Abstract
Regulation of fluid and dietary intake habits is essential in comprehensive preventive management of urolithiasis. However, despite large body of epidemiological database, there is dearth of good quality prospective interventional studies in this regard. Often there is conflict in pathophysiological basis and actual clinical outcome. We describe conflicts, controversies and lacunae in current literature in fluid and dietary modifications in prevention of urolithiasis. Adequate fluid intake is the most important conservative strategy in urolithiasis-prevention; its positive effects are seen even at low volumes. Of the citrus, orange provides the most favorable pH changes in the urine, equivalent to therapeutic alkaline citrates. Despite being richest source of citrate, lemon does not increase pH significant due to its acidic nature. Fructose, animal proteins and fats are implicated in contributing to obesity, which is an established risk factor for urolithiasis. Fructose and proteins also contribute to lithogenecity of urine directly. Sodium restriction is commonly advised since natriuresis is associated with calciuresis. Calcium restriction is not advisable for urolithiasis prevention. Adequate calcium intake is beneficial if taken with food since it reduces absorption of dietary oxalate. Increasing dietary fiber does not protect against urolithiasis. Evidence for pyridoxine and magnesium is not robust. There is no prospective interventional study evaluating effect of many dietary elements, including citrus juices, carbohydrate, fat, dietary fiber, sodium, etc. Due to lack of good-quality prospective interventional trials it is essential to test the findings of pathophysiological understanding and epidemiological evidence. Role of probiotics and phytoceuticals needs special attention for future research.
Keywords: Citrate, dietary intervention, nephrolithiasis, prevention, urolithiasis
INTRODUCTION
Urolithiasis is ubiquitous and is prevalent in relatively young and productive age group. It carries significant morbidity and imposes tremendous financial burden on healthcare system. With changing lifestyles and climate, its prevalence has shown a rise over decades, e.g., 37% rise in USA from 1976 to 1994.[1] Life-time risk of urolithiasis varies from 1-5% in Asia, 5-9% Europe, 10-15% USA and 20-25% middle-east; lowest prevalence is reported from Greenland and Japan.[2] The situation is not unlikely to be similar in various geographical regions worldwide making it alarming enough to lay more stress on preventive aspect of urolithiasis.
Majority of kidney stones are composed of calcium oxalate (CaOx) and phosphate (CaPh) crystals (~80%). Most of the rest are composed of uric acid (UA; 5-10%) and struvite (5-15%). Other rare constituents are cystine, xanthine, matrix, dihydroxy-adenosine and various drugs (e.g., indinavir, nelfinavir, efavirenz, amprenavir, triamterene).[3] Compositions vary in different geographical conditions and ethnicities; e.g., UA more prevalent among south-east Asian ethnic group (50%) and in Israel (40%) and less common in India (<1%).[1,4,5] Struvite is also rare in India (1.3%, versus a universal average ~30%); even most of the staghorn calculi are composed of CaOx.[4]
There is extensive epidemiological literature on dietary factors modulating urolithiasis risk. Prospective interventional studies are available evaluating role of various fluid/dietary elements in modulating lithogenecity of urine; however, there are not many good quality prospective dietary-interventional studies examining the effect of proposed dietary modifications on actual incidence of urolithiasis. Additionally, more often than not, discrepancy exists between cross-sectional and prospective data. In a recent meta-analysis of randomized controlled trials, Fink et al, could identify only eight good quality RCTs among a total of 579 research papers on diet in urolithiasis reviewed.[6] Genetic basis of calcium urolithiasis is being elucidated, and gene therapy is considered to hold promise for the future.
Since, good quality RCTs on diet / supplements in prevention of urolithiasis are conspicuous by their scarcity, a systematic review with meta-analysis would have to exclude elucidation of most of the dietary elements.[6] Therefore, with the aim to elucidate the strengths and limitations of current evidence in this topic, and present the direction of future research we chose to write a narrative review. Studies published in English language were searched in the Pubmed database, Cochrane database and Google-scholar without restriction of year of publication using search words including, but not exclusively, ‘nephrolithiasis’, ‘urolithiasis’, ‘renal calculi’, ‘prevention’, diet’, ‘calcium’, ‘sodium’, ‘potassium’, ‘protein’, ‘carbohydrate’, ‘fat’, ‘magnesium’, ‘fiber’, ‘phytotherapy’ and ‘probiotics’, in appropriate combinations. Since there is large heterogeneity in research publications in this area, and the type of review being narrative, strict inclusion/exclusion criteria were not laid down. Epidemiological studies (cross-sectional, prospective), case-control studies as well as randomized/non-randomized controlled trials on diet and fluid aspect of urolithiasis, all were included. Further relevant references were selected from the bibliography of the searched papers. A total of 137 research papers were reviewed, the most relevant 53 are cited in this review.
By virtue of its ubiquity, higher prevalence in relatively young and productive age, costly treatment and loss of productive hours, urolithiasis imposes tremendous financial burden on healthcare system. There are not many good quality prospective dietary-interventional studies examining the effect of proposed dietary modifcations on actual incidence of urolithiasis.
FLUIDS
Increased fluid intake for a urine-output of 2 liters/day is one of the most important and the least expensive form of conservative measure to reduce stone-recurrence. Its effect is through mechanical diuresis and decreased solute-supersaturation and formation-product ratio. Large epidemiological studies on >45,000 men (health professional follow-up study; HPFS) and 91,000 women (Nurses’ health study; NHS-I and NHS-II) found a 30-40% risk-reduction associated with doubling of fluid intake to 2.5 liters/day or more.[7,8]
Improvement in stone-recurrence fluid is a continuous process, observed even a low volumes and with small increments. Frank et al,[9] compared the incidence of urolithasis in two desert towns of Israel, Arad and Beersheba, after 3-years of education regarding fluids. They found a lower prevalence of urolithiasis in Arad (0.28% vs. 0.85%, P=0.001) with only a quarter litre increase in urine output (1071 ml vs. 804 ml). In a prospective randomized trial Borghi et al,[10] found that doubling of urine volume to >2 litre led to an over 55% decrease in stone recurrence (12/99 vs. 27/100; P=0.008) over 5 years. Improvement in stone-recurrence fluid is a continuous process, observed even a low volumes and with small increments.
HARD OR SOFT WATER?
There have been pervasive concerns regarding lithogenic potential of hard water (by virtue of being richer in calcium); however, consensus is lacking. In a large cohort of 3270 stone-patients, Schwartz et al,[11] found higher calcium and citrate excretion in patients living in areas with hard water supply versus soft water supply (P<0.0001). However, the life-time incidence of stone episodes was remarkably similar across populations (3.4 versus 3.0). In fact, epidemiological data from 10 Finnish hospitals even suggests an inverse relation (35 hard vs. 53 soft per 1,00,000 inhabitants).[12] Despite being richer in citrate, lime, lemon or lemonade generally do not increase urinary pH (0.1 point increase) presumably due to acidic nature, whereas orange juice does by 0.6-0.8 points, similar to potassium-citrate (K-cit).
WHICH BEVERAGES?
A host of clinical and epidemiological studies are available testing various common fluids ‘in vogue’ for urolithiasis prevention,[13,14] however, without consensus. In HPFS-cohort, Curham et al,[14] found that with every 8-oz/d of coffee, tea, beer and wine stone-risk decreased by 10%, 14%, 21% and 39%, respectively (inhibitory action on vasopressin probably decreases stone risk); whereas, with the same amount of apple juice and grapefruit juice, it increased by 35% and 37%, respectively. Similarly, Krieger et al,[15] found a significant protective effect of beer (OR 0.41), in a case-control study enrolling 240 cases and 392 controls. This effect is not entirely explainable, because apart from causing diuresis, it leads to hypocitraturia, uricosuria and lowering of pH.
In a cohort of 1009 treated stone-formers followed up for 3 years, Shuster et al,[13] reported 15% higher incidence of urolithiasis in men consuming at least 160 ml of soft drinks acidified by phosphoric acid (e.g., cola); citrus drinks did not affect incidence of urolithiasis. Notably, cola leads to increased oxaluria, partly explaining its lithogenic propensity. Recently, Eisner et al,[16] reported that alkali and citrate content of various commercial non-cola soda drinks (e.g., Diet 7up®, diet Canada dry®, diet mountain dew®, Fresca®, etc.) was equal or higher than that of lemonade. However, Passman et al,[17] found that one of these drinks (Fresca®), when compared to clear water (Le Bleu®) or commercial Diet coke®, did not confer any advantage in terms of increased citraturia or decreased CaOx supersaturation index.
Lemon, orange or grapefruit?
Citrus juices have long been considered to have anti-lithogenic potential, predominantly owing to increased citraturia and urinary-pH. Diets rich in citrus fruits and juices are common practice and prescription for ‘stone-prevention’. However, despite a multitude of publications evaluating citrus juices on various urinary parameters and incidence of urolithioasis, there is no consensus conclusion derived so far[14,17–26] [Table 1].
Table 1.
A summary of studies evaluating fruits and beverages in urolithiasis
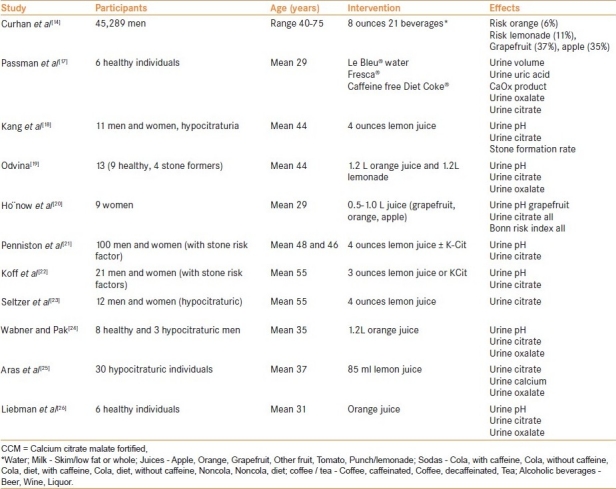
Lemon and lime juices contain the highest concentration of citric acid (1.44 g/oz, 1.38 g/oz, respectively), grapefruit juice intermediate (0.75 g/oz) and orange juice the least (0.27 g/oz).[27] Citrate is the most abundant organic ion in urine and is a potent natural inhibitor of CaOx and CaPh nucleation. Most of the orally ingested citrate gets converted to bicarbonate conferring alkali-load which may result in increased urine-pH depending concomitant acid load. Despite being richer in citrate, lime, lemon or lemonade generally do not increase urinary pH (0.1 point increase) presumably due to acidic nature, whereas orange juice does by 0.6-0.8 points, similar to potassium-citrate (K-cit).[18,24] Most of studies consistently indicate increased citraturia with ingestion of studied citrus fruits (lemon, orange, grapefruit), although in large amount. However, often prospective-observational epidemiological studies have found paradoxical results, e.g., ‘minimal protective’ effect of orange juice and ‘lithogenic’ effect of grapefruit juice.[14,18] Part of the reason may be fructose content in citrus fruits/juices which impart lithogenecity to the urine (vide infra). Therefore, the ‘best’ fluid/juice question is unclear warranting further studies.
Recently, Yilmaz et al, conducted a study on freshly blend tomato juice and found that it was high in citrate and low in sodium and oxalate content.[28] This is an interesting finding and warrants further studies in the light of dietary fad against use of tomatoes in stone formers.
DIET
Dietary fads with innumerable do's (e.g., cranberry, fish, citrus) and don’ts (e.g., calcium, oxalate, organ-meat) are pratique-commune for stone-prevention and are readily available in the multimedia. The following section we describe pathophysiological basis and current evidence of lithogenic potential of various diet components and discussed available scientific evidence for or against it.
MACRONUTRIENTS
Proteins
Increased animal-protein consumption has been considered risk-factor for urolithiasis for a number of years. The evidence is mostly indirect and observed in epidemiological surveys, e.g., almost 3 decades ago, Anderson[29] noted a four-fold higher incidence of urolithiasis in the richer northern-western states of India compared to poorer southern-eastern. The difference was ascribed to an almost twice higher intake of animal-protein in the former. A similar trend has been reported from other countries as well. Higher protein ingestion has been associated with obesity, another risk factor for urolithiasis.
A number of lithogenic metabolic changes are induced by increased protein consumption, most notably increased calciuria, uricosuria and decreased citraturia and pH; effect on oxaluria is variable. These effects result from load of acid, sulfate and purine conferred by amino-acid (AA) metabolism, particularly of animal source which is rich in sulfur-containing AA (cysteine and methionine) and in purine. It is estimated that addition of 75 g of protein to the diets of normal subjects leads to a 100 mg/d rise in urinary calcium excretion. Moreover, stone formers may be more sensitive to the calciuric effects of protein consumption than normal subjects.
Despite sound pathophysiological understanding as well as indirect epidemiological evidence neither long term observational studies[30] nor randomized controlled trials[28] suggest strongly against high animal protein diet. Borghi et al,[31] compared 2 types of diets (normal calcium, low animal protein and low salt diet versus low calcium and otherwise unspecified diet) in a randomized controlled trial and found that the incidence of new stone formation was half in the former group. However, for lack of multivariate analysis, the protective effect could not be pinpointed individual dietary modification. Dussol et al,[32] compared 3 diets (low animal-protein, high fiber and unspecified) in idiopathic calcium stone formers and found stone recurrence in 48%, 63% and 48% over 4 year follow up, respectively. They concluded against restriction of animal proteins, commenting on limitation that their patients had active stone-disease, potentially rendering dietary intervention ineffective. Hiatt et al,[33] in fact observed adverse effect of a low-protein-high-fiber diet compared to control group (adequate fluid and calcium) in a cohort of 99 patients (relative risk 5.6; 95% confidence interval 1.2-26.1). Limitation cited was possibly higher emphasis on fluid intake in the control arm.
Overall, the evidence is not strong in favor or against high protein intake. However, in view of pathophysiological basis and indirect epidemiological evidence of its lithogenic potential and its association with obesity, it may be prudent to avoid excess of proteins in diet.
It is estimated that addition of 75 g of protein to the diets of normal subjects leads to a 100 mg/d rise in urinary calcium excretion. Overall, the evidence is not strong in favor or against high protein intake.
Carbohydrates
There has been a tremendous rise in fructose-consumption over the last 4 decades, especially after 1967 with introduction of high-fructose corn-syrup. High intake of fructose has been linked to increased risk of obesity, metabolic syndrome as well as urolithiasis. It promotes insulin resistance with subsequent reduction in urine pH and increased UA-production. In HPFS and NHS (I and II) cohorts examined for 48 years, Taylor et al,[34–36] identified 4902 incidences of renal stone disease. They analyzed this data extensively and made important observations –
After adjustment for age, body mass index (BMI), calorie-intake, thiazides, fluid-intake, caffeine, alcohol, calcium-supplement, non-fructose carbohydrate intake, percentage of energy from total protein, oxalate, potassium and sodium, they found fructose to be associated with increased risk of urolithiasis (relative risk 1.37, 1.35 and 1.27, respectively; P<<0.05).[34]
A higher risk of urolithiasis associated with obesity (RR 1.90, 2.02 and 1.35, respectively; P<<0.05) and weight gain more than 35 lbs (1.70, 1.82 and 1.39, respectively; P<<0.05).[35]
A higher risk of urolithiasis associated with diabetes mellitus (RR 1.38, 1.67 and 1.31, respectively; P<<0.05).[36]
The association of fructose with urolithiasis is not only through risk of metabolic syndrome; it also causes increased calciuria, oxaluria, and uricosuria.
The lithogenic potential is not only limited to fructose. Curhan et al,[7,37] reported that high intake of sucrose was associated with this disorder in their female (but not male) group. Glucose may also lead to increased oxaluria.
Link between high fructose diet and urolithiasis complicates dietary recommendations for stone prevention, especially, with respect to (w.r.t) recommendations for citrus fruits or juices.
The association of fructose with urolithiasis is not only through risk of metabolic syndrome; it also causes increased calciuria, oxaluria and uricosuria. The lithogenic potential of carbohydrates is not only limited to fructose.
FAT
Dietary fat consumption is not considered an independent risk-factor for stone formation.[7,29] However, high fat consumption is clearly linked to incidence of obesity, which as an independent risk factor of urolithiasis.[35] Powell et al,[38] studied urine chemistries of 5942 patients with urolithiasis and found obese patients to have higher urinary excretion of calcium, oxalate, UA, sodium and sulfate; to offset this lithogenic effect, they also found a higher excretion of citrate and increased urinary volume in obese. Incidence of UA lithiasis is particularly increased in obesity due to increased production and excretion of UA and lowering of urinary pH. Lithogenic effect of weight-gain has been reported in prospective long term study by Taylor et al.[35]
There is no study evaluating protective effect of weight-reduction with respect to stone disease. There are various novel regimes used to reduce weight, which claim to be more acceptable than more conventional ‘palatable diet-restriction’ programs –
Atkin's regimen - consists of carbohydrate restriction (20 g/d), and unrestricted animal-protein and fat intake and is associated with rise in calciuria, aciduria and uricosuria and fall in citraturia.[39]
Lipase inhibitors - induce fat malabsorption which is associated with hyperoxaluria.[40]
However, it should be mentioned that there is no study on incidence of urolithiasis associated with these regimens. Nevertheless, it seems prudent to advise weight regulation without using these potentially lithogenic regimens, since weight reduction can potentially reduce risk of urolithiasis by improving urinary parameters, regaining insulin sensitivity (which has other benefits too).
Incidence of UA lithiasis is particularly increased in obesity due to increased production and excretion of UA and lowering of urinary pH.
OMEGA-3 FATTY ACID
Consumption of eicosapentaenoic acid (EPA) is associated with reduction of liothogenic factors in urine.[41] EPA is an inhibitor of arachidonic acid metabolism resulting in decreased synthesis of prostaglandin E2, a substance that is known to potentiate urinary calcium excretion. Buck et al,[41] treated hypercalciuric recurrent stone formers with an 8-week course of fish oil and found that this significantly reduced oxalate and calcium excretion. Yasui et al,[42] prospectively followed up 29 recurrent stone formers in three phases; phase-I before initiation of EPA (mean 47.8 months), phase-II of EPA supplementation (1800mg/d; mean 36.4 months) and phase-III after stopping EPA (mean 50.6 months). They observed a statistically significant reduction in stone episodes during supplementation phase than before or after (0.22, 0.07 and 0.17 times/year, respectively; RR 3.29 before and RR 2.51 after EPA). Conversely, in epidemiological NHS and HPFS studies Taylor et al,[43] did not find any significant positive association with arachidonic acid and linoleic acid, though ingestion of EPA was lower than that in Study by Yasui.[42] Moreover, as per authors this study had low statistical power for this assessment.
MICRONUTRIENTS
Sodium
Sodium restriction is widely recommended dietary modification for decreasing risk of recurrence in recurrent stone formers. Increased sodium intake leads to various changes in urine composition, some pro-lithogenic (i.e., increased calciuria and cystinuria and decreased citraturia) and some antilithogenic (increased urine volume and pH). In large cohort of 2201 stone formers and 1167 non-stone formers in the HPFS and NHS, Taylor and Curhan[44] reported 37 mg/d higher excretion of calcium in individuals with highest quartile of sodium intake. A 100 mmol/d rise in dietary sodium leads to 25 mg/d (= 0.62 mmol/d) rise in calciuria, the reverse is also true. In another NHS and HPFS analysis, Taylor et al,[45] found that DASH-type diets (Dietary approaches to stop hypertension), which composed of higher intake of potassium, magnesium, oxalate and ascorbic acid and lower intake of sodium, were associated with 40-45% reduced risk of stone formation. Epidemiological analysis suggests that among individuals consuming sodium and calcium in highest quartile, only 12-15% reduction of sodium intake (as opposed to 50% reduction of Calcium intake) is required to decrease calciuria by 6 mg/day.[44] Jaeger et al,[46] demonstrated that a sodium-dependent excretion of dibasic amino acids, including cystine, occurs at an intake of 50 mmol/d or more of sodium indicating the role of sodium restriction in cystine lithiasis.
Recently, role of sodium restriction was put into question. In 14 years follow-up of HPFS, Taylor et al, did not find sodium as an independent risk factor for urolithiasis.[30] More recently, Eisner et al,[47] retrospectively analyzed metabolic evaluation of 880 stone patients and found that despite positive association with calciuria, natriuria was associated negatively with CaOx supersaturation. They ascribed this paradox to increased urine volume and pH.
There is paucity of prospective intervention studies. In view of beneficial effect of sodium restriction on hypertension and other components of metabolic syndrome, and large body of epidemiological data in favor of stone prevention, avoidance of excessive sodium intake is desirable.
A 100 mmol/d rise in dietary sodium leads to 25 mg/d rise in calciuria, the reverse is also true.
Potassium
There is epidemiologic evidence that increased potassium intake is associated with lower incidence of urolithiasis (RR 0.49-0.54).[7] It has been estimated that every 20 meq/day increase in urinary potassium is associated with 17 mg/d (= 0.42 mmol/d) decrease in calciuria.[44] Another potential benefit is that foods high in potassium content usually are replete with alkali, which may stimulate urinary citrate excretion. Moreover, stone formers are considered to have an adversely raised sodium-to-potassium ratio.[48] Markedly beneficial effect of alkaline citrates (especially potassium salts) in prevention of recurrent urolithiasis is well established.
It has been estimated that every 20 meq/day increase in urinary potassium is associated with 17 mg/d decrease in calciuria.
Calcium
The conventional view of calcium restriction in calcium-urolithiasis, which prevailed for decades after discovery of association of hypercalciuria in calcium-oxalate urolithiasis, has been refuted based on large epidemiological as well as prospective studies. In the NHS and HPFS cohorts, prevalence of stone disease in highest quartile calcium-intake group was nearly half compared to the lowest one (RR - ♂0.56 and ♀0.49).[7,8] Moreover, a 64% decreased incidence of urolithiasis was reported in 14-year prospective study on HPFS.[30] DASH style diets which are rich in calcium (apart from other stone-prevention elements) are associated with lower incidence of stone disease.[45]
Bataille et al,[49] demonstrated that dietary calcium restriction increases urinary oxalate excretion in normocalciuric as well as hypercalciuric calcium stone formers, and controls. In a randomized prospective dietary intervention study Borghi et al,[31] found that incidence of stone incidence was twice in dietary calcium restriction group compared to normal calcium intake group. The increased oxalate excretion with calcium restriction is thought to result from less calcium available for oxalate binding in the alimentary tract, thus leaving more free oxalate for intestinal absorption.
There is a caveat in calcium intake (especially supplemental calcium); the protective alimental oxalate-complexing action of calcium is only effective only when taken with food. It should also be kept in mind that increased calcium intake does lead to increased calciuria[44] and would be beneficial only if there is a more than offsetting decrease in urinary oxalate excretion to lower the relative supersaturation of urine with calcium oxalate. Curhan group[8] found higher risk of stone disease in women on supplemental calcium and the reason cited was not taking the supplement with food. It is now believed that levels of calcium intake above the 800 mg recommended may be beneficial, but the optimum value to decrease stone risk has not been identified.
It should also be kept in mind that increased calcium intake does lead to increased calciuria and would be benefcial only if there is a more than offsetting decrease in urinary oxalate excretion to lower the relative supersaturation of urine with calcium oxalate. Decreased ingestion of calcium is known to be associated with increased risk of calcium-oxalate urolithiasis.
Oxalate
Dietary oxalate is absorbed all along the alimentary tract. Degree of absorption depends on various factors, i.e., higher proportion absorption in low oxalate intakes (predominance of ionized oxalate) and vice versa (predominance of crystalline oxalate in oxalate-rich foods), concomitant calcium intake and genetic influence.[50] Generally, contribution of dietary oxalate towards urinary oxalate is small (10-20%), but it may contribute upto 80% depending on factors discussed above.[51] The variability is also ascribed to inaccurate assessment of oxalate content of diet as well as underestimation of oxalate content by conventional indirect enzymatic or calorimetric procedures.
Although a reduction in ‘excessive’ dietary oxalate is commonly advised, definitive studies showing it as a risk factor for the disease are lacking. There are various indirect indicators, though, to suggest that decreasing oxaluria is of importance –
Decreased ingestion of calcium is known to be associated with increased risk of calcium-oxalate urolithiasis[7,31] and the most plausible mechanism is increased oxaluria since it escapes chelation with calcium in the gut.
Even small increase in the urinary excretion of oxalate is associated with increased risk of calcium-oxalate urolithiasis.[51]
Based on the available evidence, restriction of ‘excessive’ dietary oxalate intake is reasonable advice for calcium oxalate stone formers and should be coupled with advice to maintain at least the recommended daily intake of calcium and to ensure that calcium consumption accompanies the ingestion of any oxalate-rich foods.
Magnesium
Magnesium is a known inhibitor of calcium oxalate and calcium phosphate crystal nucleation, growth, and aggregation. It forms a soluble complex with oxalate in urine and binds oxalate in the gut, thus lowering the saturation of calcium oxalate in urine. Epidemiological evidence did not indicate significant association between magnesium intake and prevalence of urolithiasis.[52] Nevertheless, follow-up data from epidemiological studies[30] as well as prospective supplementation studies have shown a favorable effect.[53]
Phosphorus
Phosphorus consumption did not influence stone formation in the studies of Curhan et al.[7] Dietary phosphorus intake has been found to be similar in stone formers and normal subjects. However, supplemental phosphate administration via orthophosphate preparations has been demonstrated to decrease urinary calcium excretion.[54] This is attributed to a decrease in 1,25-dihydroxyvitamin D activity resulting in less intestinal calcium absorption.
Vitamin B6
Encouraged by efficacy of megadose vitamin B6 in treatment of primary hyperoxaluria type 1 (PH1), studies have examined role of B6 in idiopathic calcium oxalate urolithiasis. Studies have suggested that B6 deficiency in diet is associated with increased oxaluria and vice versa.[55] In two large 14-year follow-up studies, Curhan et al, found a significantly lower incidence of urolithiasis associated with high intake of B6 (>40 mg/d) in women (NHS) but not in men (HPFS).[52]
Others
High fiber consumption is associated with decreased oxaluria (by decreasing intestinal transit time) and increased citraturia (alkali load with vegetable fiber). However, dietary intervention studies have not been able to prove efficacy of high fiber diet for stone prevention.[32,33] Ascorbic acid intake has been associated with increased oxaluria; however, studies have suggested that this is mostly an artefact occurring post-micturition due to decomposition of ascorbate into oxalate. Increased intake of vitamin C has been associated with increased incidence of urolithiasis[29] which is not readily explainable with increased oxaluria (since the enzymatic process is easily saturable). Despite the association, vitamin C restriction may not be a good strategy since its vital role in body functions; nevertheless, overindulgence certainly to be avoided. Moreover, generally vitamin C rich foods are also rich in potassium which has a role in stone prevention.
FUTURE STRATEGIES
Probiotics
Various intestinal diseases have been associated with increased incidence of urolithiasis through changes in the metabolism of oxalate, calcium, and UA. Particularly, the discovery of oxalate-degrading bacteria within the human gastrointestinal tract has led to a flurry of research regarding the role of probiotics in the treatment of recurrent calcium oxalate nephrolithiasis.[56,57] Kaufman et al,[56] evaluated stool samples from 247 recurrent CaOx formers and 259 controls and found a 17 and 38% prevalence of O. Formigenes (OF), respectively; multivariate analysis revealed a 70% risk-reduction in presence of OF-colonization. OF not only degrades the luminal oxalate, but also enhances intestinal secretion of endogenous oxalates.
Although the reduced urinary oxalate levels and low side effect profile associated with this probiotic are promising, prospective trials are yet to be conducted to evaluate its protective effect in stone formers. Another potential limitation of OF therapy is its ability to control only one of the many prolithogenic factors.
Phytotherapeutic agents
Alternative treatment modalities composed of herbal remedies have been the mainstay of medical therapy for thousands of years, especially in Eastern civilizations (India, China, Brazil, Mexico, etc.). There is increasing interest in phytotherapy research exemplified by more than $1.5b/y expenditure in USA alone. A host of herbs have been studied by many investigators, in vitro as well as in vivo (animals and humans) to elucidate their mechanism(s) of action. Altered concentration of citrate, oxalate, calcium, magnesium in urine, alteration in pH, antimicrobial effect and diuretic effect, all have been reported associated with intake of various herbs.[58] Unfortunately, despite these been around for centuries, and studies on mechanism of action, there is not a single prospective study evaluating their effect on incidence of urolithiasis. Moreover, the ‘best’ herb, or a combination, the concentration of ‘effective’ constituent(s) is not known, severely limiting their current clinical role. Nevertheless, these therapies seem to hold promise in view of favorable scientific data.
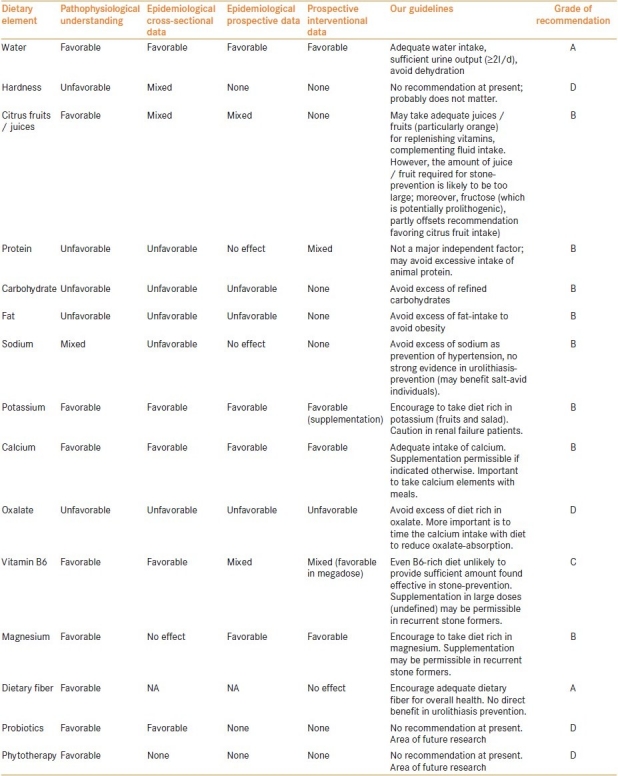
To summarize, preventive medical therapy for prevention of urolithiasis is the current need. Large epidemiological data (including prospective) with high-quality multivariate analyses have identified various risk factors associated with urolithiasis. However, prospective interventional studies have failed to confirm many of these factors, even to the point of reverse interpretation. Nevertheless, at least ‘dietary moderation’ principle seems appropriate; we propose certain fluid and dietary modifications based on our review [Table 2]. With increasing understanding of pathophysiology of the disease, various potential approaches have surfaced, e.g. probiotics and phytotherapy, which of course need to be explored further.
Table 2.
Current evidence on dietary elements resulting in decreasing incidence of urolithiasis
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ngo TC, Assimos DG. Uric acid nephrolithiasis: Recent progress and future directions. Rev Urol. 2007;9:17–27. [PMC free article] [PubMed] [Google Scholar]
- 2.Bartoletti R, Cai T, Mondaini N, Melone F, Travaglini F, Carini M, et al. Epidemiology and risk factors in urolithiasis. Urol Int. 2007;79(suppl 1):3–7. doi: 10.1159/000104434. [DOI] [PubMed] [Google Scholar]
- 3.Moe OW. Kidney stones: Pathophysiology and medical management. Lancet. 2006;367:333–44. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 4.Ansari MS, Gupta NP, Hemal AK, Dogra PN, Seth A, Aron M, et al. Spectrum of stone composition: Structural analysis of 1050 upper urinary tract calculi from northern India. Int J Urol. 2005;12:12–6. doi: 10.1111/j.1442-2042.2004.00990.x. [DOI] [PubMed] [Google Scholar]
- 5.Portis AJ, Hermans K, Culhane-Pera KA, Curhan GC. Stone disease in the Hmong of Minnesota: Initial description of a high-risk population. J Endourol. 2004;18:853–7. doi: 10.1089/end.2004.18.853. [DOI] [PubMed] [Google Scholar]
- 6.Fink HA, Akornor JW, Garimella PS, MacDonald R, Cutting A, Rutks IR, et al. Diet, fluid, or supplements for secondary prevention of nephrolithiasis: A systematic review and meta-analysis of randomized trials. Eur Urol. 2009;56:72–80. doi: 10.1016/j.eururo.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328:833–8. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- 8.Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women nurses’ health study II. Arch Intern med. 2004;164:885–91. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 9.Frank M, De Vries A, Tikva P. Prevention of urolithiasis. Arch Environ Health. 1966;13:625–30. doi: 10.1080/00039896.1966.10664630. [DOI] [PubMed] [Google Scholar]
- 10.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J Urol. 1996;155:839–43. [PubMed] [Google Scholar]
- 11.Schwartz BF, Schenkman NS, Bruce JE, Leslie SW, Stoller ML. Calcium nephrolithiasis: Effect of water hardness on urinary electrolytes. Urology. 2002;60:23–7. doi: 10.1016/s0090-4295(02)01631-x. [DOI] [PubMed] [Google Scholar]
- 12.Juuti M, Heinonen OP. Incidence of urolithiasis and composition of household water in southern Finland. Scand J Urol Nephrol. 1980;14:181–7. doi: 10.3109/00365598009179558. [DOI] [PubMed] [Google Scholar]
- 13.Shuster J, Jenkins A, Logan C, Barnett T, Riehle R, Zackson D, et al. Soft drink consumption and urinary stone recurrence: A randomized prevention trial. J Clin Epidemiol. 1992;45:911–6. doi: 10.1016/0895-4356(92)90074-w. [DOI] [PubMed] [Google Scholar]
- 14.Curhan GC, Willett WC, Rimm ED, Spiegelman D, Stampfer MJ. Prospective study of beverage use and the risk of kidney stones. Am J Epidemiol. 1996;143:240–7. doi: 10.1093/oxfordjournals.aje.a008734. [DOI] [PubMed] [Google Scholar]
- 15.Krieger JN, Kronmal RA, Coxon V, Wortley P, Thompson L, Sherrard DJ. Dietary and behavioural risk factors for urolithiasis: Potential implications for prevention. Am J Kidney Dis. 1996;28:195–201. doi: 10.1016/s0272-6386(96)90301-7. [DOI] [PubMed] [Google Scholar]
- 16.Eisner BH, Asplin JR, Goldfarb DS, Ahmad A, Stoller ML. Citrate, malate and alkali content in commonly consumed diet sodas: Implications for nephrolithiasis treatment. J Urol. 2010;183:2419–23. doi: 10.1016/j.juro.2010.02.2388. [DOI] [PubMed] [Google Scholar]
- 17.Passman CM, Holmes RP, Knight J, Easter L, Pais V, Assimos DG. Effect of soda consumption on urinary stone risk parameters. J Endourol. 2009;23:347–50. doi: 10.1089/end.2008.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang DE, Sur RL, Haleblian GE, Fitzsimons NJ, Borawski KM, Preminger GM. Long-term lemonade based dietary manipulation in patients with hypocitraturic nephrolithiasis. J Urol. 2007;177:1358–62. doi: 10.1016/j.juro.2006.11.058. [DOI] [PubMed] [Google Scholar]
- 19.Odvina CV. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin J Am Soc Nephrol. 2006;1:1269–74. doi: 10.2215/CJN.00800306. [DOI] [PubMed] [Google Scholar]
- 20.Hönow R, Laube N, Schneider A, Keßler T, Hesse A. Influence of grapefruit-, orange- and apple-juice consumption on urinary variables and risk of crystallization. Br J Nutr. 2003;90:295–300. doi: 10.1079/bjn2003897. [DOI] [PubMed] [Google Scholar]
- 21.Penniston KL, Steele TH, Nakada SY. Lemonade therapy increases urinary citrate and urine volumes in patients with recurrent calcium oxalate stone formation. Urology. 2007;70:856–60. doi: 10.1016/j.urology.2007.06.1115. [DOI] [PubMed] [Google Scholar]
- 22.Koff SG, Paquette EL, Cullen J, Gancarczyk KK, Tucciarone PR, Schenkman NS. Comparison between lemonade and potassium citrate and impact on urine ph and 24-hour urine parameters in patients with kidney stone formation. Urology. 2007;69:1013–6. doi: 10.1016/j.urology.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Seltzer MA, Low RK, McDonald M, Sham GS, Stoller ML. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J Urol. 1996;156:907–9. [PubMed] [Google Scholar]
- 24.Wabner CL, Pak CY. Effect of orange juice consumption on urinary stone risk factors. J Urol. 1993;149:1405–8. doi: 10.1016/s0022-5347(17)36401-7. [DOI] [PubMed] [Google Scholar]
- 25.Aras B, Kalfazade N, Tufcu V, Kemahli E, Ozbay B, Polat H, et al. Can lemon juice be an alternative to potassium citrate in the treatment of urinary calcium stones in patients with hypocitraturia? A prospective randomized study. Urol Res. 2008;36:313–7. doi: 10.1007/s00240-008-0152-6. [DOI] [PubMed] [Google Scholar]
- 26.Liebman M, Chai W, Harvey E, Boenisch L. Effect of supplemental ascorbate and orange juice on urinary oxalate. Nutr Res. 1997;17:415–25. [Google Scholar]
- 27.Penniston KL, Nakada SY, Holmes RP, Assimos DG. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J Endourol. 2008;22:567–70. doi: 10.1089/end.2007.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yilmaz E, Batislam E, Kacmaz M, Erguder I. Citrate, oxalate, sodium, and magnesium levels in fresh juices of three different types of tomatoes: Evaluation in the light of the results of studies on orange and lemon juices. Int J Food Sci Nutr. 2010;61:339–45. doi: 10.3109/09637480903405570. [DOI] [PubMed] [Google Scholar]
- 29.Andersen DA. Environmental factors in the etiology of urolithiasis. In: Civuentes-Delatte A, Rapado A, Hodgkinson A, et al., editors. Urinary Calculi. Basel, Karger: 1973. p. 130. [Google Scholar]
- 30.Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: New insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15:3225–32. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 31.Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 32.Dussol B, Iovanna C, Rotily M, Morange S, Leonetti F, Dupuy P, et al. A randomized trial of low-animal-protein or high-fiber diets for secondary prevention of calcium nephrolithiasis. Nephron Clin Pract. 2008;110:c185–94. doi: 10.1159/000167271. [DOI] [PubMed] [Google Scholar]
- 33.Hiatt RA, Ettinger B, Caan B, Quesenberry CP, Jr, Duncan D, Citron JT. Randomized controlled trial of a low animal protein, high fiber diet in the prevention of recurrent calcium oxalate kidney stones. Am J Epidemiol. 1996;144:25–33. doi: 10.1093/oxfordjournals.aje.a008851. [DOI] [PubMed] [Google Scholar]
- 34.Taylor EN, Curhan GC. Fructose consumption and the risk of kidney stones. Kidney Int. 2008;73:207–12. doi: 10.1038/sj.ki.5002588. [DOI] [PubMed] [Google Scholar]
- 35.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–62. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 36.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–5. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 37.Curhan GC. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497–504. doi: 10.7326/0003-4819-126-7-199704010-00001. [DOI] [PubMed] [Google Scholar]
- 38.Powell CR, Stoller ML, Schwartz BF, Kane C, Gentle DL, Bruce JE, et al. Impact of body weight on urinary electrolytes in urinary stone formers. Urology. 2000;55:825–30. doi: 10.1016/s0090-4295(99)00617-2. [DOI] [PubMed] [Google Scholar]
- 39.Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. Effect of low carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis. 2002;40:265–74. doi: 10.1053/ajkd.2002.34504. [DOI] [PubMed] [Google Scholar]
- 40.Sarica K, Akarsu E, Erturhan S, Yagci F, Aktaran S, Altay B. Evaluation of urinary oxalate levels in patients receiving gastrointestinal lipase inhibitor. Obesity. 2008;16:1579–84. doi: 10.1038/oby.2008.244. [DOI] [PubMed] [Google Scholar]
- 41.Buck AC, Davies RL, Harrison T. The protective role of eicosapentaenoic acid in the pathogenesis of nephrolithiasis. J Urol. 1991;146:188–94. doi: 10.1016/s0022-5347(17)37750-9. [DOI] [PubMed] [Google Scholar]
- 42.Yasui T, Suzuki S, Itoh Y, Tozawa K, Tokudome S, Kohri K. Eicosapentaenoic acid has a preventive effect on the recurrence of nephrolithiasis. Urol Int. 2008;81:135–8. doi: 10.1159/000144050. [DOI] [PubMed] [Google Scholar]
- 43.Taylor E, Stampfer M, Curhan G. Fatty acid intake and incident nephrolithiasis. Am J Kidney Dis. 2005;45:267–74. doi: 10.1053/j.ajkd.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 44.Taylor EN, Curhan GC. Demographic, dietary, and urinary factors and 24-h urinary calcium excretion. Clin J Am Soc Nephrol. 2009;4:1980–7. doi: 10.2215/CJN.02620409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol. 2009;20:2253–9. doi: 10.1681/ASN.2009030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaeger P, Portmann L, Saunders A, Rosenberg LE, Thier SO. Anticystinuric effects of glutamine and of dietary sodium restriction. N Engl J Med. 1986;315:1120–3. doi: 10.1056/NEJM198610303151803. [DOI] [PubMed] [Google Scholar]
- 47.Eisner BH, Eisenberg ML, Stoller ML. Impact of urine sodium on urine risk factors for calcium oxalate nephrolithiasis. J Urol. 2009;182:2330–3. doi: 10.1016/j.juro.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 48.Cirillo M, Laurenzi M, Panarelli W, Stamler J. Urinary sodium to potassium ratio and urinary stone disease: The Gubbio Population Study Research Group. Kidney Int. 1994;46:1133–9. doi: 10.1038/ki.1994.376. [DOI] [PubMed] [Google Scholar]
- 49.Bataille P, Achard JM, Fournier A, Boudailliez B, Westeel PF, el Esper N, et al. Diet, vitamin D and vertebral mineral density in hypercalciuric calcium stone formers. Kidney Int. 1991;39:1193–205. doi: 10.1038/ki.1991.151. [DOI] [PubMed] [Google Scholar]
- 50.Holmes RP, Assimos DG, Goodman HO. Genetic and dietary influences on urinary oxalate excretion. Urol Res. 1998;26:195–200. doi: 10.1007/s002400050046. [DOI] [PubMed] [Google Scholar]
- 51.Holmes RP, Goodman HO, Assimos DG. Metabolic effects of an oxalatefree formula diet. In: Pak CY, Resnick M, Preminger GM, editors. Urolithiasis. Dallas: Millet the Printer; 1996. p. 167. [Google Scholar]
- 52.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol. 1999;10:840–5. doi: 10.1681/ASN.V104840. [DOI] [PubMed] [Google Scholar]
- 53.Johansson G, Backman U, Danielson BG, Fellstrom B, Ljunghall S, Wikstrom B. Effects of magnesium hydroxide in renal stone disease. J Am Coll Nutr. 1982;1:179–85. doi: 10.1080/07315724.1982.10718985. [DOI] [PubMed] [Google Scholar]
- 54.Breslau NA, Heller HJ, Reza-Albarran AA, Pak CY. Physiological effects of slow release potassium phosphate for absorptive hypercalciuria: A randomized double-blind trial. J Urol. 1998;160:664–8. doi: 10.1016/S0022-5347(01)62749-6. [DOI] [PubMed] [Google Scholar]
- 55.Turnlund JR, Betschart AA, Liebman M, Kretsch MJ, Sauberlich HE. Vitamin B-6 depletion followed by repletion with animal- or plant-source diets and calcium and magnesium metabolism in young women. Am J Clin Nutr. 1992;56:905–10. doi: 10.1093/ajcn/56.5.905. [DOI] [PubMed] [Google Scholar]
- 56.Kaufman DW, Kelly JP, Curhan GC, Anderson TE, Dretler SP, Preminger GM, et al. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol. 2008;19:1197–203. doi: 10.1681/ASN.2007101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoppe B, Beck B, Gatter N, von Unruh G, Tischer A, Hesse A, et al. Oxalobacter formigenes: A potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int. 2006;70:1305–11. doi: 10.1038/sj.ki.5001707. [DOI] [PubMed] [Google Scholar]
- 58.Butterweck V, Khan SR. Herbal medicines in the management of urolithiasis: Alternative or complementary? Planta Med. 2009;75:1095–103. doi: 10.1055/s-0029-1185719. [DOI] [PMC free article] [PubMed] [Google Scholar]


