Abstract
Background:
In India, prostate cancer is one of the five leading sites of cancers among males in all the registries. Very little is known about risk factors for prostate cancer among the Indian population.
Objectives:
The present study aims to study the association of lifestyle factors like chewing (betel leaf with or without tobacco, pan masala, gutka), smoking (bidi, cigarette), comorbid conditions, diet, body mass index (BMI), family history, vasectomy with prostate cancer.
Materials and Methods:
This an unmatched hospital-based case-control study, comprised of 123 histologically proven prostate ‘cancer cases’ and 167 ‘normal controls. Univariate and regression analysis were applied for obtaining the odds ratio for risk factors.
Results:
The study revealed that there was no significant excess risk for chewers, alcohol drinkers, tea and coffee drinkers, family history of cancer, diabetes, vasectomy and dietary factors. However, patients with BMI >25 (OR = 2.1), those with hypertension history (OR = 2.5) and age >55 years (OR = 19.3) had enhanced risk for prostate cancer.
Conclusions:
In the present study age, BMI and hypertension emerged as risk factors for prostate cancer. The findings of this study could be useful to conduct larger studies in a more detailed manner which in turn can be useful for public interest domain.
Keywords: Age, body mass index, hypertension, India, prostate cancer
INTRODUCTION
Prostate cancer is more common in Western men, and incidence is rising rapidly in most countries, including low-risk populations.[1] Highest incidence rates for prostate cancer are reported from US, Detroit, Black (AAR = 141.5 per 105) while in the Asian subcontinent, the rates are highest in Japan, Hiroshima (AAR = 10.9 per 105).[2] In India, the age-standardized rates (per 105) vary between Delhi (11.5), Mumbai (6.3), Chennai (5.2), Bangalore (6.0) and Barshi (1.6).[3]
Prostate is one of the leading site of cancer among males in Mumbai, India. The incidence rates have risen over the past decade, though not as remarkable as in the west. Prostate cancer is known to be disease among the older people and with increasing life-expectancy, the incidence of prostate cancer are also likely to increase in the near future. The data from the Indian cancer registries are showing some increases, the increase being more distinct in Mumbai city. In India, it is one of the five leading sites of cancers among males in all the registries.[3] Very little is known about prostate cancer in terms of the risk factors in the Indian population and this has encouraged us to conduct this study for better understanding of this disease.
There are several risk factors that have been implicated in the risk for prostate cancer. Studies elsewhere have shown family history as a strong risk factor.[4] History of diabetes mellitus,[5] height, weight and obesity,[6] smoking and physical activity,[7] body mass index (BMI),[8] vasectomy[9] have been found to be associated with prostate cancer risk.
The aim of the present study is to study the association of age, diabetes, hypertension, family history, vasectomy, tobacco consumption (chewing, smoking), alcohol consumption, dietary substances like red meat or high-fat dairy products, vegetables, BMI and risk of prostate cancer.
MATERIALS AND METHODS
This is a retrospective unmatched hospital-based case-control study conducted at Tata Memorial Hospital that included subjects registered between the years 1999 and 2001. There were 123 histologically confirmed prostate ‘cancer cases’ and 167 ‘normal controls (free of cancer)’ which were considered as ‘eligible entrants’ into the study. Data on age, tobacco-habits, diabetes, hypertension, BMI, dietary factors, tea, coffee, family history of cancer and vasectomy were collected by the social investigators. Univariate and regression analysis were applied for obtaining the odds ratio (OR) for risk factors.
Patients were interviewed at the out-patient department of TMH, prior to diagnosis. All patients could not be interviewed due to various reasons. The information was recorded in a predesigned questionnaire. After interviewing the patient, cancer cases and controls were segregated based on the diagnosis. The hospital being a comprehensive cancer centre for diagnosis and treatment attracts patients from all parts of India. In general, in a year 25-30% of patients of total registrations are diagnosed as ‘free of cancer’.
Cases were histologically proven cancer cases of prostate. Controls were those that were diagnosed by histology as ‘free of cancer’. There were cases diagnosed as benign prostate hyperplasia (BPH) which were excluded to be considered as ‘controls’. During the period 1999-2001, 290 patients were interviewed. Of these, 123 histologically proven prostate cancer patients (ICD9: 1859) were included as ‘cases’ and 167 as ‘normal’ controls. Thus, there were 123 ‘prostate’ cancer ‘cases’ and 167 ‘controls’ (unmatched) that were considered as eligible entrants for this study. The questionnaire contained socio-demographic information (age, sex, religion, residence), lifestyle habits such as smoking, chewing, alcohol drinking), dietary habits, comorbid conditions including history of diabetes, hypertension, tuberculosis, height and weight for calculation of BMI, family history of cancer, history of vasectomy and number of children. The questionnaire regarding food items were based on recollection of consumption of routine food items prior to one year from the date of interview. Information on food frequency per week was collected but due to incompleteness, this was not taken into account for the analysis. The dietary items were classified as vegetarian diet and non-vegetarian diet. The non-vegetarian diet included items as dry fish, fresh fish, chicken and red meat; red meat included mutton, liver, pork, brain. Consumption of green vegetables was also recorded.
Unconditional logistic regression model was applied for obtaining the risk estimates (OR) and its 95% confidence limits using SPSS Version 15.0 software. In the analysis, independent variables were categorized into binary form and entered into the model and the results were considered for statistical significance at 5%.
RESULTS
The Table 1 shows characteristics of cases and controls. The average age was 64 years and 45 years for cases and controls, respectively. Age could not be matched since there were less men in older age who visited the hospital as controls than the cases. The difference in mean age between the cases (64 years) and controls (46 years) were distinct, essentially due to the fact that prostate cancer is known to be an elderly age disease. Literacy rate was similar in both the groups but there were differences in the distribution of the subjects by religion. An equal proportion of cases and controls (13.8%) had family history of cancer. History of diabetes was four-fold among the cases, and history of hypertension was three-fold among the cases, as compared to the controls. Proportion of those with BMI less than 25 was similar among both cases and controls. 91.1% and 86.8% did not have any children among cases and controls, respectively. History of vasectomy was predominant among cases.
Table 1.
Patient characteristics of cases and controls for prostate cancer study
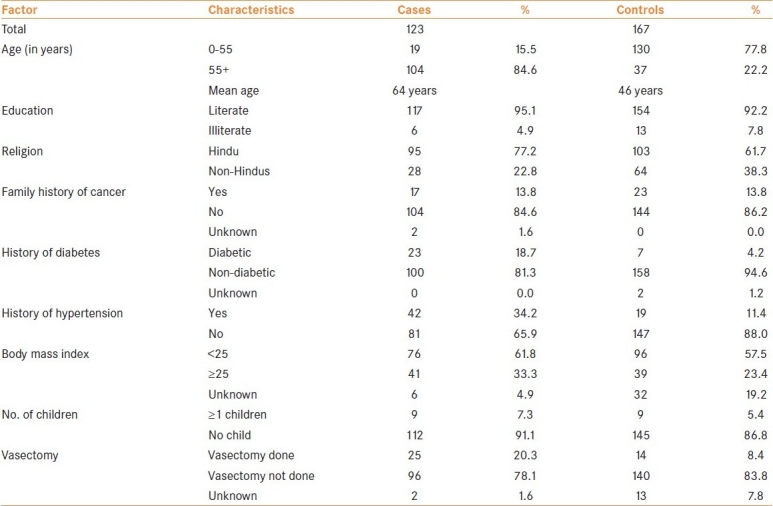
Table 2 shows the OR and confidence interval (CI) for comorbid conditions and personal history. The Table shows the crude OR and the adjusted OR estimates. The crude OR for age, education and religion had elevated risks (though education was not significant), was taken into account for estimating the adjusted risk estimates. Age was a strong determinant for prostate cancer. Those aged above 55 years had an approximately 18-fold excess risk for prostate cancer compared to those aged less than 55 years. Those with family history of cancer did not show any excess risk for prostate cancer (OR = 0.5) in the study group. Though the risk was more than two-fold (OR = 2.5) for diabetic patients compared to non-diabetic patients, it was not statistically significant. While history of hypertension showed a 2.8-fold excess risk for prostate cancer over the non-hypertensive group. Those with BMI greater than 24.9 had a two-fold enhanced risk for prostate cancer when compared to those with BMI less than 25. Number of children and vasectomy did not show any excess significant risk.
Table 2.
Odds-ratio and 95% confidence interval for age, family history, hypertension, BMI, number of children and vasectomy
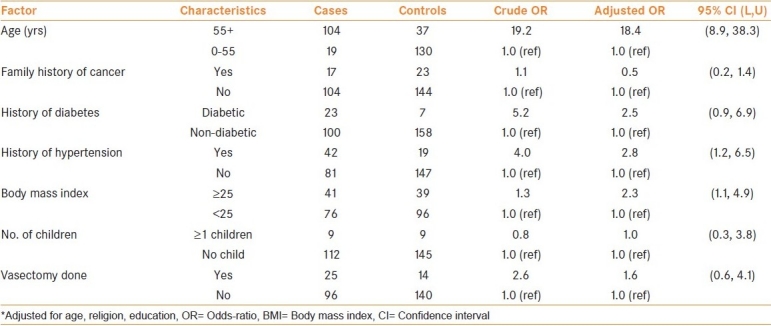
Table 3 shows the OR and 95% CI for life-style habits as chewing, smoking, alcohol drinking and dietary habits. The estimates are adjusted for age, religion and education. Smoking was prevalent in 32.5% of cases and 40% of controls, while the proportion of chewers among the cases and controls was 29% and 48.5%, respectively. Similar distribution was observed among cases and controls with alcohol drinking habit. Chewers (OR = 0.4), smokers (OR = 0.9) and alcohol-drinker (OR = 0.8) did not show any excess significant risk compared to the non-habit group, respectively.
Table 3.
Odds-ratio and 95% confidence interval for life-style and dietary factors
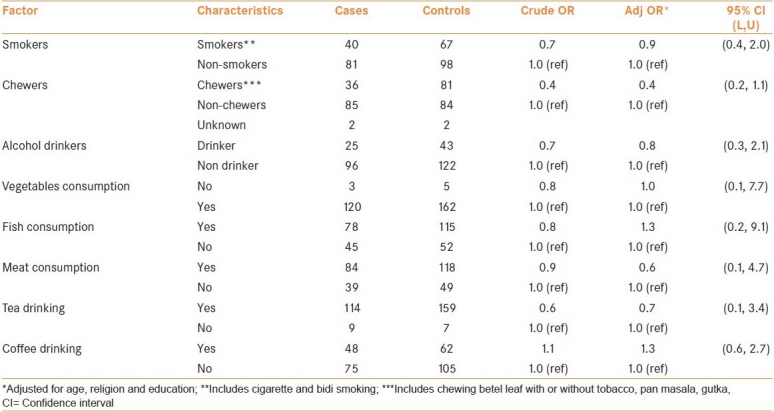
It is seen from Table 3, that the proportion of meat-eaters among controls were twice compared to those among cases while the proportion of tea and coffee drinkers, fish-eaters consumption of raw-green vegetables, were similar among both cases and controls. Consumption of ‘raw vegetables’ (OR = 1.0), meat-eating (OR = 0.6), fish-eating (OR = 1.3) did not show any significant increase/decrease in risk for prostate cancer. Similarly neither tea (OR = 0.7) nor coffee drinking (OR = 1.3) showed any additional risk for prostate cancer compared to non-drinkers.
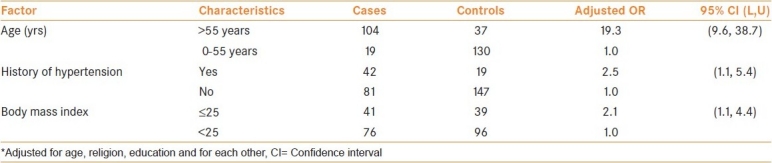
Table 4 shows the risk estimates obtained by regression analysis by adjusting for factors that were found to be significant in the univariate analysis. The Table shows that those aged above 55 years had a 19-fold excess risk, those with hypertension history had a 2.5-fold excess risk and those with BMI greater than 25 had a 2-fold excess risk for prostate cancer.
Table 4.
Odds ratio and 95% confidence limits for factors using regression method
DISCUSSION
The present study is an unmatched hospital-based case-control study conducted at TMH in Mumbai, India. Patients were interviewed prior to diagnosis thus minimizing the interviewer's bias. However, the limitations of the study are the biases that are known in a hospital-based case-control studies. All prostate cancer cases registered during the year 1999-2001 could not be interviewed due to various reasons. The social investigators collected information in a predesigned well-structured questionnaire. The questionnaire included information on demographic characteristics, lifestyle habits, dietary habits, history of diabetes, hypertension, BMI, vasectomy, number of children, etc. Since very few case- control studies reported so far from India, the present study attempts to study the association of the various factors and prostate cancer risk.
Age emerged as the strongest determinant factor for prostate cancer in our study as well, which has also been recently shown in an Iran study.[10] The difference in mean age between the cases (64 years) and controls (46 years) were distinct, essentially due to the fact that prostate cancer is known to be an elderly age disease, while those diagnosed as ‘free of cancer’ had some other ailments not linked to prostate, and also the patients were interviewed at the out-patient department. The authors agree that this is one of the drawbacks of the study, but the OR for risk factors has been adjusted for in the analysis.
Chewing of tobacco is common in India. Chewing includes chewing betel leaf with or without tobacco, pan masala or gutka. The present study showed no significant increase in risk for chewers, although tobacco chewing was more common. Although, no enhanced risk was observed for cigarette smokers in a population-based study conducted in Utah, USA;[11] there are other studies which have reported the contrary viz. no increased risk for current smoker but increased risk for pack years of smoking.[12] However, in the present study no excess risk was observed for smokers, in general, for prostate cancer. Tobacco did not emerge as a risk factor, and also due to the fact that the data on duration of tobacco use was incomplete, pack years of smoking or chewing was not considered in the analysis.
Information on alcohol drinking is difficult, but not impossible, to obtain in Indian situations because of the social stigma attached with this habit. The present study did not show any enhanced risk for alcohol drinkers and is in agreement with that reported earlier.[11]
India being a country with varied religious groups, the dietary habits also vary across the religious groups. It is well known that diet has an important role to play in cancer risk. Despite the associations with meat eating, existing studies suggest that vegetarians do not have reduced risk of breast, bowel or prostate cancer and the present study too did not show any excess risk for meat eaters.[13] Although an earlier study indicated a reduced risk for fish eaters, our study did not show any significant reduction in risk for prostate cancer.[14] Tea and coffee drinking did not show any significant risk for prostate cancer in our study.[12]
Studies in literature showed positive association of family history of cancer and prostate cancer risk; however, our study did not show any enhanced risk, even after adjustment for dietary and other risk factors.[4]
Lower risk of prostate cancer among diabetics has been suggested by many, but not all studies. The possible reason for this inconsistency could be due to the fact that studies have not accounted for ‘time since diagnosis’ of diabetes mellitus, treatment duration or have not examined confounding factors such as diet. A Health Professionals Follow-Up Study from 1986 and 1994, in which 1,369 new cases of non-stage A1 prostate cancer were documented in 47,781 men was reported.[5] Prostate cancer was not reduced in the first 5 years after diagnosis (RR = 1.24, CI = 0.87-1.77). In the present study, although patients with a history of diabetes had an elevated risk (OR = 2.5), it was not statistically significant; this could be attributed to the fact that the ‘time since diagnosis’ and also the duration of treatment taken for diabetes was not recorded. Information on history of diabetes was a part of the questionnaire and was based on interview and hospital records; however not all subjects underwent a gylcosylated hemoglobin level test. We know that there is an increase in the incidence of diabetes in India, but unless a study is carried out in detail whether the rise in prostate cancer cases is due to the increase in diabetes incidence, it cannot be said in affirmative; probably the time of diagnosis of diabetes, duration of treatment and dosage details would give an answer in this direction.
The findings on the association of hypertension and prostate cancer have not been consistent. However, the present study showed an 2.6-fold increased risk for prostate cancer for those with a history of hypertension and is in agreement with one of the earlier studies,[15] whereas an other study did not find any association with prostate cancer.[16]
It is known that obesity and physical activity can modulate the endocrine system. Cerhan et al. (1997) demonstrated that greater BMI (wt/ht2) (RR = 1.7 for BMI > 27.8 kg/m2 compared with BMI < 23.6; P trend = 0.1) was a risk factor for prostate cancer. The present study showed that those who had a BMI greater than 24.9 (obese) had a two-fold enhanced risk for prostate cancer compared to those with BMI less than 25, which is in agreement with the findings reported in other studies.[7] A Finnish study reported that middle-aged men with the metabolic syndrome were more likely to develop prostate cancer in this prospective population-based study.[17] The association between metabolic syndrome and risk of prostate cancer was stronger among overweight and obese men with a BMI ≥ 27 kg/m2 (adjusted relative risk, 3.0; 95% CI, 1.2-7.3) than in lighter men (relative risk, 1.8; 95% CI, 0.7-4.7).
Those who had undergone vasectomy showed a two-fold non-significant risk for prostate cancer, which is in concurrence with an earlier study reported from India.[9] As per the guidelines stated, men who wish to be screened for prostate cancer should have both a PSA test and a DRE.[18] Similarly there was no association between the number of children and prostate cancer risk in the present study.
The present study demonstrates age, hypertension and BMI as important determinants for prostate cancer risk. Understanding the mechanisms underlying these findings may provide biological insights into prostate carcinogenesis. BMI is directly related to the diet and physical activity. It is possible that those who are obese (BMI greater than 24.9) are eating high-fat diet and are probably less active. A detailed study addressing these factors may provide the right lead in understanding the disease process. Additional information on physical activity will provide inputs for preventing prostate cancer. The authors agree that the number of subjects are less, which is a limitation of the study; nonetheless, this can serve as a platform to launch a larger study for study the aspects in detail, since there are no case-control study on prostate cancer from India reporting on these aspects.
CONCLUSIONS
The present study is a hospital-based case-control study on prostate cancer reported from India. The study concluded that age at diagnosis, obesity (BMI > 24.9) and hypertension are risk factors for prostate cancer. Given the recent changes in lifestyles and dietary habits, increased life-expectancy and the expected rise in burden of chronic-diseases, as projected by the World Health Organization (WHO), it is likely that the incidence of prostate cancer will show an increase in the future. Detailed studies on prostate cancer in a similar research setting in a developing country will further enhance the knowledge of prostate cancer.
ACKNOWLEDGMENT
The Director, Tata Memorial Centre, Mumbai, India.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Lyon, France: International Agency for Research on Cancer; 2010. [Last accessed on 31st Oct 2010]. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Available from: http://www.globocan.iarc.fr . [Google Scholar]
- 2.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents. Vol. 8. Lyon, IARC: IARC Scientific Publications No. 155; 2002. [Google Scholar]
- 3.Nandakumar A. Consolidated Report of the Population Based Cancer Registries 2001-04. India: National Cancer Registry Programme, Indian Council of Medical Research; 2006. [Google Scholar]
- 4.Cerhan JR, Parker AS, Putnam SD, Chiu BC, Lynch CF, Cohen MB, et al. Family history and prostate cancer risk in a population-based cohort of Iowa men. Cancer Epidemiol Biomarkers Prev. 1999;8:53–60. [PubMed] [Google Scholar]
- 5.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Diabetes mellitus and risk of prostate cancer (United States) Cancer Causes Control. 1998;9:3–9. doi: 10.1023/a:1008822917449. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:557–63. [PubMed] [Google Scholar]
- 7.Cerhan JR, Torner JC, Lynch CF, Rubenstein LM, Lemke JH, Cohen MB, et al. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States) Cancer Causes Control. 1997;8:229–38. doi: 10.1023/a:1018428531619. [DOI] [PubMed] [Google Scholar]
- 8.Alyson LJ, White E, Kristal AR. Anthropometrics and prostate cancer risk. Am J Epidemiol. 2007;165:1271–9. doi: 10.1093/aje/kwm013. [DOI] [PubMed] [Google Scholar]
- 9.Platz EA, Yeole BB, Cho E, Jussawalla DJ, Giovannucci E, Ascherio A. Vasectomy and prostate cancer: A case-control study in India. Int J Epidemiol. 1997;26:933–8. doi: 10.1093/ije/26.5.933. [DOI] [PubMed] [Google Scholar]
- 10.Pourmand G, Salem S, Mehrsai A, Lotfi M, Amirzargar MA, Mazdak H, et al. The risk factors of prostate cancer: A multicentric case-control study in Iran. Asian Pacific J Cancer Prev. 2007;8:422–8. [PubMed] [Google Scholar]
- 11.Slattery ML, West DW. Smoking, alcohol, coffee, tea, caffeine and theobromine: Risk of prostate cancer in Utah (United States) Cancer Causes Control. 1993;4:559–63. doi: 10.1007/BF00052432. [DOI] [PubMed] [Google Scholar]
- 12.Huncharek M, Haddock S, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: A meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100:693–701. doi: 10.2105/AJPH.2008.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bingham SA. High-meat diets and cancer risk. Proc Nutr Soc. 1999;58:243–8. doi: 10.1017/s0029665199000336. [DOI] [PubMed] [Google Scholar]
- 14.Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357:1764–6. doi: 10.1016/S0140-6736(00)04889-3. [DOI] [PubMed] [Google Scholar]
- 15.Martin RM, Vatten L, Gunnell D, Romundstad P. Blood pressure and risk of prostate cancer: Cohort Norway (CONOR) Cancer Causes Control. 2010;21:463–72. doi: 10.1007/s10552-009-9477-x. [DOI] [PubMed] [Google Scholar]
- 16.Friedman GD. Blood pressure and heart rate: No evidence for a positive association with prostate cancer. Ann Epidemiol. 1997;7:486–9. doi: 10.1016/s1047-2797(97)00083-5. [DOI] [PubMed] [Google Scholar]
- 17.Laukkanen JA, Laaksonen DE, Niskanen L, Pukkala E, Hakkarainen A, Salonen JT. Metabolic syndrome and the risk of prostate cancer in Finnish men: A population-based study. Cancer Epidemiol Biomarkers Prev. 2004;13:1646–50. [PubMed] [Google Scholar]
- 18.AUA updates on prostate cancer - epidemiology, incidence, AUA guidelines for screening and PSA update - 2009. American Urological Association Education and Research, Inc.®, 2009


