scholar]
- 18.Rager-Zisman B, Bazarsky E, Skibin A, Chamney S, Belmaker I, Shai I, et al. The effect of measles-mumps-rubella (MMR) immunization on the immune responses of previously immunized primary school children. Vaccine. 2003;21:2580–8. doi: 10.1016/s0264-410x(03)00053-7. [DOI] [PubMed] [Google Scholar]
- 19.Bhargava I, Chhaparwal BC, Phadke MA, Irani SF, Chhapparwal D, Dhorje S, et al. Immunogenicity and reactogenicity of indigenously produced MMR vaccine. Indian Pediatr. 1995;32:983–8. [PubMed] [Google Scholar]
- 20.Yadav S, Thukral R, Chakravarti A. Comparative evaluation of measles, mumps & rubella vaccine at 9 & 15 months of age. Indian J Med Res. 2003;118:183–6. [PubMed] [Google Scholar]


