Abstract
Background & objectives:
Hyperprolactinaemia affects testicular functions by influencing hypothalamo-pituitary-testicular (HPT) axis at various levels. Available literature on the level of defect, time course of improvement of gonadal functions and its relation with decline in prolactin levels is scanty. We carried out this study to evaluate the HPT axis in patients with macroprolactinomas, before and six months after cabergoline therapy.
Methods:
Fifteen men with macroprolactinomas underwent gonadotropin and testosterone response to their respective stimuli before and after six months of cabergoline therapy.
Results:
Serum prolactin levels decreased after six months of therapy. Pretreatment, mean lutenizing and follicle stimulating hormones (LH and FSH) levels were 2.0 ± 0.4 and 1.4 ± 0.2 IU/l, respectively. However, LH and FSH responses to GnRH were preserved in majority of the patients and LH peaked to 12.1 ± 2.3 IU/l (P<0.01), while FSH to 2.9 ± 0.4 IU/l suggesting the influence of hyperprolactinaemia at the level of hypothalamus with preserved gonadotrope reserve. After cabergoline therapy, there was an increase in basal as well as stimulated LH and FSH levels, though these were not statistically significant when compared to respective pretherapy levels. Basal testosterone (T) levels significantly improved after therapy, but peak T response to hCG was similar at both pre- and post treatment. A significant correlation was observed between peak LH and peak T at baseline (r=0.53, P<0.01) and it further strengthened after therapy (r=0.70, P<0.01). After cabergoline therapy, there was significant improvement in seminal volume, sperm count and motility and sperm count correlated with peak FSH response (r=0.53, P<0.05).
Interpretation & conclusions:
Hyperprolactinaemia affects testicular functions probably by influencing at the level of hypothalamus resulting in subnormal basal secretion of gonadotropins required for optimal testicular functions.
Keywords: Hypogonadism, hypothalamus, macroprolactinomas, pituitary, testis
Impairment of gonadal functions is one of the most important clinical observation in both sexes with hyperprolactinaemia1–5. Though the clinical manifestations of hyperprolactinaemia and response to therapy with dopamine agonists in women are well described1,2, available data regarding the same in men are scant3–5. Hyperprolactinaemia in men typically leads to hypogonadism with consequent decreased libido, erectile dysfunction and abnormal semen quality4,5. However, these symptoms are frequently ignored as being considered non-specific, and most of the men present with mass effect.
Hyperprolactinaemia impairs male gonadal functions by affecting at various levels, possibly by decreasing gonadotropin releasing hormone (GnRH) pulse generator activity and /or by decreasing lutenizing hormone (LH) and follicle stimulating hormone (FSH) secretion, and might be inhibiting testosterone secretion at the level of Leydig cells as well6–9. Prolactin receptors have been demonstrated in the seminiferous epithelium, spermatogonia and spermatocytes, suggesting that prolactin might have a role to play in normal spermatogenesis7. On the other hand, hyperprolactinaemia leads to spermiogenic arrest and impairs motility and /or quality of spermatozoas10,11.
Previous studies have reported basal and stimulated gonadotropins and testosterone levels in patients with hyperprolactinaemia, may or may not improve after its normalization4,5,10 and are mainly limited to females12–14. Limited information is available to define the site of involvement at hypothalamo-pituitary-gonadal axis in men, the time course and chronology of its improvement with decline in prolactin levels. The present study was aimed to evaluate the hypothalamo-pituitary-testicular (HPT) axis in patients with macroprolactinomas, before and after 6 months of cabergoline therapy.
Material & Methods
Fifteen consecutive men with macroprolactinomas, between July 2005 to June 2006 attending Endocrine Clinic of Nehru Hospital at Postgraduate Institute of Medical Education and Research, Chandigarh, were recruited for the study. A written informed consent was obtained from all these subjects. The study protocol was approved by institute's ethics committee.
Selection criteria: Fifteen post-pubertal men with macroprolactinomas, (any tumour diameter > 10 mm) and serum prolactin level > 150 μg/l were recruited. All patients were drug naοve, although two patients, one after pituitary apoplexy and an other after pituitary surgery done three months prior to recruitment, had persistent hyperprolactinaemia with large residual pituitary tumour, were also included. The patients were assessed at monthly intervals during the six month study period. At each visit, history was noted and physical examination was carried out. Patients were investigated as per the following protocol. At the time of recruitment, at 0800 h, samples were drawn for haemogram, renal and liver function tests, and pooled samples (two aliquots of similar volume at 20 min intervals) for serum prolactin, T3, T4, TSH, cortisol, LH, FSH and testosterone. GnRH stimulation test was performed after inserting an iv catheter in antecubital vein. GnRH analogue Buserelin (100 μg) (Suprefact, Aventis Pharma, Deutschland GmbH) was injected as an iv bolus and venous samples for LH and FSH were drawn at –20, 30, 60, 90 and at 120 min. Two-fold increase in LH and one and a half fold increase in FSH from basal level after stimulation with GnRH was considered to be the optimal response15.
Five days after the GnRH stimulation test, human chorionic gonadotrophin (hCG) stimulation test was performed. After drawing basal sample for testosterone (T), intramuscular injection of 2000 IU hCG was given, and it was repeated at 24 and 48 h and at 72 h blood sample was drawn for testosterone. Post-stimulation serum testosterone level of > 9 nmol/l was regarded as the normal response16. Testosterone and prolactin levels were estimated at monthly intervals and these stimulation tests were repeated at 6 months. Semen analysis was performed after 3 days of abstinence at baseline and repeated after six months of cabergoline therapy, according to World Health Organization(WHO) Guidelines17. Semen analysis was done by a single observer. The normal ranges of sperm patterns following the WHO manual are: volume > 2 ml; pH 7.2-8.0; sperm concentration > 20×106/ml; total sperm count >40×106 spermatozoa/ejaculate; total motility > 50 per cent within 60 min of ejaculation and morphology >30 per cent sperms with normal morphology. The sperm motility was graded as follows: (i) rapid progressive motility along a linear tract covering a distance of 20 μm/sec, (ii) slow or sluggish progressive motility, (iii) non progressive motility, and (iv) immotility. Total motility defines percentage of sperms with forward progression (a+b). Total motility < 50 per cent was defined as asthenospermia. Azoospermia was defined by absence of sperms in ejaculate while sperm concentration less than normal was labelled as oligospermia. Teratozoospermia was defined as fewer than 30 per cent spermatozoa with normal morphology.
MRI of the pituitary region was done before starting therapy and after six months of initiating therapy. Tumour size was calculated by Dichiro's formula (π/6 × antero-posterior diameter × vertical diameter × transverse diameter18. Decrease in tumour size of > 25 per cent was considered as significant.
Serum prolactin, T3, T4 and TSH levels were assessed by immunoradiometric/radioimmunoassay using commercial kits (Advia Centaur, Inc, Germany) with intra- and interassay of coefficient of variation (CV) of < 8 per cent. Normal ranges for prolactin, T3, T4 and TSH levels were 5-20 μg/l, 0.6-1.81 ng/ml, 4.5-10.5 μg/dl and 0.35-5.5 mU/l, respectively. Serum testosterone (Normal range 9-27 nmol/l) and cortisol (0800 h N, > 350 nmol/l)19 were estimated with in-house radioimmunoassay. Serum LH, FSH (normal range, 1-12 IU/l) were estimated by enzyme linked immunosorbent assay (ELISA) using commercial kits (Syntron Bioresearch, Inc.).
Treatment protocol: Tablet cabergoline (Caberlin, Sun pharmaceutical, Goa, India) was prescribed at night with food as follows: for the first week it was given as 0.5 mg twice per week (e.g. Monday and Thursday). In the second week, 1.0 mg twice per week and during third week 1.5 mg twice per week as scheduled. The same dose (3 mg) was continued till 8 wk, as this is the usual effective dose shown previously3,20. At the end of the eighth week, the dose was deemed to be increased by 1.0 mg per week, if the serum prolactin levels are not normalized (<20 μg/l) by that time. Second hiking of dose (1mg/week) was planned at the end of sixteenth week if the serum prolactin levels still remained above the normal range.
Statistical analysis: Data are expressed as mean ± SEM unless otherwise specified. The statistical analysis was performed using the SPSS version 10.0 for windows, Chicago, Illinois, USA. The ANOVA was used for repeated measures to evaluate the effect of cabergoline throughout the follow up. Various hormonal parameters pre- and post-therapy were analyzed using Wilcoxon's test. Statistical significance was set at 5%. Correlations were performed by calculating the Spearman's coefficient as the data was not normally distributed.
Results
The mean age of patients was 31.7 ± 3.3 yr with duration of symptoms being 25.0 ± 3.6 months. All the patients recruited for the study completed without any dropout. The presenting symptoms and signs included headache (100%), decreased libido (100%), visual symptoms (100%), erectile dysfunction (67%), decreased shaving frequency (20%) and infertility (20%).
Mean serum prolactin at baseline was 6249.3 ±3259.2 μg/l, with a range of 186-47904 μg/l. All patients had macroprolactinomas with a mean tumour volume of 29.0 ± 8.3 cm3, ranging from 1.3 to 92.2 cm3. There was a significant correlation between tumour size and serum prolactin levels (r=0.6, P=0.05). All patients were euthyroid including one, who was on replacement with L-thyroxine therapy. Eight patients were hypocortisolic (0800 h cortisol between 100-350 nmol/l) and two of them received cortisol replacement as they were symptomatic while others were advised steroids only during stress period.
The mean dose of cabergoline required was 3.2 mg/week. Clinical symptomatology improved in all the patients with a precipitous fall in serum prolactin after one month of cabergoline therapy to 46.9 ± 14.9 μg/l (P<0.001) corresponding to a mean percentage decrease by 99 per cent from baseline. In nine (60%) patients, serum prolactin got normalized (<20 μg/l) at 2 month and it continued to decline and got normalized in all except one (93%), at 6 months of follow up with mean serum prolactin of 6.0 ± 2.7 μg/l with a range from 1.0 to 42.2 μg/l. This corresponded with a significant reduction in tumour volume to 5.8 ± 2.3 cm3 accounting for a mean reduction of 68 per cent (P<0.01). There were no new onset of hormone deficiencies and hypocortisolic state (0800 h serum cortisol > 350 nmol/l) improved in seven out of the eight patients, who were hypocortisolic at presentation.
Before therapy all these patients had low normal basal levels of LH and FSH with a respective mean of 2.0 ± 0.4 and 1.4 ± 0.2 IU/l. On stimulation with GnRH, peak LH response was 12.1 ± 2.3 IU/l (P=0.01) and was optimal (>2 fold) in all but three patients. Of these three patients, one had pituitary apoplexy and the other had pituitary surgery three months earlier and the third one had prolonged lag period between onset of symptoms and presentation (48 months). Post GnRH stimulation requisite FSH increase (>1.5 fold) was observed in all except in five patients, however, mean peak FSH levels (from 1.4 ± 0.2 to 2.9 ± 0.4 IU/l) did not attain significance. Of these five patients, three were the same mentioned above and in the other two, the probable cause could be the prolonged mass effect. After 6 months of cabergoline therapy, there was an increase in basal LH and FSH levels and the requisite stimulated LH response was achieved in all but FSH response remained below normal in these two patients with pituitary apoplexy and with prior pituitary surgery). The pre-treatment mean basal serum testosterone level was 7.4 ± 0.4 nmol/l and was low (< 9 nmol/l) in 11 (73%) patients. Peak testosterone response to hCG stimulation was optimal in all but two patients, prior to therapy. With initiation of therapy, circulating testosterone gradually increased in all patients (7.4 ± 0.4 to 12.1 ± 1.3 nmol/l, P=0.004)), and was normalized in 5 of the 11 (45%) patients after 6 months. However, pre- and post-therapy, peak T response to hCG stimulation (23.7 ± 3.3 and 24.5 ± 2.5 nmol/l, respectively) were comparable (Table I).
Table I.
Hormonal parameters at baseline and after 6 months of cabergoline therapy
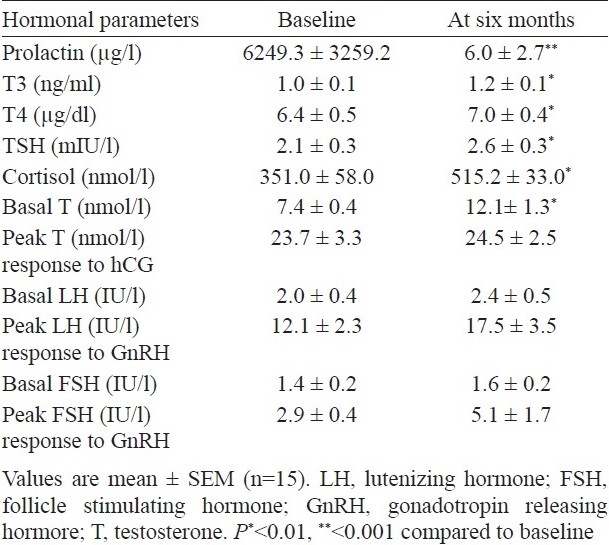
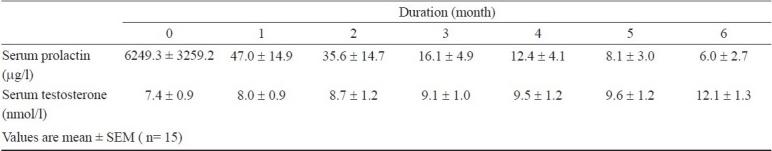
Increase in serum testosterone levels commensurated with the decrease in serum prolactin levels; however, the decrease in serum prolactin was much faster than increase in serum testosterone (Table II). There was a significant correlation between peak LH and peak T response before as well as after therapy to their respective stimuli (r=0.53, P=0.004) which further strengthened after 6 months of cabergoline therapy (r=0.70, P=0.004). A similar correlation was found between peak FSH and peak T responses to GnRH and hCG stimuli both before and after therapy (r=0.58 and 0.69; P=0.02 and 0.001 respectively).
Table II.
Serum prolactin and testosterone levels during study period
Semen analysis was done in 12 of the 15 patients at baseline, and in 3 it could not be performed because of dry ejaculate in 2 and one denied consent. Fine needle aspiration cytology of the testes was performed in the 2 patients with dry ejaculate which showed spermatogenic arrest. Total sperm count at presentation was 43.6 ± 9.4 million with a mean percentages of active sperms being 47.1 ±5.9 per cent. Before therapy, four patients had oligospermia, had asthenospermia and one had azoospermia. Sperms with abnormal morphology were less than 5 per cent (range 1-4%) in all these patients both before as well as after therapy. None of the patient had teratozoospermia. Six months of cabergoline therapy normalized seminal fluid parameters in all the patients except the one with azoospermia. After six months of therapy, seminal fluid volume significantly improved from 1.4 ± 0.3 to 2.0 ± 0.5 ml (P<0.05), mean sperm count increased to 73.7 ± 9.2 million (P<0.01) with mean percentage of active sperms being 52.7 ± 7.8 per cent (P< 0.01). A significant correlation was found between post-cabergoline peak FSH response and sperm count (r=0.63, P=0.017).
No major side effects of cabergoline were observed requiring discontinuation of the therapy. One patient each complained of nausea and sleepiness which subsided after 2 weeks of therapy. One patient developed apoplexy after four months of therapy. He was managed conservatively with steroids and improved later on.
Discussion
Our study shows hypothalamus to be the major site of affliction by hyperprolactinaemia as evidenced by preserved LH response to GnRH even prior to cabergoline therapy. The strengthening association between serum LH and testosterone during treatment substantiates subnormal secretion of gonadotropins is responsible for suboptimal testicular functions in patients with hyperprolactinaemia. The normal testosterone response to hCG at baseline denies the direct influence of hyperprolactinaemia on Leydig cells.
The site/s of involvement of HPT axis in macroprolatinomas, have not been adequately elucidated12–14. However, it has been shown that it is not only the involvement at the level of hypothalamus, but pituitary and Leydig cells have also shown to be affected6–9. This becomes more difficult in a situation, when the patient is harbouring macroadenomas that could per se compromise the gonadotrope functions. The preserved LH response to GnRH prior to therapy suggests that the hypothalamus is the main site of involvement by hyperprolactinaemia. Although GnRH stimulation with a single dose of intravenous bolus cannot clearly differentiate between hypothalamic or pituitary involvement, but preserved gonadotropin response suggests adequate pituitary reserve and that the pituitary is not the major site of affliction by hyperprolactinaemia. Evaluation of gonadotropin pulses would have further substantiated our results. Insignificant increase in FSH in response to GnRH can be explained on the basis that even in normal circumstances delta FSH response to GnRH is lesser than the delta LH response15 and probably hyperprolactinaemia more severely affects FSH than LH levels. Further improvement in LH and FSH response to GnRH, after cabergoline therapy, suggests improvement in gonadotrope functions due to normalization of prolactin levels, and decrease in compressive effect on gonadotopes by the tumour. This suggests that despite of harbouring large sized tumours, alterations in HPT axis in majority of the patients are functional. We used short acting GnRH analogue (buserelin) to assess gonadotrope reserve. This was based on a previous study with nafarelin, a short acting GnRH analogue, where results have been found to be comparable with native GnRH21, and our experience in patients with true precocious puberty as a diagnostic test is similar (unpublished data).
Prolactin receptors have been well documented, not only on Leydig cells but also on seminiferous tubular epithelial cells7. It is also proposed that, an optimal concentration of prolactin is required for normal function of Leydig cells6. It has been well documented that Leydig cell function and semen quality is impaired in patients with hyperprolactinaemia5. In the present study, the basal testosterone levels were subnormal in majority of the patients and it progressively increased in all patients with normalization of serum prolactin. However, the peak testosterone response to hCG, both pre- as well post-cabergoline therapy was normal in majority of patients suggesting that the Leydig cell response is only functionally impaired, possibly because of subnormal LH levels. It is further substantiated by progressively strengthening association observed between peak LH and peak testosterone response after cabergoline therapy. This also refutes the possibility of direct effect of hyperprolactinaemia on the Leydig cell responsiveness to LH. It has been shown earlier that the time course between the normalization of prolactin levels and recovery of Leydig cell function may not commensurate22. It was also noted that some of the patients did not have subnormal testosterone levels despite hyperprolactinaemia even before initiation of therapy. Moreover, the increase in serum testosterone level did not synchronize with decrease in serum prolactin as circulating prolactin decreased by 99 per cent at 4 wk of cabergoline therapy, while serum testosterone increased only by <10 per cent suggesting that the time course of recovery of Leydig cell is tardy but progressive as serum testosterone levels got normalized later.
Abnormalities in semen quality in patients with hyperprolactinaemia is attributed to impaired germ cell function due to low FSH and decreased intra-testicular testosterone due to low LH, which is usually reversible with treatment. An appreciable improvement in semen quality was observed during the study which is in accordance with others5.
Limitations of the study include small sample size, lack of control group, no assessment of symptomatology by any validated questionnaire and lack of estimation of estradiol levels which might also influence hypothalamo-pituitary-testicular axis.
In conclusion, impairment of HPT axis in majority of patients with hyperprolactinaemia is functional even in the presence of macroadenomas. Hyperprolactinaemia affects gonadal function possibly by acting at the level of hypothalamus resulting in subnormal basal secretion of gonadotropins required for optimal testicular functions. Recovery of gonadal functions begins soon after initiating treatment with cabergoline though duration of six months is not enough for its complete recovery.
References
- 1.Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptin in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. N Engl J Med. 1994;331:904–9. doi: 10.1056/NEJM199410063311403. [DOI] [PubMed] [Google Scholar]
- 2.Verhest J, Abs R, Maiter D, van den Bruel A, Vandeweghe M, Velkeniers B, et al. Cabergoline in treatment of hyperprolactinemia: a study in 455 patients. J Clin Endocrinol Metab. 1999;84:2518–22. doi: 10.1210/jcem.84.7.5810. [DOI] [PubMed] [Google Scholar]
- 3.Corsello SM, Ubertini G, Altomare M, Lovicu RM, Migneco MG, Rota CA, et al. Giant prolactinomas in men: efficacy of cabergoline treatment. Clin Endocrinol (Oxf) 2003;58:662–70. doi: 10.1046/j.1365-2265.2003.01770.x. [DOI] [PubMed] [Google Scholar]
- 4.De Rosa M, Colao A, Di Sarno A, Ferone D, Landi ML, Zarrilli S, et al. Cabergoline treatment rapidly improves gonadal function in hyperprolactinemic males: a comparison with bromocriptine. Eur J Endocrinol. 1998;138:286–93. doi: 10.1530/eje.0.1380286. [DOI] [PubMed] [Google Scholar]
- 5.Colao A, Vitale G, Cappabianca P, Briganti F, Ciccarelli A, De Rosa M, et al. Outcome of cabergoline treatment in men with prolactinoma: effects of a 24 months treatment on prolactin levels, tumor mass, recovery of pituitary function and semen analysis. J Clin Endocrinol Metab. 2004;89:1704–11. doi: 10.1210/jc.2003-030979. [DOI] [PubMed] [Google Scholar]
- 6.Horseman ND, Gregerson KA. Prolactin. In: DeGroot LJ, Jameson JL, editors. Endocrinology. Philadelphia: Elsevier Saunders; 2006. pp. 309–21. [Google Scholar]
- 7.Hondo E, Kurohmaru M, Sakai S, Ogawa K, Hayashi Y. Prolactin receptor expression in rat spermatogenic cells. Biol Reprod. 1995;52:1284–90. doi: 10.1095/biolreprod52.6.1284. [DOI] [PubMed] [Google Scholar]
- 8.Bex FJ, Bartke A. Testicular LH binding in the hamster : modification by photo period and prolactin. Endocrinology. 1977;100:1223–6. doi: 10.1210/endo-100-4-1223. [DOI] [PubMed] [Google Scholar]
- 9.Bere F, Bartke A, Goldman BD and Dalterio S. Prolactin, growth hormone, luteinizing hormone receptors and seasonal changes in testicular activity in the golden hamster. Endocrinology. 1978;103:2069–80. doi: 10.1210/endo-103-6-2069. [DOI] [PubMed] [Google Scholar]
- 10.Carter JN, Tyson JE, Tolis G, Van Vliet S, Faiman C, Friesen HG. Prolactin-screening tumors and hypogonadism in 22 men. N Engl J Med. 1978;299:847–52. doi: 10.1056/NEJM197810192991602. [DOI] [PubMed] [Google Scholar]
- 11.Segal S, Yaffe H, Laufer N, Ben-David M. Male hyperprolactinemia: effects on fertility. Fertil Steril. 1979;32:556–61. doi: 10.1016/s0015-0282(16)44359-1. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki T, Azyuma K, Irahara M, Yasuit T, Aono T. Mechanism of anovulation in hyperprolactinemic amenorrhea determined by pulsalite gonadotropin-releasing hormone injection combined with human chorionic gonadotropin. Fertil Steril. 1994;62:1143–9. doi: 10.1016/s0015-0282(16)57176-3. [DOI] [PubMed] [Google Scholar]
- 13.Moult PJ, Rees LH, Besser GM. Pulsalite gonadotrophin secretion in hyperprolactinaemic amenorrhoea and the response to bromocriptine therapy. Clin Endocrinol (Oxf) 1982;16:153–62. doi: 10.1111/j.1365-2265.1982.tb03159.x. [DOI] [PubMed] [Google Scholar]
- 14.Koizumi K, Aono T, Koike K, Kurachi K. Restoration of LH pulsatility in patients with prolactinomas after transphenoidal surgery. Acta Endocrinol (Copenh) 1984;107:433–8. doi: 10.1530/acta.0.1070433. [DOI] [PubMed] [Google Scholar]
- 15.Bhasin S, Jameson JL. Harrisons’principles of internal medicine. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Disorders of the testes and male reproductive system. New York: The McGraw Hill Companies, Inc.; 2005. pp. 2185–97. [Google Scholar]
- 16.Kauschansky A, Dickerman Z, Phillip M, Weintrob N, Strich D. Use of GnRH agonist and human chorionic gonadotrophin tests for differentiating constitutional delayed puberty from gonadotrophion deficiency in boys. Clin Endocrinol. 2002;56:603–7. doi: 10.1046/j.1365-2265.2002.01520.x. [DOI] [PubMed] [Google Scholar]
- 17.WHO Laboratory manual for the examination of human semen and sperm-cervical mucus interactions. Cambridge, UK: Cambridge University Press; 1992. World Health Organization. [Google Scholar]
- 18.Lundin P, Pedersen F. Volume of pituitary macroadenomas : assessment by MRI. J Comput Assist Tomogr. 1992;16:518–28. doi: 10.1097/00004728-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Inder WJ, Hunt PJ. Glucocorticoid replacement in pituitary surgery: Guidelines for perioperative assessment and management. J Clin Endocrinol Metab. 2002;87:2745–50. doi: 10.1210/jcem.87.6.8547. [DOI] [PubMed] [Google Scholar]
- 20.Bhansali A, Walia R, Dutta P, Khandelwal N, Sialy R, Bhadada S. Efficacy of cabergoline on rapid escalation of dose in men with macroprolactinomas. Indian J Med Res. 2010;131:530–5. [PubMed] [Google Scholar]
- 21.Chaplin MD. Bioavailability of nafarelin in healthy volunteers. Am J Obstet Gynecol. 1992;166:726–5. doi: 10.1016/0002-9378(92)91710-r. [DOI] [PubMed] [Google Scholar]
- 22.Biller BMK, Molitch ME, Vance ML, Cannistraro KB, Davis KR, Simons JA, et al. Treatment of prolactin–secreting macroadenomas with the once weekly dopamine agonist cabergoline. J Clin Endocrinol Metab. 1996;81:2338–43. doi: 10.1210/jcem.81.6.8964874. [DOI] [PubMed] [Google Scholar]


