Abstract
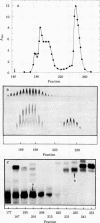
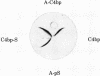
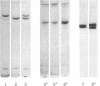
Protein S, a recently described vitamin K-dependent plasma protein, is shown to exist in two forms in plasma--free protein and in complex with C4b-binding protein. C4b-binding protein is involved in the regulation of the rate of complement activation. A major proportion of C4b-binding protein in plasma is in complex with protein S. The complex is a major and previously unrecognized component of the group of plasma proteins that adsorbs to barium citrate. The complex dissociates in the presence of NaDodSO4, indicating that C4b-binding protein and protein S are held together by noncovalent bonds. Uncomplexed C4b-binding protein was purified from the supernatant after barium citrate adsorption. On NaDodSO4/polyacrylamide gels without reduction, it appeared to have a slightly faster migration rate than the C4b-binding protein dissociated from the complex with protein S. After reduction, the subunits of the two forms of C4b-binding protein appeared to have identical molecular weights. Furthermore, there is an equilibrium between free and bound protein S in plasma. The role of protein S in the complex is unknown.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Di Scipio R. G., Hermodson M. A., Yates S. G., Davie E. W. A comparison of human prothrombin, factor IX (Christmas factor), factor X (Stuart factor), and protein S. Biochemistry. 1977 Feb 22;16(4):698–706. doi: 10.1021/bi00623a022. [DOI] [PubMed] [Google Scholar]
- DiScipio R. G., Davie E. W. Characterization of protein S, a gamma-carboxyglutamic acid containing protein from bovine and human plasma. Biochemistry. 1979 Mar 6;18(5):899–904. doi: 10.1021/bi00572a026. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Fernlund P., Stenflo J., Roepstorff P., Thomsen J. Vitamin K and the biosynthesis of prothrombin. V. Gamma-carboxyglutamic acids, the vitamin K-dependent structures in prothrombin. J Biol Chem. 1975 Aug 10;250(15):6125–6133. [PubMed] [Google Scholar]
- Fujita T., Gigli I., Nussenzweig V. Human C4-binding protein. II. Role in proteolysis of C4b by C3b-inactivator. J Exp Med. 1978 Oct 1;148(4):1044–1051. doi: 10.1084/jem.148.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Nussenzweig V. The role of C4-binding protein and beta 1H in proteolysis of C4b and C3b. J Exp Med. 1979 Aug 1;150(2):267–276. doi: 10.1084/jem.150.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Fujita T., Nussenzweig V. Modulation of the classical pathway C3 convertase by plasma proteins C4 binding protein and C3b inactivator. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6596–6600. doi: 10.1073/pnas.76.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Ichihara C., Stroud R. M. Cleavage of C4b by C3b inactivator: production of a nicked form of C4b, C4b', as an intermediate cleavage product of C4b by C3b inactivator. J Immunol. 1980 Aug;125(2):578–582. [PubMed] [Google Scholar]
- Nelsestuen G. L., Kisiel W., Di Scipio R. G. Interaction of vitamin K dependent proteins with membranes. Biochemistry. 1978 May 30;17(11):2134–2138. doi: 10.1021/bi00604a017. [DOI] [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. Activation of the complement system by antibody-antigen complexes: the classical pathway. Adv Protein Chem. 1979;33:1–71. doi: 10.1016/s0065-3233(08)60458-1. [DOI] [PubMed] [Google Scholar]
- Scharfstein J., Ferreira A., Gigli I., Nussenzweig V. Human C4-binding protein. I. Isolation and characterization. J Exp Med. 1978 Jul 1;148(1):207–222. doi: 10.1084/jem.148.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenflo J. A new vitamin K-dependent protein. Purification from bovine plasma and preliminary characterization. J Biol Chem. 1976 Jan 25;251(2):355–363. [PubMed] [Google Scholar]
- Stenflo J., Jönsson M. Protein S, a new vitamin K-dependent protein from bovine plasma. FEBS Lett. 1979 May 15;101(2):377–381. doi: 10.1016/0014-5793(79)81048-0. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Walker F. J. Regulation of activated protein C by a new protein. A possible function for bovine protein S. J Biol Chem. 1980 Jun 25;255(12):5521–5524. [PubMed] [Google Scholar]