Abstract
Background & objectives:
WNIN/Ob (obese and euglycaemic) and WNIN/GR-Ob (obesity with impaired glucose tolerance), were isolated and established from Wistar rat stock (WNIN). Both strains showed physical, physiological and biochemical indices related to obesity. We present here haematology, histology and pathophysiological changes between the phenotypes of these strains, lean (+/+), carrier (+/-) and obese (-/-).
Methods:
A total of 72 animals of equal gender consisting of three phenotypes were used for the study. Haematology, organ weights were measured and histopathology of the tissues studied using standard procedures. In 12 lean and obese rats (equal gender) of WNIN/GR-Ob group morphometry of pancreatic islets was done immunohistochemically (IHC).
Results:
Obese rats of both the strains showed normal haematology (except low platelet count), but exhibited changes in the organ weights and in histopathology in organs like liver, kidney, brain and testis/ovary. Hyperplasia of adipocytes was seen in obese rats as compared to lean and carrier. IHC of obese rat pancreas showed that both islet density and volume were significantly (P<0.05) increased compared to lean littermates.
Interpretation & conclusions:
The histological and pathophysiological changes seen in these mutants were in tune with obese phenotype exhibited by these animals.
Keywords: Fat cell, glomerular necrosis, hepatomegaly, hyperplasia of adipocytes, insulin resistance, islet density
Obesity apart from insulin resistance (IR) is a major risk factor associated with diabetes and is a major public health problem affecting more than 1 billion people all over the world1. Several rodent models, either induced or spontaneous have been used to study the mechanistic basis behind the development of human type II diabetes associated with obesity. Some of the frequently used rodent models are Otsuka Long Evans Tokushima fatty (OLETF), Goto-Kakizaki (GK), Zucker Diabetes Fatty (ZDF), and Bio-Breeding Zucker diabetes-resistant (BBZDR/Wor) rats2.
Most of these animal models have several features of human type II diabetes, including glucose intolerance with hyperinsulinaemia, IR and obesity3. Wistar diabetic fatty rat (WDF/fa-fa) is a well-known animal model, which is hyperglycaemic, hyperinsulinaemic with glucose intolerance and shows decreased insulin sensitivity4. Spontaneous hypertensive/NIH-Corpulent (SHR/N-cp) and Spontaneous hypertension and heart failure/Mcc-corpulent (SHHF/Mcc-cp) strains developed from SHR/Ob rat share the corpulent gene for obesity. In these animals, glucose intolerance is associated with insulin and glucagon resistance, decreased insulin binding5, insulin stimulated glucose uptake by adipocytes and increased gluconeogenic activity in the liver and kidney6. Additionally, SHHF/Mcc-cp male rats exhibit hypertension and congestive heart failure. Among other corpulent non-insulin dependent diabetic mellitus (NIDDM) rat models, Jcr:LA and LA/N-cp also show IR and glucose intolerance7,8. All these mutants have been developed in the west from a random bred or outbred stock, and their mutations were found to be due to deficit in leptin receptor gene.
In our laboratory unique obese mutant strains designated as WNIN/Ob and WNIN/GR-Ob were developed from the parental WNIN [Wistar inbred strain maintained at National Institute of Nutrition (NIN)] stock of rats9,10. Though both males and females were obese, the trait was preponderant in males of both the strains. The animals showed visible obesity from 35 days of age with hyperphagia, polydypsia, polyuria and additionally glycosuria in WNIN/GR-Ob mutants. Biochemically the obese mutants of both strains showed hyperinsulinaemia, hypertriglyceridaemia, hypercholesterolaemia and hyperleptinaemia. And additionally hyperglycaemia on challenge with oral glucose was seen in WNIN/GR-Ob mutants. The homozygous obese animals from both strains were infertile and propagation of these rats was carried out, by mating heterozygous carriers (+/-).
The animals being overweight for their age, weight-associated changes in the various organs and some adaptive changes especially in the major organs like heart, liver, kidney, and brain are anticipated and the present study was carried out for characterizing the obese mutants, in-terms of parameters like haematology, organ weights, histopathology and adipocyte density. In the present study we also carried out the morphometry of islets in WNIN/GR-Ob obese and lean rats to see the ß-cell population by immunohistochemistry in the pancreas to confirm whether these animals inherited the IGT trait from its parental stock.
Material & Methods
The rats used in the study were housed individually in standard polycarbonate cages with top grill (Techniplast, Italy) and given a standard rodent chow developed at National Centre for Laboratory Animal Sciences, National Institute of Nutrition, Hyderabad containing 56 per cent carbohydrate, 18.5 per cent protein, 8 per cent fat, 12 per cent fiber and recommended levels of minerals and vitamins. UV sterilized drinking water (Aqua guard online water filter-cum-purifier, Eureka Forbes Ltd, India) in polycarbonate bottles with stainless steel sipper tubes were provided to rats. Rats were housed at a temperature of 24 ± 2°C with 14-16 air changes per hour and relative humidity (60 ± 5%) and kept on a 12 h light-dark cycle. The animals had free access to food and water.
For haematological studies, 6 males and 6 females from both lean and obese rats belonging to WNIN/Ob and WNIN/GR-Ob were taken, along with parental WNIN rats as the control. For histological studies, organ weights and morphometry of pancreatic islets (in WNIN/GR-Ob), a total of 72 animals (6 male and 6 female of each phenotype i.e., lean, carrier and obese) of 105 days age were taken from WNIN/Ob and WNIN/GR-Ob groups.
Haematology: After overnight fast blood was drawn from one of the supra orbital sinus via the inner canthus by using heparanized microcapillary tubes11. Blood (1 ml) was collected in tubes containing 1 per cent EDTA-potassium containing vials was used for haemoglobin (Hb%), red blood cell (RBC), total white blood cell (WBC) and platelet counts as well as packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), monocyte (%) and lymphocyte (%) on an automated cell counter (SERONO BAKER System 9120 CP+, UK).
Organ weights and histopathology: Rats were fasted for 24 h and euthanized by CO2inhalation and subjected to gross necropsy. External features suggesting any abnormality were looked into. After opening the viscera an in situ examination was done. The major organs like brain, thyroid, heart, lungs, liver, spleen, kidneys, adrenals, pancreas and sex organs were collected. After detailed gross necropsy examination, these organs were trimmed of fat and blotted on filter paper and weighed (Essae Digi analytical balance, ES-DIGI, India) and organ to body weight ratio was measured12. The organs slices (5 mm thickness) were fixed in 10 per cent neutral buffered formalin/Bouin's fluid overnight. All the tissues were subjected to routine standard histopathological processing and staining procedures. Five-micron paraffin sections were stained with hematoxylin and eosin and examined under a light microscope (Leitz Diaplan, USA).
Adipocyte distribution and density: For adipocyte distribution and density, representative samples from cutaneous, subcutaneous, retroperitoneal, abdominal wall, omentum and mesenteric, pelvic and femoral sites were taken from each phenotype of WNIN/Ob group. White adipose tissue was collected and fat cell density was measured in a calibrated microscope eyepiece graticule at X 400 magnification. The number of cells within the marked area were counted and expressed as cells per sq.mm.
Immunohistochemistry (IHC) of pancreas: IHC of pancreas was carried out by Avidin: Biotin complex method (ABC) for identification of ß-cells containing insulin (by specific antigen-antibody interaction), wherein the antibody has been tagged with a visible label i.e., chromogen13–15.
For obtaining the islet number, slides were examined under a magnification of 10 X. The number of islets was then counted in given field. Area was calculated using the calibrated ocular grid. Islet number was expressed as a number of islets/sq.mm area. Islet size was given as an average islet area in sq.mm, by taking one representative sample from each phenotype, i.e., lean and obese. The entire slide was analyzed for the area occupied by each islet and the average of all these observations was calculated. For obtaining ß-cell number, the number of cells showing brownish granules (indication of insulin stained with the chromogen di-amino benzidene) were counted. The results were expressed as (i) average number of ß-cells per observed area, (ii) as a derived number per sq.mm of the grid.
The study protocol was reviewed and approved by the Institutional Animal Ethical Committee (IAEC), and was conducted in accordance with the internationally accepted principles for laboratory animal use and care.
Statistical analysis: Statistical significance of differences between the groups was determined by student's unpaired “t” test. ANOVA was carried out with multiple comparisons using Duncan's multiple range test. P<0.05 was considered significant.
Results
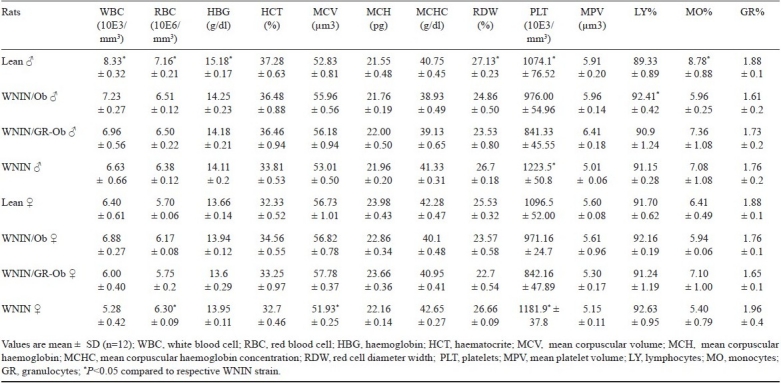
Haematology: The female rats of all groups exhibited lower values compared to males for all the haematological parameters analyzed (Table I). However, there was no significant differences among the groups for any of the parameters, except for platelet counts, (expressed as 10E3/mm3 of blood) which were significantly (P<0.01) low in mutant rats of both groups and both sexes compared to WNIN strain in both the genders (P<0.05).
Table I.
Haematological values of lean and obese phenotypes of WNIN/Ob and WNIN/GR-Ob rats with normal WNIN rats (n=12)
Organ weights: In both the mutant strains, males showed significantly higher organ weights with respect to brain, thyroid, heart, liver and kidney than females in all the three phenotypes (P<0.05). In both the strains of mutants, except for testis, weights of all the other organs were comparable between lean and carrier of both sexes (Tables II, III). Major organs like liver, heart, lungs, kidneys, testis/ovary and brain weights were calculated for organ-to-body weight ratio and given in (Table IV). When the organ weights were calculated for their ratio, the obese rats of both groups were found to be lower compared to their lean and carrier counterparts. There is up to 50 per cent of reduction in their organ-to-body weight ratio in obese rats of both groups.
Table II.
Body weights and various organ weights (g) of three phenotypes of WNIN/Ob group (n=6)
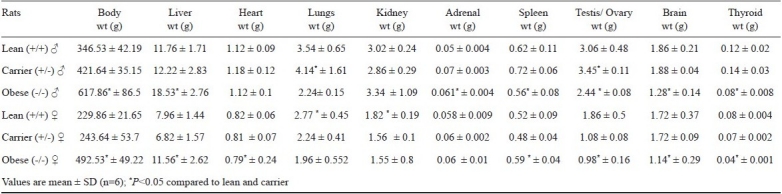
Table III.
Body weights and various organ weights (g) of three phenotypes of WNIN/GR-Ob group (n=6)
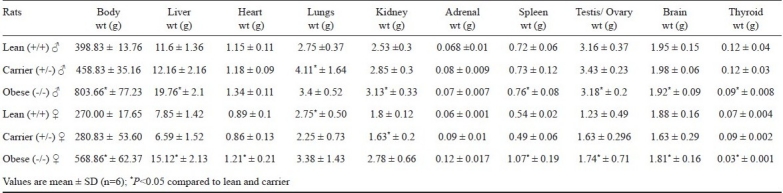
Table IV.
Organ to body weight ratio in three phenotypes of WNIN/Ob and WNIN/GR-Ob groups (n=6)
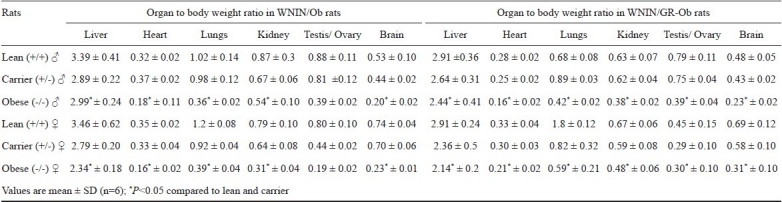
In obese phenotypes, the brain and thyroid weights were significantly low compared to other phenotypes; however, the lean and carrier had more or equal weights. Heart weights in females of both the groups of obese mutants were significantly less, when compared to lean and carrier (P<0.05). In lean rats of WNIN/GR-Ob, there was no difference in lung weights between males and females. However, significant differences were seen in obese and carrier rats of both strains between genders. In both the strains, the carrier (+/-) males had higher lung weight than females. The liver weight was significantly high in both genders of the mutants compared to lean (+/+) and carrier (+/-) rats (P<0.05). In +/- and +/+ rats of both strains, spleen weight was found to be higher in males compared to females. However, in obese phenotype (-/-), it was reverse. No sexual dimorphism was seen in adrenal weights of mutant rats.
In obese mutants of both strains, all males and only females of WNIN/Ob show higher kidney weight compared to lean and carrier. The WNIN/GR-Ob obese rats had lower weight than lean and carrier (P<0.05). Obese phenotypes of WNIN/Ob and WNIN/GR-Ob had low testis weight compared to lean littermates. The heterozygous carrier rats had significantly higher testis weight compared to homozygous obese and lean phenotypes. In WNIN/GR-Ob, ovary weight of obese (-/-) was higher compared to lean (+/+) and carrier (+/-), while in WNIN/Ob, the obese mutants; it was equal to carrier (+/-) and lower to lean (+/+).
Histopathology: Histopathological changes were observed in lungs, liver, and reproductive organs of both the obese mutants and additionally in kidney and adrenal of WNIN/GR-Ob. Other organs like brain, heart, trachea, spleen, foregut and glandular stomach were normal. Lungs of all animals (-/-, +/- and +/+) showed chronic interstitial pneumonitis grade III. The liver was pale in colour with hepatomegaly, as reflected by organ weights and showed periportal round cell infiltration. The circular clear spaces observed in the hepatocytes in obese rats represent areas previously occupied by fat and dissolved out by xylene during processing of the tissue. The liver of obese rats showed both micro- and macro-vesicular steatosis of moderate to severe degree in most of the hepatic lobules. In WNIN/GR-Ob rats, additionally some of the animals showed focal areas of necrosis with sinusoidal congestion (Fig. 1A). Kidneys of WNIN/GR-Ob rats (2/6) showed focal and partial glomerular necrosis (Fig. 1B) while in the rest, kidneys showed focal peritubular round cell infiltration with interstitial fibrosis and focal tubular necrosis. Adrenals in WNIN/GR-Ob (3/6) showed fatty changes in zona fasciculata, and the thyroid showed micro- and macro-follicles (Fig. 1C). Shrunken testis with normal spermatogenesis was observed in both the obese phenotypes. The uterine cavity of obese mutants of both groups had purulent material inside (Fig. 1D). The ovaries in WNIN/GR-Ob mutants (2/6) showed cystic corporaluteae and congestion (Fig. 1E). Except for changes in lung histology, the lean and carrier animals of both mutants presented a normal histological picture.
Fig. 1.
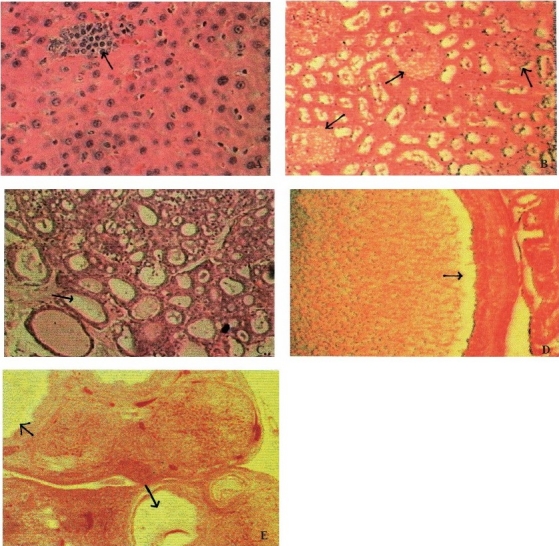
Photomicrograph of lesions observed in various organs of WNIN/GR-Ob rats. (A) Focal areas of necrosis in liver; (B) Glomerular necrosis in kidney; (C) Follicles devoid of colloid in thyroid; (D) Pus cells in uterus cavity; (E) Cystic ovary with corporalutea. (Lesions represented by arrows).
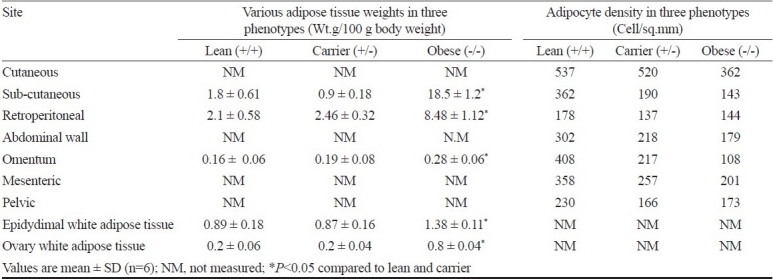
Adipocyte studies: The distribution of fat (white adipose tissue) in all the three phenotypes in WNIN/Ob group was in the cutaneous, subcutaneous, retroperitoneal, abdominal wall, omentum, mesenteric, pelvic and femoral areas. Maximum distribution of fat was seen in cutaneous and subcutaneous areas followed by abdominal fat represented by retroperitoneal, omental and mesenteric areas. Irrespective of the area, more fat was seen in the obese (-/-) phenotype, followed by carrier (+/-) and lean (+/+) animals.
The white adipose tissue weight at 6 major areas and adipocyte density at 7 major areas and of the body for three phenotypes of WNIN/Ob (lean, carrier and obese) is given in Table V. In general, the density was high in +/+, followed by +/- and -/-. In +/- rats, the density in specified areas was close to that of the +/+ (cutaneous) and -/- (retroperitoneal and pelvic), and in other areas, it was less than +/+ but higher than -/- (subcutaneous, omentum). The adipocyte density in -/- animals was much less compared to +/+ at every site examined, and also in most of the areas compared to +/- animal. Similar results were seen in WNIN/GR-Ob rats.
Table V.
Adipocyte density in three phenotypes of WNIN/Ob group (n=6)
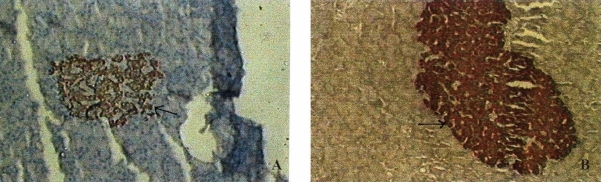
Immunohistochemistry: The islet density as well as the area in lean rats was significantly less compared to obese rats. The islet density in lean rats was 1.03±0.02/sq.mm and in WNIN/GR-Ob mutant rats 4.12 ± 0.08/sq. mm (Fig. 2A & 2B). The average area of islet in lean rats was 0.006 ± 0.00001/sq.mm, while it was 0.14 ± 0.002/sq.mm in obese mutants. The average number of β-cells per islet was significantly higher (P <0.01) in obese rats (221.59 ± 66.23) compared to lean rats (45.34 ± 9.82) (Fig. 3A & 3B). However, when the number was expressed per sq. mm, there was no significant difference between the two phenotypes. Apart from increase in number, the islets in obese rats were mostly irregular and larger, in contrast to lean animals, where the islets shape and size were normal.
Fig. 2.
Photomicrograph of immunohistochemistry details of pancreatic islets of WNIN/GR-Ob rats. (A) Islet density in lean; (B) Islet density in obese rats. (Islets represented by arrows).
Fig. 3.
Photomicrograph of ß - cell density in islet of WNIN/GR-Ob lean (A), and obese rats (B) (Number of ß - cell represented by arrows).
Discussion
Most of the haematological parameters analyzed were within the normal range with evident differences between males and females in all the phenotypes of both the mutant strains. The platelet counts were significantly decreased in both the obese mutant strains compared to WNIN parent strains, the physiological implications of which need to be assessed by detailed analysis of related parameters.
The higher liver weights seen in obese animals were due to hepatomegaly, which was confirmed by gross body weight exhibited by these animals. This is a common condition seen in all obese rodents arising genetically as well as when experimentally induced16–18. Liver from obese SHR/N-cp rats showed widespread deposition of fat globules of different size inside parenchymal cells. Microvescicles of fat appeared to be localized in both pericentral and periportal hepatocytes with a slight preponderance in pericentral zones19. Similar observations were seen in the present study in obese rat liver of WNIN obese mutant rats.
SHR/N-cp obese males and females exhibited glomerular lesions characterized by segmental diffusion and nodular lesions20. Progressive kidney failure is prevalent in fa/fa rats and is manifested by polydypsia and polyurea21. Glomerulosclerosis was seen as early as 5 months of age in ZDF rats. It is associated with glomerular hypertrophy and mild mesangial expansion. Diabetic nephropathy features like diffuse glomerulosclerosis, nodular lesion, and thickening of basement membrane, mesangial proliferation and fibrin cap were observed in OLETF rat22. Glomerular and other lesions were observed in WNIN/GR-Ob mutants in the present study. Thus, it can be used as a model to understand the mechanism behind the development of obesity associated diabetic complications, especially related to kidney.
Reduction in brain weight was also seen in other obese rodents and this seems to be specific to obesity23. Heart weights in present study showed sexual dimorphism, i.e. only females showed lower weights compared to lean and control, which is not reported in any other model. Usually the reported weights are either high or equal to other phenotypes24. The lung weights in obese mutant rats compared to other phenotypes were almost the same and all the animals had varying grades of pneumonitis. Fatty changes were observed in adrenals of obese mutant rats in zona fasiculata indicative of active and higher production of glucocorticoids. Thyroid function also could be altered in these obese rats as significant reduction in thyroid weights was seen, unlike the other obese rodents16. The reduction in gonadal weights in WNIN obese mutants was also expected and this was shown by many other genetically obese rodents also25,26. However, while the testis showed normal spermatogenesis on histology, the ovarian histopathology showed changes, implying varying levels of gonadal hormone deficiency among the genders, being more severe in females compared to males.
The distribution of fat in WNIN obese rats was like other obese models, distributed in all the major sites such as cutaneous, subcutaneous and abdominal regions. Obesity in these animals appears to be due to hypertrophy of adipocytes as seen at all major sites examined. Hypertrophy of fat cells has been shown in at least four forms of inherited obesity as well as in two forms resulting from injury to hypothalamus27,28, and this could be due to a general response to hyperphagia and hypertriglyceridaemia they exhibit. In models like Zucker rats, both hyperplasia as well as hypertrophy of the fat cells was also seen16.
Increased production of insulin can be achieved by hyperplasia of islets as seen in many NIDDM models29,30 or ß-cells hyperplasia itself as shown by db/db mice31. IHC study showed an increase in the islet density in WNIN/GR-Ob rats along with an increase in islet area. Subsequently an increase in number of the ß-cells was also seen. But when the number of ί-cells were expressed per sq.mm area, no difference between lean and obese rats could be seen, indicating that the compensatory mechanism for hyperinsulinaemia is not by hyperplasia of ß-cells, but due to an overall increase in islet density.
WNIN/GR-Ob rats are similar to lean GK rat in terms of onset of pre-diabetic conditions and also to pre-diabetic Chinese hamsters. However, WNIN/GR-Ob rats are only glucose intolerant and not frankly diabetic. In many well-established laboratory diabetes rodent models, hyperplasia of the ß-cell is followed by degranulation along with reduction in number and even total absence of ß-cells32,33. It will be worthwhile to look at the islet morphology as and when the WNIN/GR-Ob rats develop frank diabetes like fasting hyperglycaemia and other characteristics. The preliminary studies carried out in our laboratory show that these rats are diet sensitive and develop frank diabetes by 4-6 wk of feeding purified carbohydrate diets (unpublished data). Thus diabetes can be produced in these animals by dietary manipulation without treatment of alloxan and streptozotocin.
In conclusion, the data presented in terms of gross organ weights, histopathology and immunohistochemistry equivocally prove the obese nature of the mutants established from the parental Wistar stock (WNIN) in our animal facility. The life span of these animals is reduced to half, and as they cross one year develop opportunistic infections, cataract and retinal degeneration (10-15%)34, mammary tumours and lipomas (over 50-60%) and kidney abnormalities in most of them. Recently, these mutants have been shown to develop hypertension35, thus making it an ideal model to study metabolic syndrome with all its variations.
Acknowledgments
Authors acknowledge the Indian Council of Medical Research (ICMR) and Department of Biotechnology (DBT), Government of India, New Delhi, for the financial support, and thank Shriyut E. Ganesh and N. Yadagiri animal caretakers, for their co-operation in the maintenance of obese rat colony. Ms. K. Sharada for technical assistance. Authors also thank Dr Kamala Krishnaswamy, former director of National Institute of Nutrition for her support in this work.
References
- 1.Kumanyika SK, Obarzanek E, Stettler N, Bell R, Field AE, Fortmann SP, et al. Population-based prevention of obesity. Circulation. 2008;118:428–64. doi: 10.1161/CIRCULATIONAHA.108.189702. [DOI] [PubMed] [Google Scholar]
- 2.Rebecca ST, Joan FF, Tiangen Wu, Edward HK, Paul JB, Dennis LG. The BBZDR/Wor Rat model for investigating the complications of type 2 diabetes mellitus. Int Lab Anim Res News. 2004;45:292–302. [Google Scholar]
- 3.Shafrir E. Diabetes in animals. In: Rifkin T, Porte D, editors. Ellenberg & Rifkins diabetes mellitus. 4th ed. New York: Elsevier; 1990. pp. 231–56. [Google Scholar]
- 4.Ikeda H, Shino A, Matsuo T. A new genetically obese hyperglycemic rat (Wistar fatty) Diabetes. 1981;30:1045–50. doi: 10.2337/diab.30.12.1045. [DOI] [PubMed] [Google Scholar]
- 5.Bhathena SJ, Kennedy BW, Jones J, Smith PM, Michaelis OE, 4th, Carswell N, et al. Effect of dietary carbohydrate on insulin and glucagon receptors in a new model of NIDD-SHR/N-Cp rat. Proc Soc Exp Biol Med. 1989;192:66–71. doi: 10.3181/00379727-192-42957. [DOI] [PubMed] [Google Scholar]
- 6.Baly DL, Zarnowski MJ, Carswell N, Michaelis OE., IV Insulin resistant glucose transport activity in adipose cells from the SHR/N-Cp rat. J Nutr. 1989;119:628–32. doi: 10.1093/jn/119.4.628. [DOI] [PubMed] [Google Scholar]
- 7.Russell JC, Koeslag DG. Jcr:LA - Corpulent rat; A strain with spontaneous vascular and myocardial disease. Int Lab Anim Res News. 1990;32:27–32. [Google Scholar]
- 8.Tulp OL. Characteristics of thermogenesis, obesity and longevity in LA/N-cp rat. Int Lab Anim Res News. 1990;32:32–8. [Google Scholar]
- 9.Giridharan NV, Harishankar N, Satyavani M, Lakshmi CN. WistarNIN/Igt, WistarNIN/Ob, WistarNIN/Ob-Igt. Three mutant animal models for diabetes and obesity from a Wistar inbred colony. Rat News Lett. 1993;29:5–7. [Google Scholar]
- 10.Giridharan NV, Harishankar N, Satyavani M. WNIN/Ob, A new rat model for the studies of obesity. Scan J Lab Anim Sci. 1996;23:131–8. [Google Scholar]
- 11.Riley V. Adaptation of orbital bleeding technique to rapid serial blood studies. Proc Soc Exp Biol Med. 1960;109:751–4. doi: 10.3181/00379727-104-25975. [DOI] [PubMed] [Google Scholar]
- 12.Bratthauer GL. Avidin -Biotin labeling of cellular antigens in cryostat-sectioned tissue. In: Javois LC, editor. Methods in molecular biology. Vol. 4. Clifton JC; 1994. pp. 175–84. [DOI] [PubMed] [Google Scholar]
- 13.Van Noorden S, Polak JM. Practical applications in pathology and biology. In: Polak JM, Van Noorden S, editors. Bristol England: Wright PSG; 1983. pp. 11–42. [Google Scholar]
- 14.Hsu SM, Cossman J, Jaffe ES. Unlabelled antibody and conjugated immunohistochemical methods with monoclonal and polyclonal antibodies-an examination of germinal center of tonsils. Am J Clin Pathol. 1983;80:429–35. doi: 10.1093/ajcp/80.4.429. [DOI] [PubMed] [Google Scholar]
- 15.Bindhu Michael, Barry Yano, Rani S, Sellers, Perry Rick, Morton Daniel, Roome Nigel, et al. Evaluation of organ weights for rodent and non-rodent toxicity studies: A review of regulatory guidelines and a survey of current practices. Toxicol Pathol. 2007;35:742–50. doi: 10.1080/01926230701595292. [DOI] [PubMed] [Google Scholar]
- 16.Bray GA, York DA. Genetically transmitted obesity in rodents. Physiol Rev. 1971;51:598–646. doi: 10.1152/physrev.1971.51.3.598. [DOI] [PubMed] [Google Scholar]
- 17.Bray GA. The Zucker-fatty rat: a review. Fed Proc. 1977;36:148–53. [PubMed] [Google Scholar]
- 18.Deb C, Mortin RJ. Effects of exercise and food restriction on the development of spontaneous obesity in rats. J Nutr. 1975;105:543–9. doi: 10.1093/jn/105.5.543. [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Lin HZ, Hwang J, Chacko VP, Diehl AM. Hepatic hyperplasia in noncirrhotic fatty livers: is obesity-related hepatic steatosis a premalignant condition? Cancer Res. 2001;61:5016–23. [PubMed] [Google Scholar]
- 20.Gross ML, Ritz E, Schoof A, Hemke B, Parkman A, Tulp O, et al. Renal damage in the SHR/N-Cp type 2 diabetes model: comparison of an angiotensin-converting enzyme inhibitor and endothelin receptor blocker. Lab Invest. 2003;83:1267–77. doi: 10.1097/01.lab.0000085188.23709.29. [DOI] [PubMed] [Google Scholar]
- 21.Michaelis OE, IV, Carswell H, Hansen CT, Canary JJ, Kimmel P. A new genetic model of non-insulin dependent diabetes mellitus (type II) and hypertension: The spontaneous hypertensive/NIH-corpulent rat. Frontiers in Diabetes Research. In: Shafrir E, Renold AE, editors. Lessons from animal diabetes II. London: John Libbey; 1988. pp. 257–64. [Google Scholar]
- 22.Sachi Hoshi, Yujing Shu, Fusayo Yoshida, Tomoko Inagaki, Jiro Sonoda, Teruo Watanabe, et al. Podocyte injury promotes progressive nephropathy in Zucker Diabetic Fatty Rats. Lab Invest. 2002;82:25–35. doi: 10.1038/labinvest.3780392. [DOI] [PubMed] [Google Scholar]
- 23.Cleary PM, Vaselli JR, Greenwood MRC. Development of obesity in Zucker obese (fafa) rat in the absence of hyperphagia. Am J Physiol. 1980;238:E-284–92. doi: 10.1152/ajpendo.1980.238.3.E284. [DOI] [PubMed] [Google Scholar]
- 24.Mecune SA, Baker PA, Stills HF., Jr Model of obesity, non-insulin dependent diabetes and congestive heart failure. Int Lab Anim Res News. 1990;32:23–7. [Google Scholar]
- 25.Silberberg R, Silberberg M. Lesions in yellow mice fed stock, high fat or high carbohydrate diets. Yale J Biol Med. 1957;29:525–39. [PMC free article] [PubMed] [Google Scholar]
- 26.Bray GA, Saiduddin S, York DA, Batt RAL, Swerdloff RS. Effect of estradiol on uterine weight, thyroid function, food intake and pituitary weight of genetically obese (fatty-Zucker) and lean rats. Proc Soc Exp Biol Med. 1976;153:88–91. doi: 10.3181/00379727-153-39486. [DOI] [PubMed] [Google Scholar]
- 27.Swerdloff RA, Batt RA, Bray GA. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology. 1976;98:1359–64. doi: 10.1210/endo-98-6-1359. [DOI] [PubMed] [Google Scholar]
- 28.Edmonds ES, Withyachumnarnkal B. Sexual behavior of the obese male Zucker rat. Physiol Behav. 1980;24:1139–41. doi: 10.1016/0031-9384(80)90060-8. [DOI] [PubMed] [Google Scholar]
- 29.Johnson PR, Zucker LM, Cruce JA, Hirsch J. Cellularity of adipose depots in the genetically obese Zucker rat. J Lipid Res. 1971;12:706–14. [PubMed] [Google Scholar]
- 30.Hattori M, Yamato E, Matsumoto E, Itoh N, Toyonaga T, Petruzzelli M, et al. Occurrence of Pretype I Diabetes (Pre-IDDM) and Type II Diabetes (NIDDM) in BC1 [(NODxMus spretus)F1xNOD] Mice. In: Shafrir E, editor. Lessons from animal diabetes. VI. John Libbey; 1996. pp. 83–95. [Google Scholar]
- 31.Bloch K, Vardi P. In: Lessons from animal diabetes. VI. Shafrir E, editor. John Libbey; 1996. pp. 317–32. [Google Scholar]
- 32.Adler JH, Lazarovici G, Marton M, Ben-Sasson R, Ziv E, Bar-On H, et al. Pattern of hyperglycemia, hyperinsulinemia and pancreatic insufficiency in sand rats (Psammomys obesus) In: Shafrir E, Renold AE, editors. Frontiers in diabetes research: Lessons from animal Diabetes II. London: John Libbey & Company; 1988. pp. 384–8. [Google Scholar]
- 33.Guberski DL, Butler L, Like AA. The BBZ/Wor rat: an obese animal with autoimmune diabetes. In: Shafrir E, Renold AE, editors. Lessons from animal diabetes II. London: John Libbey & Company; 1988. pp. 268–71. [Google Scholar]
- 34.Reddy GB, Vidyulatha V, Nawajes Md, Mandal A, Mridula T, Wang A novel rat model with obesity associated retinal degeneration. Invest Opthalmol Visc Sci. 2009;50:3456–63. doi: 10.1167/iovs.08-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giridharan NV, Sailaja P, Harishankar N. A new obese rat model to study obesity and cardiovascular risks. Proceedings of CMJ journal. 2010:96. (Abstract) [Google Scholar]