Abstract
Objectives:
Pelvic fracture urethral distraction defect (PFUDD) may be associated with disabling complications, such as recurrent stricture, urinary incontinence, and erectile dysfunction. In this article we review the current concepts in the evaluation and surgical management of PFUDD, including redo urethroplasty.
Materials and Methods:
A PubMed™ search was performed using the keywords “pelvic fracture urethral distraction defect, anastomotic urethroplasty, pelvic fracture urethral stricture, pelvic fracture urethral injuries, and redo-urethroplasty.” The search was limited to papers published from 1980 to March 2010 with special focus on those published in the last 15 years. The relevant articles were reviewed with regard to etiology, role of imaging, and the techniques of urethroplasty.
Results:
Pelvic fracture due to accidents was the most common etiology of PFUDD that usually involved the membranous urethra. Modern cross-sectional imaging, such as sonourethrography and magnetic resonance imaging help assess stricture pathology better, but their precise role in PFUDD management remains undefined. Surgical treatment with perineal anastomotic urethroplasty yields a success rate of more than 90% in most studies. The most important complication of surgical reconstruction is restenosis, occurring in less than 10% cases, most of which can be corrected by a redo anastomotic urethroplasty. The most common complication associated with this condition is erectile dysfunction. Urinary incontinence is a much rarer complication of this surgery in the present day.
Conclusions:
Anastomotic urethroplasty remains the cornerstone in the management of PFUDD, even in previously failed repairs. Newer innovations are needed to address the problem of erectile dysfunction associated with this condition.
Keywords: Anastomotic urethroplasty, posterior urethral stricture, redo-urethroplasty, urethral injury
INTRODUCTION
Posterior pelvic fracture urethral distraction defect (PFUDD) is a challenging urologic problem that may result in complications, such as urinary incontinence and inability to void due to recurrent stricture leading to a lifelong disabling condition. As the understanding of the disease process has improved with evolution of better imaging in the form of magnetic resonance imaging (MRI) and Doppler ultrasound and with better surgical techniques, the success rates of posterior anastomotic urethroplasty have improved worldwide. In this article, we review the current concepts in the evaluation and surgical management of PFUDD, including redo-urethroplasty based on the comprehensive review of published literature. This review is confined to the management of male urethral injury.
MATERIALS AND METHODS
A PubMed™ search was performed using the keywords “pelvic fracture urethral distraction defect, anastomotic urethroplasty, pelvic fracture urethral stricture, pelvic fracture urethral injuries, and redo-urethroplasty.” The search was limited to papers published from 1980 to March 2010. The search was further focused, especially on the articles published in the last 15 years. The relevant articles were reviewed with regard to etiology, preoperative evaluation with emphasis on the role of imaging studies, surgical techniques in primary cases, and in redo anastomotic urethroplasty.
INCIDENCE
The incidence of urethral injury in men with pelvic fracture varies widely. The results of 2 extensive reviews have shown that the incidence of posterior urethral injury varied from 1.6% to 25% (mean 9.9%).[1,2] The incidence of posterior urethral injuries in pelvic fractures in another series had been estimated to be 5–10%.[3] This variation is due to the differences in age group and the type of pelvic fracture in different series and due to the prospective and retrospective nature of different series.
ETIOLOGY
The most common etiology of posterior urethral injury is motor vehicle accidents.[4] In a study by Morey et al. on 82 patients undergoing urethroplasty for posterior urethral injury, the main causes were car to pedestrian in 40%, car to motorcycle in 26%, and falling down and crash injuries in 26% of the cases. In vehicular accidents, the pedestrians were more involved rather than the occupants of the vehicle.[5] Other uncommon causes include gunshot injuries without pelvic fracture and explosive blasts.[4,6]
The risk factors for urethral injury along with concomitant pelvic fracture are influenced by the sex, age, and the type of pelvic fracture. In a series of 234 patients with pelvic fracture, 12 of the 109 men sustained urethral injury, whereas none of the 125 women had urethral injury.[7] This is due to the longer length, fixed urethra, and the rigid attachments to the pubic bone in males. There is a high risk of urethral injury with straddle fracture with diastasis of the sacroiliac joint and there is a low risk with single ramus and ipsilateral rami fractures.
MECHANISM OF URETHRAL INJURY
Traditionally, it has been accepted that urethral rupture in men occurs at the prostatomembranous junction by the shearing forces that avulse the prostatic apex from the urogenital diaphragm.[8] But recent evidence from various cadaveric studies has shown that there is no distinct superior membrane of the urogenital diaphragm separating the sphincter muscle from the prostate. The urethral sphincter extends from the bladder base to the perineal membrane and is associated throughout the prostate although the bulk of the sphincter is displaced distally as the prostate grows, especially during puberty.[9] The muscles lining and surrounding the membranous urethra are directly continuous with similar muscles of the prostatic urethra, which end at the perineal membrane and are not in the bulbar urethra. Hence, it is the bulbomembranous junction, which is the weak spot at which the posterior urethra is prone to injury.[10] This is observed intraoperatively as the fibrous process of posterior urethral disruption involves the proximal bulbar and membranous region. Uncommonly, the prostatic urethra and the bladder neck are directly lacerated by the sharp edges of bone fragments, which are seen in young boys due to the insufficient protection offered by the small prostate.[11] In 1977, Colapinto and McCallum proposed a classification for posterior urethral injuries comprising 3 types.[12] Recently, Goldman et al. have proposed a new classification system, which allows us to compare different therapeutic strategies and their outcomes.[13] But these classifications do not have a role in determining the management strategies of these injuries at present.
Posterior urethral injuries often take a low priority in the management of patients with pelvic fracture injuries as these individuals almost always have multiple injuries of more serious consequence. Most patients are best treated by a suprapubic catheter initially followed 3 months later by an end-to-end anastomotic urethroplasty in those who have developed urethral occlusions. Although there are roles for delayed primary repair and for endourologic management in selected patients, these procedures require considerable technical expertise that may not be available in all centers. Their exact roles have yet to be defined.
PREOPERATIVE EVALUATION
The successful results of urethroplasty will depend on the accurate estimation of strictured length. A combination of voiding cystourethrography (VCUG) and retrograde urethrography (RGU) helps in the assessment of the strictured segment. This combination also helps in the assessment of the coronal displacement of the prostatic urethra. Some authors have advocated the use of perineal ultrasonography for stricture assessment.[14] Recently, MRI has been employed in the evaluation of this disease. MRI can provide additional information on the lateral displacement of the prostate and the severity of the posterior urethral defect. In addition, this technique can detect bone fragments between the 2 ends of the urethra after pelvic fracture.[15] MRI should be used in conjunction with RGU and VCUG and not as a sole method of evaluation. Some authors believe that the information provided by MRI is not of much utility.[16]
Flexible cystoscopy is an important adjunct in the evaluation of strictured segment. Hosseini et al. in a series of 11 patients have described the role of flexible cystoscopy in the assessment of bladder, bladder neck, and posterior urethra. This can reveal the anatomical configuration with regard to the urethral ends and the complications related to previous management.[17]
SURGICAL CONSIDERATIONS
The principles in the surgical management of posterior urethral distraction defect include complete excision of scar tissue involving the membranoprostatic region, lateral fixation of pliable prostatic mucosa, and creation of a tension-free mucosa to mucosa anastomosis.[18] This can be accomplished by perineal approach in most cases, with abdominoperineal approach being required only in a few select patients. The most important maneuver to achieve tension-free anastomosis is the mobilization of bulbar urethra, which is sufficient in many cases to bridge a gap of 2–3 cm. An elaborated perineal approach including separation of corporal bodies, inferior pubectomy, and supracrural rerouting of the urethra or a perineoabdominal approach with superior or total pubectomy is required in longer defects or complex cases [Figure 1]. The purpose of these techniques is to straighten the normally curved course of the urethra and to achieve a shorter distance to the prostatic urethra. These techniques are described in standard operative urology textbooks.
Figure 1.
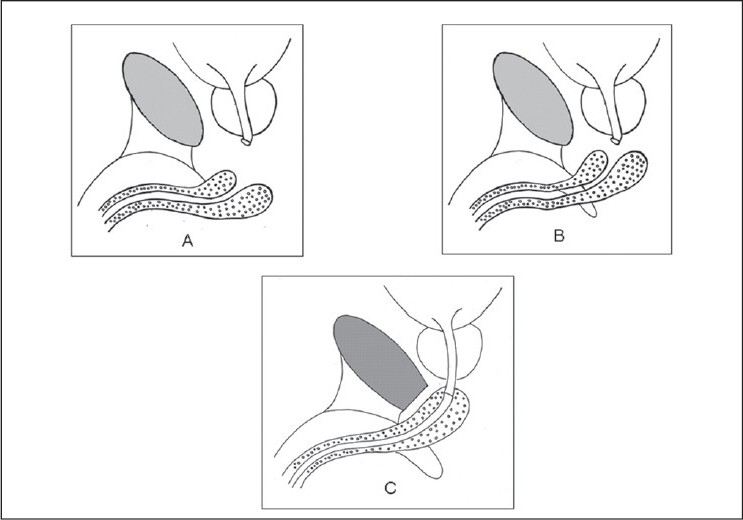
A schematic representation of the key steps of bulbar urethral mobilization; (A) mobilization of the bulbar urethra; (B) crural separation bridges the additional gap; (C) inferior pubectomy straightens the course of the bulbar urethra to the prostatic apex.
TYPES OF PUBECTOMY AND ITS ROLE IN URETHROPLASTY
In complex posterior urethral distraction defects, that include defects exceeding 3 cm, strictures associated with perineal fistulas, rectourethral fistulas, periurethral cavities, false passages, associated anterior urethral stricture or an open bladder neck, and strictures with previously failed repair,[19] some form of pubectomy is needed to achieve adequate exposure and a tension-free anastomosis. Pierce was credited with the earliest description of transpubic urethroplasty in 1962.[20] Waterhouse et al. popularized this technique after his successful results in 1973.[21] In cases where a formal transpubic repair is contemplated the authors’ preference is the abdominal transpubic perineal urethroplasty.[22] Through a midline perineal incision, the anterior urethra is dissected and the fibrotic tissue of the stricture is completely excised. A midline subumbilical incision is made that extends over the symphysis. The attachments of the rectus abdominis muscles are cleared off the outer surface of the pubis about 2 cm from each side of the symphysis pubis. A wedge of bone is removed from the pubis. The prostate is freed from the retropubic callus. The prostatic apex and distal urethra are anastomosed. Such procedures are needed in less than 5% of cases.[23]
However, in current day practice the extent of pubectomy has changed from total to partial pubectomy in most cases due to complications, such as profuse bleeding and problems arising out of the large dead space,[24] including the cosmetic deformity of an externally visible depression in the prepubic region. Partial pubectomy may be in the form of a superior or inferior pubectomy. The technique of perineoabdominal partial superior pubectomy is advocated by Koraitim et al. in the management of complex posterior urethral distraction defects.[25] In superior pubectomy, about 1.5 × 0.5 inch of bone is resected along with the arcuate ligament. This provides an excellent exposure for a tension-free bulboprostatic anastomosis. The preference for the superior pubectomy is that it greatly facilitates exposure of the normal urethra proximal to the injury site and thereby downward mobilization of the superiorly displaced prostate. This approach helps in managing the associated adverse events, such as the fistulous communication to the surrounding organs and bladder neck incompetence at the same time.[14] Moreover, a pedicled omental graft can be brought down to obliterate the perianastomotic dead space, reducing the inflammatory response and reducing the fibrotic response.[26] This is also helpful in managing the defects in prepubescent boys who may have a narrow body habitus and in whom the distal urethral mobilization may be limited by the insufficient blood supply to the glans.[22]
Many authors have advocated the technique of perineal approach with inferior pubectomy. Webster et al. have successfully treated about 120 patients of distraction defect through progressive perineal approach comprising inferior pubectomy, with a success rate close to 97% even with bridging urethral defects as long as 10 cm. But a combined abdominoperineal approach was carried out only in patients with urethral fistulae.[22] Many authors agree that the classical abdominoperineal transpubic approach is best reserved for the more complex posterior urethral distraction defects and probably in children. Basiri et al. have described anastomotic urethroplasty after symphysiotomy rather than using a transpubic approach in children.[27]
OTHER TECHNICAL NUANCES IN URETHROPLASTY
Several nuances and innovations have been described by various authors to improve the results of anastomotic urethroplasty. A few of these are discussed further here. Hossieni et al. in their experience have described the role of flexible cystoscopy in the intraoperative localization of the proximal healthy urethra.[17] In this technique, cystoscope is passed through the bladder neck from the prostatic urethra and the tip of the cystoscope is placed on the end of the stricture. The scar tissue is resected under the guidance of cystoscopic light. Then a needle is passed through the perineum into the proximal urethral end under the guidance of the flexible cystoscope light. The scar tissue is removed till the tip of the needle. According to the authors, this technique has 2 distinct advantages in comparison to using a 20-F van Buren sound for the determination of the true end of the urethra. First, it does not allow creation of a false passage and second, the true end point of the urethra is opened, and opening of the urethra proximal to the point of obstruction is avoided. Moreover, flexible cystoscope helps in determining the abnormal and nonanatomical placement of the proximal end of the urethra and its deviation to the rectum, lateral and behind the pubis.
Al-Rifaei et al. have suggested a modified surgical approach in that during the repair of the distal end of the urethra, dissection should be performed only inside the bulb (cutting within the bulb) without disturbing the region outside the bulb. This is suggested in order to preserve the bulbar arteries and to preserve erection. By this so called “midline approach,” the authors have achieved a 4.5% erectile dysfunction rates in patients who were sexually active before urethroplasty. Also, during the exposure of the prostatic urethra, dissection in the lateral surface of the prostate is not recommended and it is preferred only in the anterior surface of the prostate.[28] Similarly, Jordan et al. have described a technique, proximal bulbous urethral reconstruction, preserving the proximal blood supply to the bulbar urethra.[29] Their series did not include reconstruction of PFUDD, but the technique can be potentially used in at least some cases of reconstruction of PFUDD. However, technically demanding, the preservation of proximal blood supply has the theoretic advantage of being important should an artificial sphincter placement become necessary in future in the same patient.
Wang et al. described a modified technique of urethral pull-through operation for posttraumatic posterior urethral stricture.[30] In a review of their experience of 113 patients with PFUDD who underwent the modified urethral pull-through operation, including 29 cases of previously failed urethroplasty, they reported a recurrence of stricture only in 4 patients yielding a primary success rate of 96.5%. All treatment failures occurred within the first 8 months postoperatively and failed repairs were successfully managed endoscopically or by urethral dilation in 2 and by repeating the pull-through operation in the remaining 2 resulting in a final success rate of 100%. All patients were continent. Erectile dysfunction was noted postoperatively only in 5 patients (3.7%).
Dalpiaz et al. have investigated the anatomy of the male rhabdosphincter and the relationship between the membranous urethra, the rhabdosphincter, and the neurovascular bundles to provide the anatomical basis for surgical approach of the posterior urethra for successful outcomes in urethral reconstructive surgery using cadaveric models. They found that there exists a thin connective layer between the membranous urethral wall and the rhabdosphincter. The meticulous dissection of this connective tissue sheath between the membranous urethra and the rhabdosphincter leads to separation of both structures, thus providing the basis for a safe surgical approach to the anterior wall of the posterior urethra. This anatomical approach helps preserve the muscular structures involved in the continence mechanism.[31]
For the treatment of long defects or complicated urethral distraction defect after pelvic trauma, an alternative posterior sagittal pararectal approach has been described as an alternative to the transpubic approach. Abdalla reported a successful result in 6 of the 7 cases in their series treated by this technique.[32] They reported a better visualization of the apex of the prostate and surgical field by this approach.
Mathur et al. have described a novel technique of “U” shaped anastomosis between the bulbar urethra and the prostatic apex. After the strictured segment is excised, the sutures are taken between both the urethral ends sparing the region extending between 10 o’clock to 2 o’clock positions. The authors propose that this technique has lesser restenosis rates as the urethral blood supply is not hampered. Near the apex of the prostate, the neurovascular bundle divides into 2 parts: a larger anterior part and a smaller posterior part. The anterior part crosses the membranous urethra, then the bulb of the penis at the 1 o’clock and 11 o’clock positions and finally enters the corpus cavernosum. The posterior part crosses the membranous urethra more posterior to enter the bulb of the penis at the 2 o’clock position, thus avoiding stitches at anterior aspect of anastomosis reducing the chances of compromising blood supply to urethra and less chances of ischemia, fibrosis, and restricture as well as impotence. They also propose that since their anastomosis is not in the form of a ring, the chances of postanastomotic stricture is less. They suggest that by their technique, the floor is formed by the anastomosed bulbar urethra and the prostatic apex and the roof is formed by the urogenital membrane and tunica of the corpora cavernosa. The overall success rate was 97.05% in their series.[33]
SURGICAL OUTCOMES OF PRIMARY END-TO-END ANASTOMOTIC URETHROPLASTY
The success rates of urethroplasty varied widely from 77% to 95% in various series,[34,35] which was due to various definitions of surgical success. Restricture after anastomotic urethroplasty occurs in about 15% of cases.[36] But most of these can be successfully corrected by 1 or 2 sessions of endoscopic internal urethrotomy. The results of these endoscopic urethrotomy are durable in most cases treated, and most authors accept these cases as successful urethroplasty.
INCONTINENCE AND SEXUAL DYSFUNCTION AFTER URETHROPLASTY
In a review of 60 cases of anastomotic urethroplasty for PFUDD, Corriere describes both early and late complications. Surgical complications included rectal injuries (3%), repeat strictures that required dilation or visual internal urethrotomy (32%), and repeat strictures that required reoperation (5%). By 1 year after surgery, all patients had a patent urethra (100%). At 1 year, 43 (72%) patients voided normally, 5 (8.3%) were areflexic and performed self-catheterization, 5 (8.3%) had urge incontinence, and 5 (8.3%) had mild stress incontinence requiring no treatment. Moderate stress incontinence responded to imipramine in one case and collagen injection in one. The risk of incontinence due to sphincter weakness following anastomotic urethroplasty is very low as continence depends on the intact bladder neck. Open bladder neck seen cystoscopy and/or cystography before urethroplasty may herald postoperative incontinence. At present, the preferred option is to manage the PFUDD and bladder neck problem sequentially.[37] Bladder neck reconstruction provides good postoperative continence rates, although some patients may require a sling procedure or implantation of an artificial urinary sphincter.
Of the patients who were potent preoperatively only 52% remained potent postoperatively. Of the 29 (48%) patients who were impotent preoperatively and immediately postoperatively 9 regained potency at 1 year. However, at 1 year, the quality of erections of the 40 potent men was normal in only 22 (37%) and fair to poor in 18 (30%).[38] Anger et al. reported 54% of patients with PFUDD had erectile dysfunction of some degree, including severe dysfunction in 31%.[39] They found that the risk of erectile dysfunction was much higher in patients with PFUDD as compared with patients with pelvic fracture alone. It appears that while most men with erectile dysfunction have it consequent to the pelvic fracture itself, a number of men suffer erectile dysfunction consequent to urethroplasty, although the incidence varies from 2% to as high as 52%. It should also be noted that though a few patients reported improvement of sexual function following urethroplasty and a substantial number developed erectile dysfunction progressively several months after the urethral reconstruction. Thus men with PFUDD injuries represent a target population for early penile rehabilitation programs.
URETHROPLASTY FOR PFUDD IN CHILDREN
Reconstruction of pediatric PFUDD represents a significant surgical challenge because of the smaller pelvic confines, smaller caliber of the urethra, the less developed and therefore less elastic nature of the preadolescent corpus spongiosum, and the increased tissue fragility. Resection with end-to-end anastomosis is the usual procedure in the face of a short segment stricture. Most posttraumatic posterior urethral strictures in children can be managed through the perineal route.[40,41] Inferior pubectomy or transpubic urethroplasty is uncommonly required (about 15%) as seen in contemporary series.[42] However, some series in the past have reported a larger percentage of use of the transpubic technique ranging from 35% to 75%.[19,43,44] Transpubic approach may be needed in children with defects more than 3 cm long.[43]
The success rate of anastomotic urethroplasty in children is in the range of 89–98%.[40–43] In about 10–11% of children, anastomotic urethroplasty fails. In these cases redo-urethroplasty still has a very high success rate of more than 95%.[40,43] Previous urethral manipulations did not seem to affect the intermediate-term results of anastomotic urethroplasty.[40] Incontinence has been reported to occur in 6–13% of cases.[41,43] Most cases of incontinence were attributed to bladder neck injury sustained at the time of original trauma.[43] No penile curvature, shortening, or urethral diverticulae were noted during follow-up.[45]
It has been reported that in some children the urethral disruption occurred within the prostate itself and not at the prostatomembranous junction. In such cases the proximal sphincteric mechanism may be at risk and immediate repair of the injury is advisable.[44] However, this is an uncommon occurrence. Most strictures are inferior to the verumontanum.[19,44]
MANAGEMENT OF FAILED URETHROPLASTY
Gupta et al. have reported a large retrospective series comparing outcomes in fresh cases and in redo cases. The success rate in both the groups were similar (excellent or acceptable result in 95%), but the redo cases required a longer operative time due to the more frequent need for the transpubic approach and the need for meticulous dissection and additional maneuvers to achieve successful urethroplasty.[46] Similar conclusions were drawn by Singla et al. in their series of pediatric anastomotic urethroplasties.[40] Culty and Boccon-Gibod in a series of 51 patients, reported a satisfactory outcome of 95% in primary cases compared with 60% in patients with previous failed urethroplasty.[15] Singh et al. concluded that previous intervention in the form of railroading and urethroplasty affected the outcome of redo urethroplasty but previous core through internal urethrotomy did not affect the outcome significantly.[47] Lumen et al. also concluded that the failures and complications were higher after reconstruction following failed urethroplasty.[48] These studies indicate that in cases of failed anastomotic urethroplasty, redo anastomotic urethroplasty is the treatment of choice giving the best and most durable results in terms of urethral patency. However, these redo cases require greater expertise and often need an elaborated perineal approach with a greater need for pubectomy than primary anastomotic urethroplasty. The patency results in redo cases, although very good, are marginally inferior to primary anastomotic urethroplasty in most series thereby emphasizing the need to do as good a urethroplasty as possible in the first attempt itself.
On occasion a patient with a failed urethroplasty or rarely even in a primary PFUDD the gap between the bulbar urethra and the prostatic apex may be so long that an anastomotic urethroplasty may not be feasible. In these cases there is no option but to perform a substitution urethroplasty using a perineoscrotal fasciocutaneous flap.[49] The authors prefer to do this in a staged manner creating a perineal urethrostomy first and then 6 months later, if the urethroplasty remains stable, performing the second stage to complete the reconstruction of the urethra. These procedures are associated with a high complication rate, which includes recurrent stricture, diverticulum formation, and formation of calculi.[49] There are anecdotal reports of the successful use of innovative techniques for reconstruction of the posterior urethra, such as using a pedicled appendix graft[50] or a microvascular free flap, such as the radial forearm free flap.[51]
MANAGEMENT OF PFUDD ASSOCIATED WITH RECTAL FISTULA
Urethrorectal fistulas in the setting of urethral distraction defect is uncommon and a difficult problem to treat. The key points in the surgical technique include excellent exposure of the diseased segment, separation of the urethra and rectum, closure of the defect, and interposition of a well vascularized tissue. The surgical approach may depend on the site of the fistula, the length of the stricture, and the number of previous unsuccessful operative attempts. Two surgical approaches are described in the literature.[6,23,52,53] The first is the perineal approach that is suitable for fistula near the anus. In this approach inferior pubectomy may be required in long segment strictures of more than 2.5 cm length. The second is the combined transpubic and perineal approach, which is useful in long segment defects and when the fistula is located far from the anus. In both approaches, once adequate exposure of the stricture and fistula sites are obtained, the prostate and proximal urethra is separated from the rectum and the rectum is repaired first. Some vascularized tissues, such as the gracilis, rectus abdominis, bulbocavernous muscle flaps, or a dartos flap is tacked over the rectal repair to separate the rectal sutures from the urethral anastomotic site and then the urethral anastomosis is completed. In one of the largest series on PFUDD with rectal fistula published so far, Xu et al. reported their experience in treating 31 patients.[54] They found it useful to interpose a rectus abdominis flap in the transpubic-perineal approach, while they preferred using the gracilis, bulbocavernous muscle flaps, or subcutaneous dartos in the perineal approach. They had an overall success rate of 87%. Their restructure rate and recurrence of fistula were 6.5% each. The authors concluded that the transperineal–inferior pubectomy approach may be the most appropriate as a first-line procedure.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Levine JI, Crampton RS. Major abdominal injuries associated with pelvic fractures. Surg Gynecol Obstet. 1963;116:223–6. [PubMed] [Google Scholar]
- 2.Wilkinson FO. Rupture of the posterior urethra with a review of twelve cases. Lancet. 1961;1:1125–9. doi: 10.1016/s0140-6736(61)92062-1. [DOI] [PubMed] [Google Scholar]
- 3.Cass AS, Godec CJ. Urethral injury due to external trauma. Urology. 1978;11:607–11. doi: 10.1016/0090-4295(78)90013-4. [DOI] [PubMed] [Google Scholar]
- 4.Pratap A, Agrawal CS, Tiwari A, Bhattarai BK, Pandit RK, Anchal N. Complex posterior urethral disruptions: management by combined abdominal transpubic perineal urethroplasty. J Urol. 2006;175:1751–4. doi: 10.1016/S0022-5347(05)00974-2. [DOI] [PubMed] [Google Scholar]
- 5.Morey AF, McAninch JW. Reconstruction of posterior urethral disruption injuries: outcome analysis in 82 patients. J Urol. 1997;157:506–10. [PubMed] [Google Scholar]
- 6.Das K, Charles AR, Alladi A, Rao S, D’Cruz AJ. Traumatic posterior urethral disruptions in boys: experience with the perineal/perineal-transpubic approach in ten cases. Pediatr Surg Int. 2004;20:449–54. doi: 10.1007/s00383-004-1174-y. [DOI] [PubMed] [Google Scholar]
- 7.Antoci JP, Schiff M., Jr Bladder and urethral injuries in patients with pelvic fractures. J Urol. 1982;128:25–9. doi: 10.1016/s0022-5347(17)52734-2. [DOI] [PubMed] [Google Scholar]
- 8.Devine PC, Devine CJ., Jr Posterior urethral injuries associated with pelvic fractures. Urology. 1982;20:467–70. doi: 10.1016/0090-4295(82)90114-5. [DOI] [PubMed] [Google Scholar]
- 9.Oelrich TM. The urethral sphincter muscle in the male. Am J Anat. 1980;158:229–46. doi: 10.1002/aja.1001580211. [DOI] [PubMed] [Google Scholar]
- 10.Colapinto V. Trauma to the pelvis: urethral injury. Clin Orthop Relat Res. 1980;151:46–55. [PubMed] [Google Scholar]
- 11.Devine CJ, Jr, Jordan GH, Devine PC. Primary realignment of the disrupted prostatomembranous urethra. Urol Clin North Am. 1989;16:291–5. [PubMed] [Google Scholar]
- 12.Colapinto V, McCallum RW. Injury to the male posterior urethra in fractured pelvis: a new classification. J Urol. 1977;118:575–83. doi: 10.1016/s0022-5347(17)58110-0. [DOI] [PubMed] [Google Scholar]
- 13.Goldman SM, Sandler CM, Corriere JN, Jr, McGuire EJ. Blunt urethral trauma: a unified, anatomical mechanical classification. J Urol. 1997;157:85–9. doi: 10.1016/s0022-5347(01)65291-1. [DOI] [PubMed] [Google Scholar]
- 14.Koraitim MM. On the art of anastomotic posterior urethroplasty: a 27-year experience. J Urol. 2005;173:135–9. doi: 10.1097/01.ju.0000146683.31101.ff. [DOI] [PubMed] [Google Scholar]
- 15.Culty T, Boccon-Gibod L. Anastomotic urethroplasty for posttraumatic urethral stricture: previous urethral manipulation has a negative impact on the final outcome. J Urol. 2007;177:1374–7. doi: 10.1016/j.juro.2006.11.092. [DOI] [PubMed] [Google Scholar]
- 16.Jordan GH, Schlossberg SM. Surgery of the penis and urethra. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 9th ed. Philadelphia: Saunders; 2007. pp. 1054–84. [Google Scholar]
- 17.Hosseini SJ, Kaviani A, Jabbari M, Hosseini MM, Haji-Mohammadmehdi-Arbab A, Simaei NR. Diagnostic application of flexible cystoscope in pelvic fracture urethral distraction defects. Urol J. 2006;3:204–7. [PubMed] [Google Scholar]
- 18.Santucci RA, McAninch JW, Mario LA, Rajpurkar A, Chopra AK, Miller KS, et al. Urethroplasty in patients older than 65 years: indications, results, outcomes and suggested treatment modifications. J Urol. 2004;172:201–3. doi: 10.1097/01.ju.0000128810.86535.be. [DOI] [PubMed] [Google Scholar]
- 19.Koraitim MM. Posttraumatic posterior urethral strictures in children: a 20-year experience. J Urol. 1997;157:641–5. [PubMed] [Google Scholar]
- 20.Pierce JM., Jr Exposure of the membranous and posterior urethra by total pubectomy. J Urol. 1962;88:256–8. doi: 10.1016/S0022-5347(17)64779-7. [DOI] [PubMed] [Google Scholar]
- 21.Waterhouse K, Abrahams JI, Gruber H, Hackett RE, Patil UB, Peng BK. The transpubic approach to the lower urinary tract. J Urol. 1973;109:486–90. doi: 10.1016/s0022-5347(17)60459-2. [DOI] [PubMed] [Google Scholar]
- 22.Flynn BJ, Delvecchio FC, Webster GD. Perineal repair of pelvic fracture urethral distraction defects: experience in 120 patients during the last 10 yrs. J Urol. 2003;170:1877–80. doi: 10.1097/01.ju.0000091642.41368.f5. [DOI] [PubMed] [Google Scholar]
- 23.Webster GD, Ramon J. Repair of pelvic fracture posterior urethral defects using an elaborated perineal approach: experience with 74 cases. J Urol. 1991;145:744–8. doi: 10.1016/s0022-5347(17)38442-2. [DOI] [PubMed] [Google Scholar]
- 24.Waterhouse K, Laungani G, Patil U. The surgical repair of membranous urethral strictures: experience with 105 consecutive cases. J Urol. 1980;123:500–5. doi: 10.1016/s0022-5347(17)55990-x. [DOI] [PubMed] [Google Scholar]
- 25.Koraitim MM. Transpubic urethroplasty revisited: Total, superior, or inferior pubectomy? Urology. 2010;75:691–4. doi: 10.1016/j.urology.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Turner-Warwick R. Prevention of complications resulting from pelvic fracture urethral injuries and from their surgical management. Urol Clin North Am. 1989;16:335–58. [PubMed] [Google Scholar]
- 27.Basiri A, Shadpour P, Moradi MR, Ahmadinia H, Madaen K. Symphysiotomy: a viable approach for delayed management of posterior urethral injuries in children. J Urol. 2002;168:2166–9. doi: 10.1016/S0022-5347(05)64345-5. [DOI] [PubMed] [Google Scholar]
- 28.Al-Rifaei MA, Zaghloul S, Al-Rifaei AM. Bulboprostatic anastomotic urethroplasty with preservation of potency: anatomical study, operative approach and clinical results. Scand J Urol Nephrol. 2005;39:163–8. doi: 10.1080/00365590310019972. [DOI] [PubMed] [Google Scholar]
- 29.Jordan GH, Eltahawy EA, Virasoro R. The technique of vessel sparing excision and primary anastomosis for proximal bulbous urethral reconstruction. J Urol. 2007;177:1799–802. doi: 10.1016/j.juro.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Fan M, Zhang Y, Huang C, Feng J, Xiao Y. Modified urethral pull-through operation for posterior urethral stricture and long-term outcome. J Urol. 2008;180:2479–85. doi: 10.1016/j.juro.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 31.Dalpiaz O, Mitterberger M, Kerschbaumer A, Pinggera GM, Bartsch G, Strasser H. Anatomical approach for surgery of the male posterior urethra. BJU Int. 2008;102:1448–51. doi: 10.1111/j.1464-410X.2008.07772.x. [DOI] [PubMed] [Google Scholar]
- 32.Abdalla MA. A posterior sagittal pararectal approach for repair of posterior urethral distraction injuries. Eur Urol. 2008;53:191–6. doi: 10.1016/j.eururo.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Mathur RK, Aggarwal H, Odiya S, Lubana PS. U- shaped prostatobulbar anastomosis for urethral injury after pelvic trauma. ANZ J Surg. 2008;78:605–9. doi: 10.1111/j.1445-2197.2008.04584.x. [DOI] [PubMed] [Google Scholar]
- 34.Webster GD, Sihelnik S. The management of strictures of the membranous urethra. J Urol. 1985;134:469–73. doi: 10.1016/s0022-5347(17)47243-0. [DOI] [PubMed] [Google Scholar]
- 35.Koraitim MM. The lessons of 145 posttraumatic posterior urethral strictures treated in 17 years. J Urol. 1995;153:63–6. doi: 10.1097/00005392-199501000-00024. [DOI] [PubMed] [Google Scholar]
- 36.Cooperberg MR, McAninch JW, Alsikafi NF, Elliott SP. Urethral reconstruction for traumatic posterior urethral disruption: outcomes of a 25-year experience. J Urol. 2007;178:2006–10. doi: 10.1016/j.juro.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Iselin CE, Webster GD. The significance of the open bladder neck associated with pelvic fracture urethral distraction defects. J Urol. 1999;162:347–51. [PubMed] [Google Scholar]
- 38.Corriere JN. 1-Stage delayed bulboprostatic anastomotic repair of posterior urethral rupture: 60 patients with 1-year followup. J Urol. 2001;165:404–7. doi: 10.1097/00005392-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Anger JT, Sherman ND, Dielubanza E, Webster GD, Hegarty PK. Erectile function after posterior urethroplasty for pelvic fracture-urethral distraction defect injuries. BJU Int. 2009;104:1126–9. doi: 10.1111/j.1464-410X.2009.08589.x. [DOI] [PubMed] [Google Scholar]
- 40.Singla M, Jha MS, Muruganandam K, Srivastava A, Ansari MS, Mandhani A, et al. Posttraumatic posterior urethral strictures in children--management and intermediate-term follow-up in tertiary care center. Urology. 2008;72:540–3. doi: 10.1016/j.urology.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 41.Hafez AT, El-Assmy A, Sarhan O, El-Hefnawy AS, Ghoneim MA. Perineal anastomotic urethroplasty for managing post-traumatic urethral strictures in children: the long-term outcome. BJU Int. 2005;95:403–6. doi: 10.1111/j.1464-410X.2005.05309.x. [DOI] [PubMed] [Google Scholar]
- 42.Orabi S, Badawy H, Saad A, Youssef M, Hanno A. Post-traumatic posterior urethral stricture in children: how to achieve a successful repair. J Pediatr Urol. 2008;4:290–4. doi: 10.1016/j.jpurol.2008.01.209. [DOI] [PubMed] [Google Scholar]
- 43.Podestá ML, Medel R, Castera R, Ruarte A. Immediate management of posterior urethral disruptions due to pelvic fracture: therapeutic alternatives. J Urol. 1997;157:1444–8. [PubMed] [Google Scholar]
- 44.al-Rifaei MA, Gaafar S, Abdel-Rahman M. Management of posterior urethral strictures secondary to pelvic fractures in children. J Urol. 1991;145:353–6. doi: 10.1016/s0022-5347(17)38337-4. [DOI] [PubMed] [Google Scholar]
- 45.El-Sheikh MG, Ziada AM, Sadek SZ, Shoukry I. Pediatric and adolescent transperineal anastomotic urethroplasty. J Pediatr Urol. 2008;4:333–6. doi: 10.1016/j.jpurol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Gupta NP, Mishra S, Dogra PN, Hemal AK, Seth A, Kumar R. Does a previous end-to-end urethroplasty alter the results of redo end-to-end urethroplasty in patients with traumatic posterior urethral strictures? Int J Urol. 2008;15:885–8. doi: 10.1111/j.1442-2042.2008.02135.x. [DOI] [PubMed] [Google Scholar]
- 47.Singh BP, Andankar MG, Swain SK, Das K, Dassi V, Kaswan HK, et al. Impact of prior urethral manipulation on outcome of anastomotic urethroplasty for post-traumatic urethral stricture. Urology. 2010;75:179–82. doi: 10.1016/j.urology.2009.06.081. [DOI] [PubMed] [Google Scholar]
- 48.Lumen N, Hoebeke P, Troyer BD, Ysebaert B, Oosterlinck W. Perineal anastomotic urethroplasty for posttraumatic urethral stricture with or without previous urethral manipulations: a review of 61 cases with long-term followup. J Urol. 2009;181:1196–200. doi: 10.1016/j.juro.2008.10.170. [DOI] [PubMed] [Google Scholar]
- 49.Koraitim MM. Post-traumatic posterior urethral strictures: preoperative decision making. Urology. 2004;64:228–31. doi: 10.1016/j.urology.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Aggarwal SK, Goel D, Gupta CR, Ghosh S, Ojha H. The use of pedicled appendix graft for substitution of urethra in recurrent urethral stricture. J Pediatr Surg. 2002;37:246–50. doi: 10.1053/jpsu.2002.30265. [DOI] [PubMed] [Google Scholar]
- 51.Khazanchi RK, Dorairajan LN, Dogra PN, Nanda V, Chahal R. Free-flap urethroplasty for a complex, long-segment stricture of the bulbomembranous urethra. J Reconstr Microsurg. 1998;14:223–5. doi: 10.1055/s-2007-1000172. [DOI] [PubMed] [Google Scholar]
- 52.Golimbu M, al-Askari S, Morales P. Transpubic approach for lower urinary tract surgery: a 15-year experience. J Urol. 1990;143:72–6. doi: 10.1016/s0022-5347(17)39869-5. [DOI] [PubMed] [Google Scholar]
- 53.Podestá ML. Use of the perineal and perineal-abdominal (transpubic)approach for delayed management of pelvic fracture urethral obliterative strictures in children: long-term outcome. J Urol. 1998;160:160–4. [PubMed] [Google Scholar]
- 54.Xu YM, Sa YL, Fu Q, Zhang J, Jin SB. Surgical treatment of 31 complex traumatic posterior urethral strictures associated with urethrorectal fistulas. Eur Urol. 2010;57:514–21. doi: 10.1016/j.eururo.2009.02.035. [DOI] [PubMed] [Google Scholar]


