Abstract
Introduction and Objectives:
There is a paucity of information in the literature about the characteristics of prostate cancer in the Asian-Indian population. We wanted to evaluate the oncological outcomes of Asian-Indians and Caucasians. We also derived a nomogram for prediction of extraprostatic extension (EPE) and presented biochemical recurrence (BCR) rates in the Asian-Indian population.
Materials and Methods:
A total of 2367 D’Amico low-risk patients underwent robotic-assisted radical prostatectomy (RARP) for clinically localized prostate cancer between January 2005 and July 2010 by a single surgeon. Of these 56 (2.4%) patients were Asian-Indians and 2025 were Caucasians (85.6%). Univariate and multivariate models were created for predicting EPE. A multivariate logistic regression model was used to develop a predictive nomogram. BCR was defined as a prostate-specific antigen ≥0.2 at any postoperative time point. Kaplan-Meier survival analysis was used to investigate BCR rates.
Results:
A significantly greater percentage of Asian-Indians compared to Caucasians had EPE (32.3 vs. 16.5; P = 0.01). In multivariate analysis adjusted for significant variables from univariate analyses, Asian-Indian race (P = 0.028), age (P = 0.050), maximum percentage cancer on biopsy (P < 0.001), and pathology prostate weight (P = 0.047) were independent predictors of EPE. Kaplan-Meier analysis demonstrated BCR free rates of 94.6% and 95.4%, for Asian-Indians and Caucasians, respectively, at a median follow-up of 16 months (range 2-70 months). There was no statistically significant difference in BCR rates across the two cohorts (log-rank P-value = 0.405).
Conclusions:
This study highlights that while Asian-Indians have more advanced cancer variables, their risk of BCR after surgery is similar to Caucasian patients. Further work is required to better understand the social, genetic and environmental factors that affect the biology of prostate cancer in men of Asian-Indian descent.
Keywords: Asian-Indian, nomogram, prostate cancer, race, robotic prostatectomy
INTRODUCTION
Prostate cancer is the commonest nondermatological cancer in men in the Western world.[1] However, it is reported far less frequently in developing countries, at least in part due to racial predilections.[2,3] While several other racial groups have been studied in detail, there is a paucity of published literature regarding histopathological features and biology of prostate cancer among Asian-Indian men. Some information regarding prostate cancer in Asian-Indians suggests that the incidence in five major Indian cities appears to be rising.[4] This trend could be secondary to improved diagnostic abilities and increases in risk due to the adoption of a Western diet and /or increased exposure to other environmental carcinogens.[3] One of the reasons for a lack of information regarding the biology of prostate cancer in Asian-Indians is the infrequent use of radical prostatectomy in this population; hence, there is a lack of histological data. Since most Asian-Indian patients present with advanced stage cancer, they hardly ever go through the curative treatment and thus few surgical series are available to highlight unique pathological variables in Asian-Indian men with prostate cancer.[5,6]
At our center, due to a high surgical volume we were uniquely positioned to gather a sizeable cohort of Indian men with clinically localized prostate cancer. Presented herein are the findings of our study involving 56 patients treated and followed-up by a single surgeon (AT). We have also derived a nomogram for prediction of extraprostatic extension (EPE) or pT3 disease as well as presented five-year BCR-free survival rates in Asian-Indians after surgery. Comparisons have been made with an age-matched Caucasian cohort for pathological and biochemical outcomes.
MATERIALS AND METHODS
Patient population
A total of 2367 patients underwent robotic-assisted radical prostatectomy for clinically localized adenocarcinoma of the prostate between January 2005 and July 2010 by a single surgeon (AT). Of these, 56 (2.4%) patients were Asian-Indians and 2025 were Caucasians (85.6%). Surgery was performed using a DaVinci Robotic System™(Intuitive Surgical Inc, Sunnyvale, CA) by a previously described technique.[7–12]
Patients underwent a standardized preoperative workup including serum prostate-specific antigen (PSA), digital rectal examination, systematic TRUS-guided prostate biopsy and an endorectal MRI. All the preoperative needle biopsies were re-analyzed by one of our institutional uropathologists (MS). Pathology slides were re-reviewed and additional blocks were analyzed wherever necessary for all the patients who had their biopsies done outside our institution. In select cases (those with PSA>10 ng/ml and/or Gleason of >7, and/or clinical T3 prostate cancer) bone scans and computerized tomography (CT) scans of the abdomen and pelvis were also obtained.
Preoperative information was collected on a custom-designed data-sheet and included age, prior abdominal surgery, comorbidities, body mass index (BMI), serum PSA and clinical stage. Prostate biopsy data included the number and location of positive cores, Gleason score, percent of cancer involvement in positive biopsy cores and percent of positive biopsy cores. Pathological data, including specimen weight, Gleason grades, EPE of tumor (if any) and margin status, were recorded.
Radical prostatectomy specimen pathological analysis
All the specimens were weighed and analyzed by the uropathology service at our institution. The surgical specimen was inked and processed for standard histopathological analysis. Margins were considered positive if there was tumor present at the inked margin. Patients with extension of tumor through the prostatic capsule were considered to have EPE.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 (SPSS Inc, Chicago, IL). χ2 analysis was performed for comparison of groups with nominal variables while continuous variables were compared using Student's t-tests. Univariate and multivariate models were also created for predicting EPE in D’Amico low-risk category patients as these patients should, by conventional criteria, have a low risk of EPE and we wanted to investigate whether Asian-Indian ethnicity impacted on this.[13] BCR was defined as a PSA ≥ 0.2 at any postoperative time point, and patients were censored if and when this happened. Kaplan-Meier survival analysis was used to compare BCR rates in Asian-Indians versus Caucasians, and the log rank test used to determine if the BCR-free survival differences were statistically significantly different between the racial groups.
Multivariable logistic regression modeling was used to develop the nomogram to predict pT3 disease using the Regression Modeling Strategies package version 2.9.1. Any possible nonlinear relationship between the predictive factors and outcome of pT3 disease was evaluated using the multiple fractional polynomial method.[14,15] The predictive accuracy of the model was assessed in terms of discrimination as well as calibration. Discrimination is the ability to differentiate between individuals with and without pT3 disease and is measured by the receiver operating characteristic (ROC) curve, which is summarized by the area under the curve (AUC), i.e., the concordance (c-) index. Bootstrap methods were used to correct for over-optimism of the predictive model during validation.[16] Calibration is the correlation between the number of pT3 diseased individuals predicted and the number found to actually have pT3 disease, and was assessed by fitting LOESS (locally weighted scatterplot smoothing) curves to the observed proportion versus the predicted probability of pT3 disease. Confidence intervals were evaluated using the bootstrap method with 95% coverage.
RESULTS
Table 1 demonstrates the baseline demographics, preoperative variables, and systemic biopsy data comparing Asian-Indians with Caucasians. A total of 56 Asian-Indians and 2025 Caucasians with clinically localized prostate cancer were retrospectively evaluated. The mean patient age in Asian-Indians was 60.9 years (range 44-72) and 59.7 years (range 36-82) in Caucasians. Asian-Indians patients had significantly lower BMIs when compared to Caucasians (24.9 vs. 27; P < 0.001). Mean preoperative PSA was 6.9 ng/ ml in Asian-Indians, which was not significantly different from Caucasians who had a mean preoperative PSA of 6.1 ng/ ml. Of the 56 Asian-Indians, the preoperative serum PSA level was 0 to 4 ng/ml in 10 (17.9%), 4.1 to 10 ng/ml in 38 (67.9%) and greater than 10 ng/ml in 8 (14.2%). The median number of biopsy cores taken in each patient was 12 in both groups. The median number of positive cores per patient was three in Asian-Indians and two in Caucasians. The percentage of positive cores ranged from 2 to 100% (median 28.6) in Asian-Indians and from 1.3 to 100% (median 20) in Caucasians. The median maximum percentage of tumor in a core in each sextant biopsy was significantly higher in Asian-Indians compared to Caucasians (35% versus 20%; P = 0.011). There was no significant difference in Gleason score on needle prostate biopsies between the two groups.
Table 1.
Preoperative variables, baseline demographics and systemic biopsy data comparing Asian-Indians vs. Caucasians
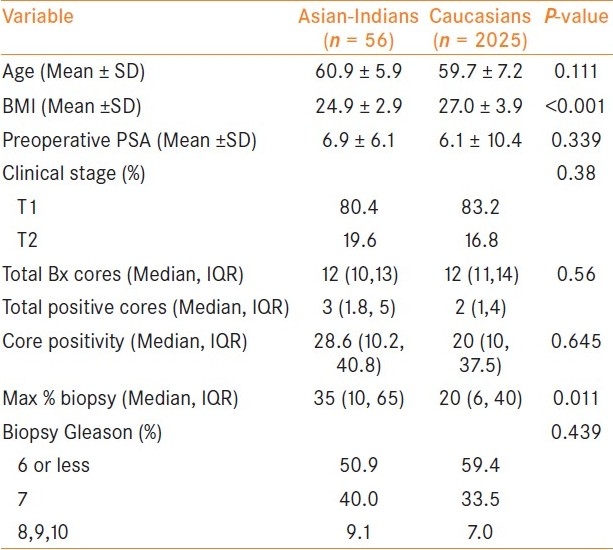
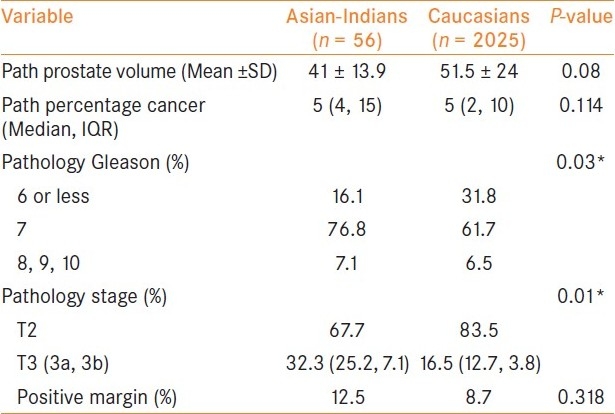
Table 2 demonstrates the pathology data in the two groups. The average prostate weight was lower in Asian-Indians compared to Caucasians; however, this was not statistically significant (41 vs. 51.5 g; P = 0.08). The median percentage cancer in radical prostatectomy specimen was the same between the two groups. Asian-Indians had significantly higher Gleason grade on RP specimen with 83.9% having Gleason 7 or more compared to 68.2% in Caucasians (P = 0.03). A significantly greater percentage of Asian-Indians compared to Caucasians also had EPE (32.1 vs. 16.5; P = 0.01). 7.1% of Asian-Indians had seminal vesicle involvement compared to 3.8% in the Caucasians (P = 0.01). When we compared positive surgical margin status, Asian-Indians had more incidences than Caucasians but the difference was not statistically significant (12.5% vs. 8.7%; P = 0.318).
Table 2.
Pathological data and biochemical recurrence data comparing Asian-Indians vs. Caucasians
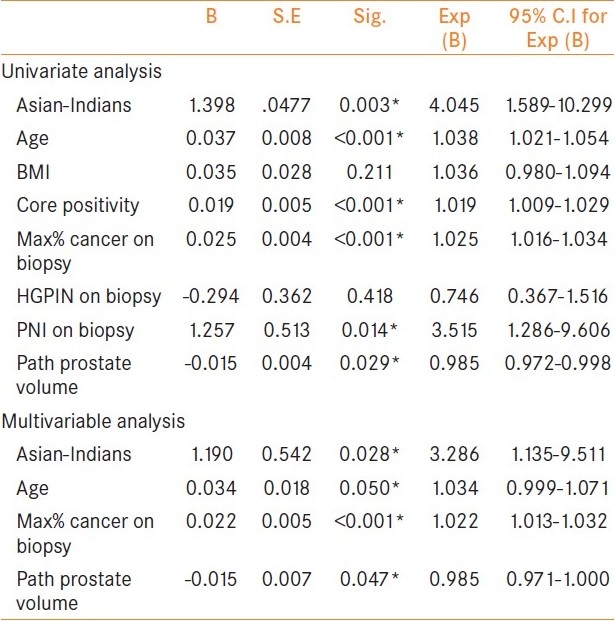
Univariate and multivariate analyses for the prediction of EPE incidence on RP specimens are shown in Table 3. We did these analyses only in patients who met D’Amico low-risk criteria (29 Asian-Indians vs. 1134 Caucasians). Univariate analysis showed a significant association between EPE and age (P < 0.001), being of Asian-Indian decent, core positivity percentage (P < 0.001), maximum percentage cancer on biopsy (P < 0.001), perineural invasion on biopsy (P = 0.014), and pathological prostate volume (P = 0.029). In multivariate analysis using significant variables from univariate analyses, Asian-Indian race (P = 0.028), age (P = 0.050), maximum percentage cancer on biopsy (P < 0.001), and pathology prostate weight (P = 0.047) remained independent predictors of EPE.
Table 3.
Univariate and multivariable model for predicting extracapsular extension in D’Amico low-risk category patients
Kaplan-Meier analysis demonstrated BCR Indians and Caucasians, respectively, [Figure 1] at a median follow up of 16 months (range 2-70 months). There was no statistically significant difference in biochemical-free survival across the two cohorts (log-rank P-value = 0.405). The nomogram showed Indian race as a significant contributor to the risk of having pT3 disease (contributing 50 points; Figure 2). The nomogram was reasonably well calibrated (Figure 3 as evidenced by the closeness of the Loess fit to the line that starts from the southwest corner and ends at the northeast corner of the plot. However, the nomogram underpredicted the risk of pT3 disease in those at higher risk (observed risk >40%).
Figure 1.
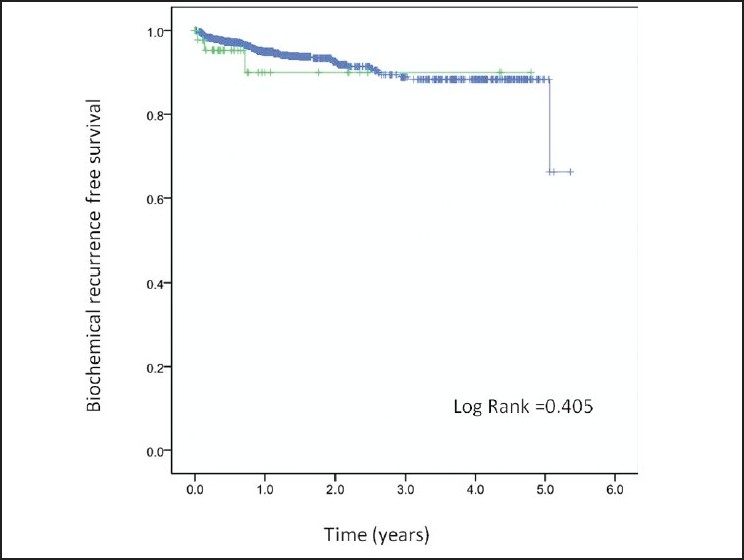
Biochemical recurrence-free survival following radical prostatectomy comparing Asian-Indians vs. Caucasians: Green: Asian-Indians; Blue: Caucasians
Figure 2.
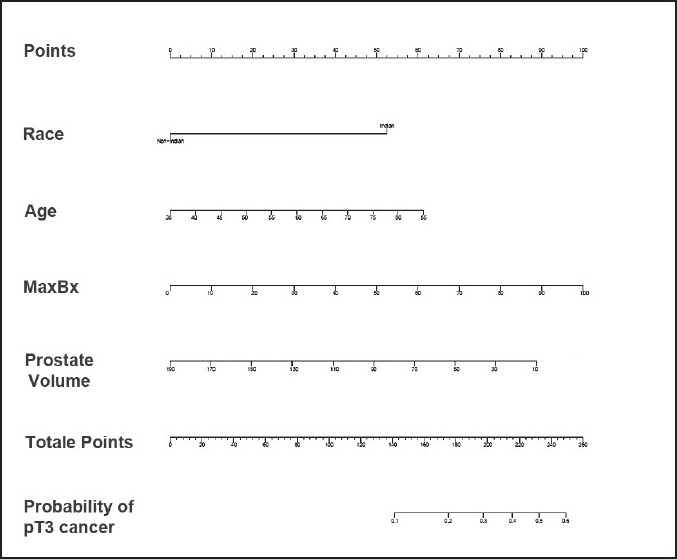
Nomogram [race (Asian-Indian vs. Caucasians), age, maximum% cancer on biopsy (MaxBx), prostate volume] for predicting extraprostatic extension (EPE; T3 disease). The nomogram is used by locating the patient position on each factor. Each factor has corresponding prognostic points (top axis). The points for each factor are added and the probability of EPE is estimated from the bottom line
Figure 3.
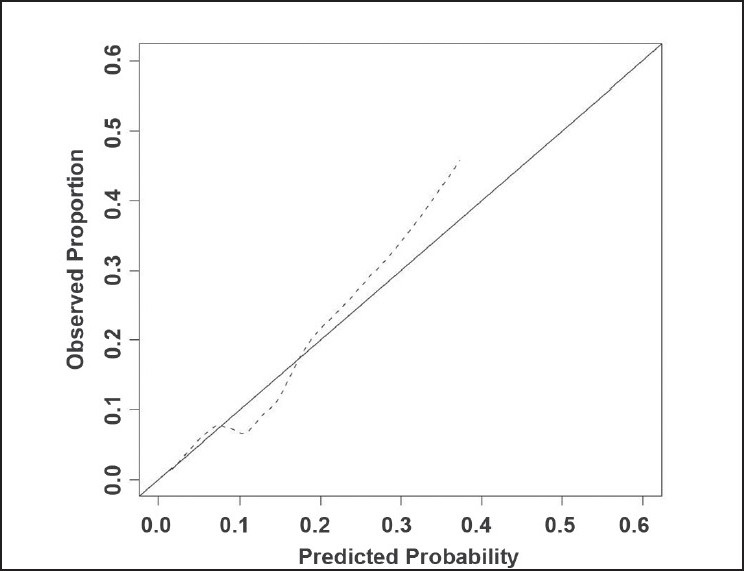
Calibration of nomogram. ------- Apparent, Ideal
DISCUSSION
The purpose of this study was to analyze the pathological features of specimens following radical prostatectomy in Asian-Indians and compare these to Caucasians. We found that Asian-Indians had a greater tendency for higher Gleason scores (7 or more) in both the preoperative prostate biopsy and final histopathological analysis. A significantly higher incidence of EPE was found in Asian-Indians versus Caucasians (32.3% versus 16.5%, respectively).
Since many Asian-Indians present with advanced prostate cancer and are essentially not candidates for radical prostatectomy, our study provides unique insight into those uncommon patients who presented early enough to be surgical candidates. This allowed us to make comparisons between Asian-Indians and Caucasians with regards to various pathological variables in early prostate cancer. Interestingly, we noted that even in the clinically localized stage, these patients have more aggressive pathological parameters. In addition to the low percentage of pathologically organ-confined cancer, Asian-Indians also had a high incidence of seminal vesicle invasion compared to Caucasians (7.1% versus 3.8%, respectively).[17,18] So why do Asian-Indians have more aggressive pathological features? While genetics may play a part, the contribution of delayed diagnosis cannot be excluded. This could be due to the prevalent notion that Asian-Indians have a lower incidence of prostate cancer, resulting in lower awareness among these men, and them being diagnosed at a later stage as a result. Secondly, while there are known racial differences in PSA highlighted in studies on African-American men, the PSA profile of Asian-Indians have never been established.[3,6] This study showed a slightly higher PSA on average in Asian-Indian men compared to Caucasians, but this was not statistically significant. This may be due to this study being underpowered to detect a difference and larger studies will be required to evaluate this further. The cut-off PSA of 4 ng/ml that is commonly used to trigger the need for biopsy was developed on a predominantly Caucasian population, and it may be that lower cut-offs, free: total PSA ratios, or PSA kinetics such as PSA velocity would be better guides to trigger biopsy in Asian-Indian men. It may also be interesting to look at urinary substances such as PCA-3 and others which are currently being investigated as diagnostic markers.[19] There could also be differences in the location of cancer (anterior versus peripheral), which could interfere with biopsy detection using the conventional transrectal approach and we need to investigate this in future studies.
On a positive note, our study shows that while Indian men have more aggressive pathological features at the time of surgery, they had comparable BCR-free survival. Hence, there appears to be a window of opportunity during which there is no systemic spread and timely intervention can provide cure to this group of patients. The use of our nomogram may help physicians in appropriate patient counseling to maximize cancer control and not be baffled by unexpected EPEs.
We recognize that there are several shortcomings in the study, which must be considered. One of the foremost is the small cohort size. We strongly recommend multi-institutional studies to increase subject numbers such that our findings can be verified or dismissed. Further, there is a significant difference between the health care practice and the health care seeking behavior between the United States and India. Our findings may not be directly applicable to patients residing in the Indian subcontinent. However, our own patient pool has significant representation of Asian-Indians who flew from India to New York for their surgery, and on subgroup analysis, we did not find any difference between these two cohorts (data not shown).
CONCLUSIONS
This study highlights that while Asian-Indians have more advanced cancer variables, their risk of BCR after surgery is similar to Caucasian patients. Further work is required to better understand the social, genetic, and environmental factors that affect the biology of prostate cancer in men of Asian-Indian descent.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Farkas A, Marcella S, Rhoads GG. Ethnic and racial differences in prostate cancer incidence and mortality. Ethn Dis. 2000;10:69–75. [PubMed] [Google Scholar]
- 3.Reddy S, Shapiro M, Morton R, Jr, Brawley OW. Prostate cancer in black and white Americans. Cancer Metastasis Rev. 2003;22:83–6. doi: 10.1023/a:1022216119066. [DOI] [PubMed] [Google Scholar]
- 4.Yeole BB. Trends in the prostate cancer incidence in India. Asian Pac J Cancer Prev. 2008;9:141–4. [PubMed] [Google Scholar]
- 5.Hebert JR, Ghumare SS, Gupta PC. Stage at diagnosis and relative differences in breast and prostate cancer incidence in India: Comparison with the United States. Asian Pac J Cancer Prev. 2006;7:547–55. [PubMed] [Google Scholar]
- 6.Zeigler-Johnson CM, Rennert H, Mittal RD, Jalloh M, Sachdeva R, Malkowicz SB, et al. Evaluation of prostate cancer characteristics in four populations worldwide. Can J Urol. 2008;15:4056–64. [PMC free article] [PubMed] [Google Scholar]
- 7.Tewari A, Srivasatava A, Menon M Members of the VIP Team. A prospective comparison of radical retropubic and robot-assisted prostatectomy: Experience in one institution. BJU Int. 2003;92:205–10. doi: 10.1046/j.1464-410x.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava A, Grover S, Sooriakumaran P, Tan G, Takenaka A, Tewari AK. Neuroanatomic basis for traction-free preservation of the neural hammock during athermal robotic radical prostatectomy. Curr Opin Urol. 2011;21:49–59. doi: 10.1097/MOU.0b013e32834120e9. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen L, Jhaveri J, Tewari A. Surgical technique to overcome anatomical shortcoming: Balancing post-prostatectomy continence outcomes of urethral sphincter lengths on preoperative magnetic resonance imaging. J Urol. 2008;179:1907–11. doi: 10.1016/j.juro.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 10.Tewari AK, Bigelow K, Rao S, Takenaka A, El-Tabi N, Te A, et al. Anatomic restoration technique of continence mechanism and preservation of puboprostatic collar: A novel modification to achieve early urinary continence in men undergoing robotic prostatectomy. Urology. 2007;69:726–31. doi: 10.1016/j.urology.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Tewari AK, Srivastava A, Mudaliar K, Tan GY, Grover S, El Douaihy Y, et al. Anatomical retro-apical technique of synchronous (posterior and anterior) urethral transection: A novel approach for ameliorating apical margin positivity during robotic radical prostatectomy. BJU Int. 2010;106:1364–73. doi: 10.1111/j.1464-410X.2010.09318.x. [DOI] [PubMed] [Google Scholar]
- 12.Tewari A, Takenaka A, Mtui E, Horninger W, Peschel R, Bartsch G, et al. The proximal neurovascular plate and the tri-zonal neural architecture around the prostate gland: Importance in the athermal robotic technique of nerve-sparing prostatectomy. BJU Int. 2006;98:314–23. doi: 10.1111/j.1464-410X.2006.06266.x. [DOI] [PubMed] [Google Scholar]
- 13.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 14.Royston P, Sauerbrei W. Building multivariable regression models with continuous covariates in clinical epidemiology--with an emphasis on fractional polynomials. Methods Inf Med. 2005;44:561–75. [PubMed] [Google Scholar]
- 15.Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26:5512–28. doi: 10.1002/sim.3148. [DOI] [PubMed] [Google Scholar]
- 16.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman and Hall; 1993. pp. 16–436. [Google Scholar]
- 17.Stamey TA, Donaldson AN, Yemoto CE, McNeal JE, Sözen S, Gill H. Histological and clinical findings in 896 consecutive prostates treated only with radical retropubic prostatectomy: Epidemiologic significance of annual changes. J Urol. 1998;160:2412–7. doi: 10.1097/00005392-199812020-00009. [DOI] [PubMed] [Google Scholar]
- 18.Epstein JI, Amin M, Boccon-Gibod L, Egevad L, Humphrey PA, Mikuz G, et al. Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol Suppl. 2005;216:34–63. doi: 10.1080/03008880510030932. [DOI] [PubMed] [Google Scholar]
- 19.de Kok JB, Verhaegh GW, Roelofs RW, Hessels D, Kiemeney LA, Aalders TW, et al. DD3(PCA3), a very sensitive and specific marker to detect prostate tumors. Cancer Res. 2002;62:2695–8. [PubMed] [Google Scholar]


