Sir,
Sperm DNA integrity is necessary for accurate transmission of genetic information and birth of healthy offspring1. It is critical for normal embryogenesis, foetal well being and success of assisted reproductive techniques (ART)2,3. Earlier studies reported DNA fragmentation index (DFI) threshold value by sperm chromatin structure assay (SCSA) with respect to ART outcome in the range of 27-30 per cent4,5. Till date, diagnostic work up of men with idiopathic infertility involves mainly semen analysis, but standard semen parameters are modest predictors of fertility potential. In an ongoing study in our laboratory we found that 15.5 per cent men with idiopathic infertility had normal semen parameters (unpublished data). We have explained in our previous studies that the standard semen analysis should be coupled with analysis of oxidative stress and DNA integrity assessment6,7 as sperm with normal morphology and motility (collected after swim up) also harbour DNA damage. Studies have shown that reactive oxygen species (ROS) levels are elevated in about 68 per cent of infertile men6,8 and this is one of the most important factors in the aetiology of DNA damage, thus tests for sperm DNA quality are more informative and have diagnostic and prognostic implications9,10. Therefore, this pilot study was planned with aim to assess ROS levels and DFI in cases with normal sperm parameters and highlight the need to assess ROS levels and DFI in infertile couples opting for ART or experiencing recurrent miscarriages.
In this study we analysed 28 men with idiopathic infertility, who had normal semen parameters and 30 fertile controls for ROS levels by chemiluminescence assay and DNA damage assessment by SCSA. All the men were recruited from the Departments of Urology and Obstetrics and Gynaecology after obtaining their informed consent and Institute Ethics Committee approval.The study was conducted in the laboratory for molecular reproduction and genetics, Department of Anatomy from March 2009 to March 2010. Semen samples were obtained by masturbation in a sterile plastic container after 4 days of sexual abstinence. After liquefaction at 37°C, semen analysis was performed manually as per WHO (1999) guidelines11. For morphology, 10 μl of semen smear was prepared in a clean slide and fixed with 90 per cent ethanol and stained with Giemsa. Atleast 200 sperm per sample were evaluated for morphological defects. Four hundred μl of raw semen was taken in duplicate for ROS estimation and 100 μl was stored at -80°C for SCSA analysis. To 400 μl of liquefied neat semen, 10 μl of luminol (5-amino-2, 3, -dihydro-1,4-phthalazinedione; Sigma, USA), prepared as 5 mM stock in dimethyl sulphoxide (DMSO), was added. Ten μl of 5 mM luminol in DMSO served as blank. Twenty five μl H2O2 with 10 μl luminol was used as a positive control. All the samples were measured in duplicate and the average of the readings was taken. Levels of ROS were assessed by measuring the luminol-dependant chemiluminescence with the single detector luminometer (Sirius, Berthold Detection Systems GmbH, Pforzheim, Germany) in the integrated mode for 15 min. The values were expressed as × 104 relative light unit per minute (RLU/min) per 20 × 106 spermatozoa. The SCSA was performed as described elsewhere12. The aliquot from each ejaculate was thawed in a water bath at 37°C for 30 sec and diluted to a concentration of 2 × 106 sperm/ml in tris sodium chloride-EDTA (TNE) buffer to a total of 200 μl in a falcon tube. Immediately, 0.4 ml of acid detergent solution (0.08 M HCl, 0.15 M NaCl, 0.1% v/v Triton X-100, pH 1.2) was added to the Falcon tube. After exactly 30 sec, 1.2 ml of acridine orange (AO)-staining solution [6 μg AO (chromatographically purified) (Polysciences, Inc. – USA) per ml citrate buffer (0.037M citric acid, 0.126M Na2HPO4, 1.1mM EDTA disodium, 0.15M NaCl, pH 6.0] was added. For every six test samples, one standard reference sample was analysed to ensure instrument stability. The samples were analysed using a FAC Scan flow cytometer (BD Biosciences, USA), with an air-cooled argon laser operated at 488 nm and a power of 15 mW. The green fluorescence (FL1) was collected through a 515-545 nm bandpass filter, and the red fluorescence (FL3) was collected through a 650 nm longpass filter. The sheath/sample was set on ‘low’, adjusted to a flow rate of 200 events/s when analysing a sample containing 2×106 sperm/ml. Immediately after the addition of the AO staining solution, the sample was placed in the flow cytometer and run through the flow system. All the samples were assessed in duplicate at one month interval and the average was taken. After complete analysis of sample, the X-mean (red fluorescence) and Y-mean (green fluorescence) values were recorded manually after selecting gate for sperm cells using FlowJo software (Oregon, USA). Strict quality control was maintained throughout the experiment. Post-acquisition, DFI calculation was performed offline using Flowjo software. The sperm cells are gated after excluding debris and high DNA stainability (HDS) cells and mean values of red and green fluorescence were recorded manually. The DNA fragmentation index was then calculated by the formula, DFI = mean red fluorescence/(mean red fluorescence+ mean green fluorescence). Statistical analysis of data was performed between infertile and fertile groups using Student's t test and Mann Whitney test.
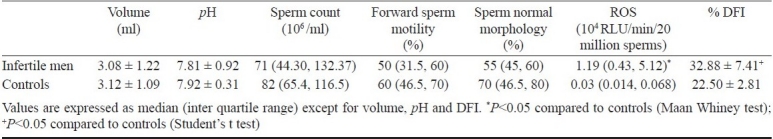
No significant differences in the semen parameters between the normozoospermic infertile men and controls were observed. However, significantly higher (P<0.05) ROS and DFI levels were observed in normozoospermic infertile men compared to controls (Table). Female partner in all these cases were normal on gynaecological and genetic examination. Of these, 22 (78.5%) infertile men had elevated ROS levels (>0.8 RLU/min) and 25 (89.2%) had higher sperm DNA fragmentation index (>30%). Our previous study13 established interquartile range of ROS levels in fertile controls as 0.014 - 0.11 × 104 RLU/min/ 20 million sperms which is similar to the findings by Fingerova et al14 (0.12- 0.55) × 103 RLU per 20 million cells. Relative light unit (RLU) and counted photons per minute (CPM) (1 CPM = 10 RLU) are widely used to represent ROS levels in the semen and presented as 103 or 104 RLU/min per either 10 or 20 million sperm cell. However, these values of normozoospermic infertile men in the current study are of significance compared to controls. This emphasizes the fact that in sperm with normal and abnormal morphology, motility may harbour oxidative DNA damage. This may be the underlying aetiology of pre-post implantation pregnancy loss and poor ART success rate despite professional expertise and use of state of art technology and also recurrent miscarriages after spontaneous or assisted conception. Pregnancy with sperm with DNA damage may lead to birth of offspring with increased prenatal/postnatal morbidity, major and minor congenital malformations and increased incidence of childhood cancer15. High ROS levels are also known to cause pronuclear block and impair cleavage and lead to production of morphologically abnormal blastomeres16. One of the chief causes of DNA damage is high ROS levels17. Supraphysiological ROS levels damage both mitochondrial and nuclear DNA9. The other causes of DNA damage and oxidative stress are exposure to xenobiotics, environmental pollutants, varicocele, abortive apoptosis, infection, inflammation, high testicular temperature and electromagnetic radiation17–19. Apart from ROS levels some of the important oxidative stress parameters such as malondialdehyde (MDA)20, 8-hydroxy deoxyguanosine (8-OHdOG)21, 8-isoprostane22, catalase, superoxide dismutase (SOD) and seminal total antioxidant capacity (TAC) are also used to predict oxidative stress in the semen23.
Table.
Semen parameters, reactive oxygen species (ROS) levels and DNA fragmentation index (DFI) in normozoospermic infertile men and controls
Repeated pregnancy losses either following spontaneous or assisted conception are financially and emotionality crippling for the couple and matter of great concern for the doctor. Despite detailed work up of such couples, a large number of cases experience recurrent spontaneous abortion or ART failure. It is possible that sperm DNA damage and seminal OS is the underlying factor for successive pregnancy losses and assisted conception failure. Thus all couples experiencing recurrent ART failure or RSA must be evaluated for sperm DNA integrity and ROS levels. This study highlights the need to assess all infertile men with normal or abnormal semen parameters for sperm DNA damage and ROS levels. This will help to predict future pregnancy outcome or explain for previous ART failure or early pregnancy losses.
References
- 1.Ward WS. Function of sperm chromatin structural elements in fertilization and development. Mol Hum Reprod. 2010;16:30–6. doi: 10.1093/molehr/gap080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Micinski P, Pawlicki K, Wielgus E, Bochenek M, Tworkowska I. The sperm chromatin structure assay (SCSA) as prognostic factor in IVF/ICSI program. Reprod Biol. 2009;9:65–70. doi: 10.1016/s1642-431x(12)60095-3. [DOI] [PubMed] [Google Scholar]
- 3.Dada R. Recurrent pregnancy loss: Male factor. In: Deka D, Malhotra N, editors. An introduction to genetics & fetal medicine. New Delhi: Japee Publisher; 2010. pp. 31–7. [Google Scholar]
- 4.Larson-Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET, Evenson DP. Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril. 2003;80:895–902. doi: 10.1016/s0015-0282(03)01116-6. [DOI] [PubMed] [Google Scholar]
- 5.Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81:1289–95. doi: 10.1016/j.fertnstert.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 6.Venkatesh S, Riyaz AM, Shamsi MB, Kumar R, Gupta NP, Mittal S, et al. Clinical significance of reactive oxygen species in semen of infertile Indian men. Andrologia. 2009;41:251–6. doi: 10.1111/j.1439-0272.2009.00943.x. [DOI] [PubMed] [Google Scholar]
- 7.Shamsi MB, Venkatesh S, Tanwar M, Talwar P, Sharma RK, Dhawan A, et al. DNA integrity and semen quality in men with low seminal antioxidant levels. Mutat Res. 2009;665:29–36. doi: 10.1016/j.mrfmmm.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl. 2002;23:737–52. [PubMed] [Google Scholar]
- 9.Venkatesh S, Deecaraman M, Kumar R, Shamsi MB, Dada R. Role of reactive oxygen species in the pathogenesis of mitochondrial DNA (mtDNA) mutations in male infertility. Indian J Med Res. 2009;129:127–37. [PubMed] [Google Scholar]
- 10.Shamsi MB, Venkatesh S, Tanwar M, Singh G, Mukherjee S, Malhotra N, et al. Comet assay: a prognostic tool for DNA integrity assessment in infertile men opting for assisted reproduction. Indian J Med Res. 2010;131:675–81. [PubMed] [Google Scholar]
- 11.WHO Laboratory manual for the examination of human semen and semen-cervical mucus interaction. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- 12.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14:1039–49. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 13.Venkatesh S, Shamsi MB, Dudeja S, Kumar R, Dada R. Reactive oxygen species measurement in neat and washed semen: comparative analysis and its significance in male infertility assessment. Arch Gynecol Obstet. 2011;283:121–6. doi: 10.1007/s00404-010-1645-4. [DOI] [PubMed] [Google Scholar]
- 14.Fingerova H, Oborna I, Novotny J, Svobodova M, Brezinova J, Radova L. The measurement of reactive oxygen species in human neat semen and in suspended spermatozoa: a comparison. Reprod Biol Endocrinol. 2009;7:118. doi: 10.1186/1477-7827-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aitken RJ, De Iuliis GN. Value of DNA integrity assays for fertility evaluation. Soc Reprod Fertil Suppl. 2007;65:81–92. [PubMed] [Google Scholar]
- 16.Goto Y, Noda Y, Narimoto K, Umaoka Y, Mori T. Oxidative stress on mouse embryo development in vitro. Free Radic Biol Med. 1992;13:47–53. doi: 10.1016/0891-5849(92)90165-d. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal A, Varghese AC, Sharma RK. Markers of oxidative stress and sperm chromatin integrity. Methods Mol Biol. 2009;590:377–402. doi: 10.1007/978-1-60327-378-7_24. [DOI] [PubMed] [Google Scholar]
- 18.Tremellen K. Oxidative stress and male infertility - a clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 19.Dada R, Shamsi MB, Venkatesh S, Gupta NP, Kumar R. Attenuation of oxidative stress & DNA damage in varicocelectomy: implications in infertility management. Indian J Med Res. 2010;132:728–30. [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar R, Venkatesh S, Kumar M, Tanwar M, Shasmsi MB, Kumar R, et al. Oxidative stress and sperm mitochondrial DNA mutation in idiopathic oligoasthenozoospermic men. Indian J Biochem Biophys. 2009;46:172–7. [PubMed] [Google Scholar]
- 21.Shen H, Ong C. Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med. 2000;28:529–36. doi: 10.1016/s0891-5849(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 22.Khosrowbeygi A, Zarghami N. Levels of oxidative stress biomarkers in seminal plasma and their relationship with seminal parameters. BMC Clin Pathol. 2007;7:6. doi: 10.1186/1472-6890-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamsi MB, Venkatesh S, Kumar R, Gupta NP, Malhotra N, Singh N, et al. Antioxidant levels in blood and seminal plasma and their impact on sperm parameters in infertile men. Indian J Biochem Biophys. 2010;47:38–43. [PubMed] [Google Scholar]


