Abstract
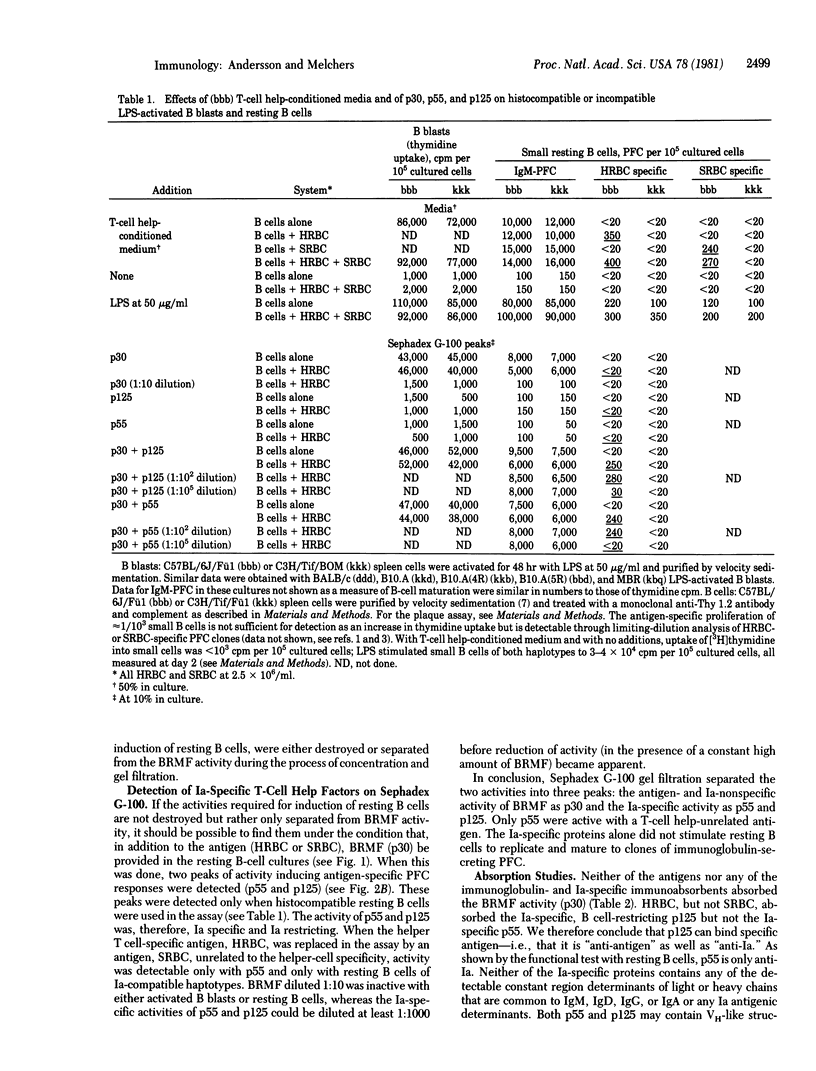
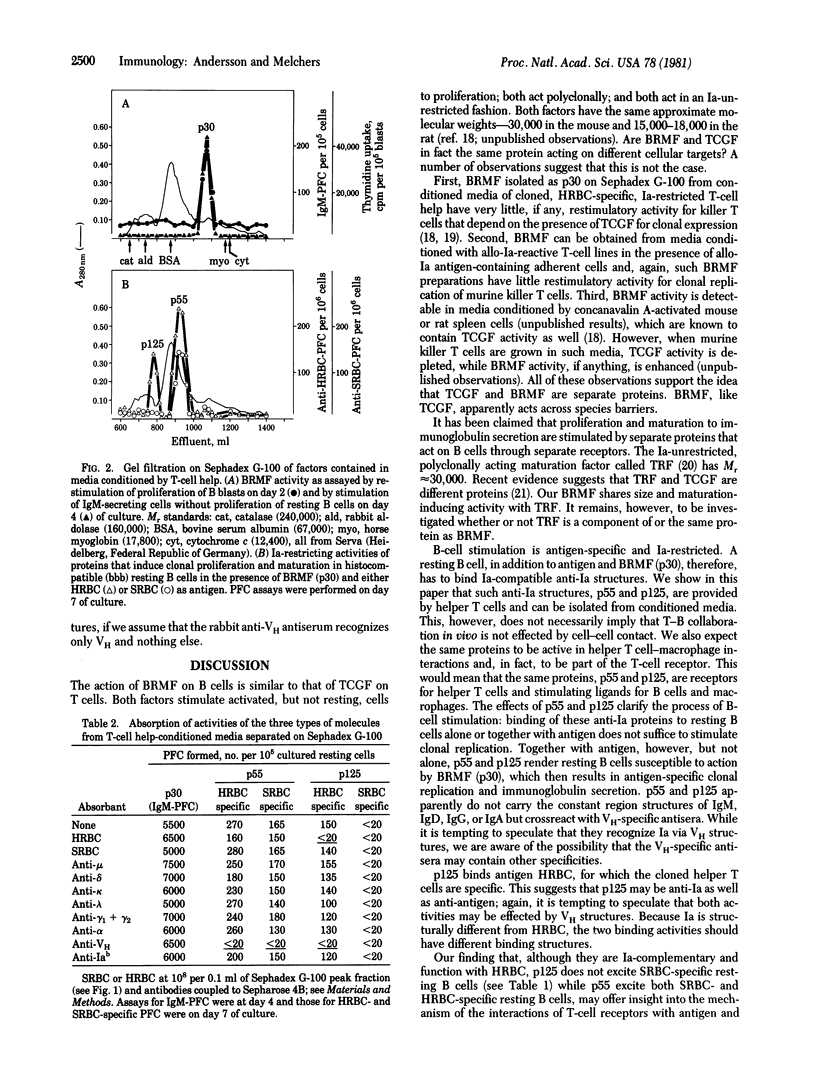
Cloned murine helper T cells, restricted to the Iab antigens of the major histocompatibility locus and specific for horse erythrocytes as a foreign antigen, produce, in cooperation with antigen and histocompatible adherent cells, soluble factors that replace the helper T cells in their action on B cells. Three types of factors can be distinguished on the basis of molecular weight: proteins having apparent Mr 30,000 (p30) that act antigen- and Ia-nonspecifically as replication- and maturation-inducing factors and proteins having apparent Mr 55,000 (p55) and 125,000 (p125) that act on resting B cells in an Ia-specific, restricted fashion. Neither horse erythrocytes (a T-cell specific antigen) nor p55 and p125, alone or together, stimulate resting B cells to proliferation and maturation. Double occupancy by antigen and p55 or p125, however, renders Ia-compatible, but not Ia-incompatible, resting B cells susceptible to stimulation. The subsequent addition of p30 to these "excited" B cells then results in the proliferation and maturation of clones of horse erythrocyte-specific resting B cells, which then replicate under the stimulatory action of p30. p30 do not bind antigen, nor do they bind anti-Ia or anti-immunoglobulin antibodies. p55 are bound by anti-heavy chain variable region antibodies. p55 are bound by anti-heavy chain variable region antibodies, but not by anti-heavy or anti-light chain constant region antibodies or anti-Ia antibodies. p125 molecules bind horse but not sheep erythrocytes and are bound by anti-heavy chain variable region, but not by anti-heavy or light chain constant region or anti-Ia antibodies. p55 and p125 are likely to be soluble analogues of the antigen-specific, Ia-restricted T-cell receptors of the cloned helper T cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Lernhardt W., Melchers F. Clonal growth and maturation to immunoglobulin secretion in vitro of every growth-inducible B lymphocyte. Cell. 1977 Jan;10(1):27–34. doi: 10.1016/0092-8674(77)90136-2. [DOI] [PubMed] [Google Scholar]
- Andersson J., Coutinho A., Melchers F. Frequencies of mitogen-reactive B cells in the mouse. I. Distribution in different lymphoid organs from different inbred strains of mice at different ages. J Exp Med. 1977 Jun 1;145(6):1511–1519. doi: 10.1084/jem.145.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Coutinho A., Melchers F. Mitogen-activated B-cell blasts reactive to more than one mitogen. J Exp Med. 1979 Mar 1;149(3):553–564. doi: 10.1084/jem.149.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Lernhardt W., Melchers F. The purified protein derivative of turberculin, a B-cell mitogen that distinguishes in its action resting, small B cells from activated B-cell blasts. J Exp Med. 1979 Dec 1;150(6):1339–1350. doi: 10.1084/jem.150.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Schreier M. H., Melchers F. T-cell-dependent B-cell stimulation is H-2 restricted and antigen dependent only at the resting B-cell level. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1612–1616. doi: 10.1073/pnas.77.3.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armerding D., Eshhar Z., Katz D. H. Activation of T and B lymphocytes in vitro. VI. Biochemical and physicochemical characterization of the allogeneic effect factor. J Immunol. 1977 Oct;119(4):1468–1474. [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. II. Biological and biochemical properties of an allogeneic effect factor (AEF) active in triggering specific B lymphocytes. J Exp Med. 1974 Jul 1;140(1):19–37. doi: 10.1084/jem.140.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y., Wuilmart C., Lonai P., Givol D. Preparation and characterization of anti-framework antibodies to the heavy chain variable region (VH) of mouse immunoglobulins. Eur J Immunol. 1978 Nov;8(11):797–801. doi: 10.1002/eji.1830081109. [DOI] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Delovitch T. L., Watson J., Battistella R., Harris J. F., Shaw J., Paetkau V. In vitro analysis of allogeneic lymphocyte interaction. V. Identification and characterization of two components of allogeneic effect factor, one of which displays H-2-restricted helper activity and the other, T cell-growth factor activity. J Exp Med. 1981 Jan 1;153(1):107–128. doi: 10.1084/jem.153.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hübner L., Müller G., Schimpl A., Wecker E. Partial characterization and purification of murine T cell-replacing factor, TRF. II. Biochemical characteristics. Immunochemistry. 1978 Jan;15(1):33–39. doi: 10.1016/0161-5890(78)90023-8. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J Exp Med. 1978 Mar 1;147(3):923–933. doi: 10.1084/jem.147.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Andersson J., Lernhardt W., Schreier M. H. H-2-unrestricted polyclonal maturation without replication of small B cells induced by antigen-activated T cell help factors. Eur J Immunol. 1980 Sep;10(9):679–685. doi: 10.1002/eji.1830100905. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Replacement of T-cell function by a T-cell product. Nat New Biol. 1972 May 3;237(70):15–17. doi: 10.1038/newbio237015a0. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Andersson J., Lernhardt W., Melchers F. Antigen-specific T-helper cells stimulate H-2-compatible and H-2-incompatible B-cell blasts polyclonally. J Exp Med. 1980 Jan 1;151(1):194–203. doi: 10.1084/jem.151.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J., Monticone V., Paetkau V. Partial purification and molecular characterization of a lymphokine (costimulator) required for the mitogenic response of mouse thymocytes in vitro. J Immunol. 1978 Jun;120(6):1967–1973. [PubMed] [Google Scholar]
- Watson J., Aarden L. A., Shaw J., Paetkau V. Molecular and quantitative analysis of helper T cell-replacing factors on the induction of antigen-sensitive B and T lymphocytes. J Immunol. 1979 May;122(5):1633–1638. [PubMed] [Google Scholar]



